?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to understand the willingness of and affecting factors of non-national immunization program (non-NIP) vaccines among children’s parents during the COVID-19 era in Shanghai, China. A cross-sectional survey was conducted with parents who attended vaccination clinics in four out of 16 districts in Shanghai, China. Data was obtained using a self-administered structured questionnaire. A multivariate logistic regression model was used to analyze factors associated with vaccination acceptability. In total, 1691 valid questionnaires were obtained. Of the participants, 69.5% (1,176/1,691) reported being interested in non-NIP vaccines for their children. Further, respondents were more likely to be willing to get non-NIP vaccines for their children if they had an income of 10,000–20,000CNY or more, an educational level of college or above, and if getting the vaccination was moderately convenient or convenient. Respondents were less likely to be willing to get the vaccines if they were in the 30–39 age group and had moderate or low satisfaction with the vaccine. Many parents are willing to get non-NIP vaccines for their children. However, some demographic factors, perceived convenience and satisfaction of vaccination, perceived necessity, safety and price barrier of non- NIP influenced the acceptability of non-NIP vaccines in Shanghai. Our findings can help guide future efforts to increase non-NIP vaccines acceptability.
Introduction
Vaccines are one of the greatest advancements in the history of public health.Citation1 Since the launch of the National Immunization Program (NIP), significant achievements have been made and the incidence of infectious diseases has sharply declined.Citation2 In China, vaccination programs are divided into NIP and non-NIP. NIP vaccines are free and mandatory and the budget for NIP vaccines is covered by the Chinese central government, while non-NIP vaccines are optional and self-paid.Citation3 As a result, the coverage of NIP vaccines is high.Citation4,Citation5
As an effective supplement to the NIP vaccines, the non-NIP vaccines have played an important role in disease outbreak control and prevention, helping curtail infection spread and build herd immunity. Due to limited health financing, the types of vaccines included in NIP are relatively limited. Some non-NIP vaccines that are typically included in many countries have not been included in China. For example, according to the position paper of the World Health Organization position paper, the streptococcus pneumoniae vaccine is the first choice among the high-priority vaccines, and the Haemophilus influenzae type B vaccine is also the first recommended vaccine for young children.Citation6 However, both of these are considered optional and constitute caregivers’ out-of -pocket expenses in China.
In recent years, the coverage rate of NIP vaccines among children has been higherCitation7 than the coverage rate of non-NIP vaccines.Citation8 Some investigations show regional differences in the coverage rate of non-NIP vaccines, which is the highest in eastern China, followed by the central region, and the lowest in Western China.Citation7,Citation8 Moreover, a longitudinal follow-up study showed a remarkable decline in the willingness to receive vaccination in China since the outbreak of coronavirus disease 2019 (COVID-19).Citation9 Shanghai is the largest and most developed city in China with approximately 24 million people. There are more than 390 vaccination clinics that can provide non-NIP vaccines. Although the amount of non-NIP vaccines increased yearly from 2010 to 2018, few studies have investigated the acceptance of non-NIP vaccines in Shanghai since the outbreak of COVID-19.
Parents are the decision-makers for their children’s vaccination, particularly regarding administering non-NIP vaccines. This study aimed to examine the acceptability of non-NIP vaccines and the influencing factors for choosing non-NIP vaccines among parents of children in Shanghai city, so as to provide references for improving the immunization rate of non-NIP vaccines.
Methods
Study setting and subjects
This study was conducted in four of the 16 districts in Shanghai city, including Pudong, Qingpu, Xuhui, and Huangpu districts. According to the Shanghai Bureau of Statistics data, the 16 districts were stratified into two groups by their economic development, for this study, two districts were randomly selected from each group. In each district, three health service centers were selected. Most non-NIP vaccines are administered to children under the age of 6 years under the guardianship of their parents, who decide their children’s vaccines. Hence, parents with children aged 0–6 years, were recruited for this study from March 2021 to July 2021.
The sample size of this cross-sectional studyCitation10 was calculated using the formula:
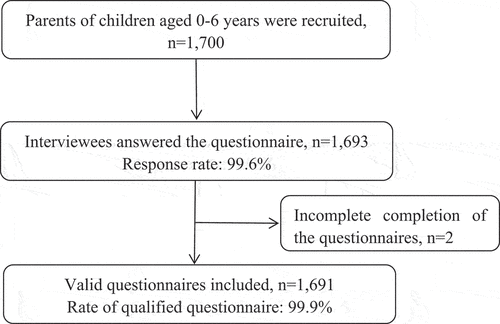
. The parameters for the calculation were set as follows: a two-tailed ɑ of 5%, the proportion of acceptance of non-NIP among parents was 50%, and a permission odds ratio (OR) or (d) of .05. The minimum sample size for each district was 385, and the total sample size was at least 1,540 for the entire city. As shown , a total of 1700 subjects were enrolled and 1,693 interviewees volunteered to answer the self-administered questionnaire. The response rate was 99.6%. Of these volunteers, 2 were excluded because of incomplete completion of the questionnaires. Finally, 1,691 questionnaires with valid date were included in this study.
Data collection and quality management
The study was approved by the Ethics Committee of the Shanghai Center for Disease Control and Prevention. All subjects provided informed consent before the survey. A cross-sectional survey was conducted using a self-administered questionnaire. The questionnaire was developed based on previous studiesCitation11,Citation12 of the Shanghai Center for Disease Control and Prevention. The internal consistency reliability of the questionnaire calculated using Cronbach’s ɑ was 0.71. The questionnaire’s contents were as follows:
Socio-demographic characteristics, such as age, sex, education, family income
Knowledge, perceived safety, efficacy, and the necessity for non-NIP vaccines. (Six items determined the knowledge of non-NIP vaccines, ranging from 0 to 1, and three items determined the perceiving safety, efficacy, and necessity, on a 5-point response scale ranging from “not at all” to “very”)
Two items determined the convenience and satisfaction of vaccination
Acceptance of and attitude toward non-NIP vaccines. Parents who wanted to get non-NIP vaccines for their children were considered as willing to accept.
Trained fieldworker conducted face-to-face interviews with the parents of children aged 0–6 years A total of 1700 subjects were randomly selected.
Statistical analysis
SPSS 17.0 (SPSS Inc, Chicago, IL) was used for the data analysis. Descriptive statistics were generated for all variables. The chi-squared test was used for univariate analysis. Multivariate logistic regression was then performed between the different acceptability groups to identify the influencing factors. Significance was assessed at an alpha = .05 level and 95% confidence interval (CI).
Results
Sociodemographic characteristics
A total of 1,691 valid questionnaires, of the 1,700 total responses from parents, were analyzed, with a valid questionnaire return ratio of 99.5%. The age distribution of participants was as follows: 35.5% of ages lay in 20–29 years, 57.6% in 30–39 years, and 6.9% were 40 years and above. Among the present sample, 50.8% (859/1,691) were residents in Shanghai, and 49.2% (832/1,691) were migrants. Further, 11.3% (191/1,691), 37.4% (168/1,691), and 51.3% (262/1,691) of the participants had studied till junior high school or below, high school or technical secondary school, college or above, respectively. Additionally, 61.0% (1,032/1,691) participants had only one child and 39.0% (659/1,691) had more than one child. A total of 6.6% (121/1,691), 41.4% (700/1,691), 22.0% (372/1,691) and 30.0% (507/1,691) of the surveyed households had a monthly income per capita of less than 2,000 CNY, 2,000–5,000 CNY, 5,000–10,000 CNY, 10,000–20,000 CNY, and more than 20,000 CNY, respectively ().
Table 1. The socio-demographics of the participants (N = 1,691).
Acceptability of childhood non-NIP vaccines
The results revealed that 69.5% (1,176/1,691) of participants would opt for non-NIP vaccines for their children, and the acceptability would increase to 86.3% if non-NIP vaccines were covered by health insurance. The chi-squared test results revealed that the proportion of acceptability varied by gender (χ2 = 10.70, p < .001), age group (χ2 = 189.74, p < .001), educational level (χ2 = 24.05, p < .001), immigration status (χ2 = 16.66, p < .001), and household income (χ2 = 19.75, p < .001). Most participants [76.6% (1296/1691)] thought it is very convenient to the immunization clinic, and 82.1% (1389/1691) rated their previous vaccination experience as good. Perceived convenience (χ2 = 85.77, p < .001) and satisfaction of vaccination (χ2 = 6.95, p = .031) showed a significant relationship with acceptability of non-NIP vaccines for their child ().
Table 2. Association between categorical variables and acceptance of childhood non-NIP vaccines.
Non-NIP vaccines knowledge and attitude
Of the 1,691 respondents, most (87.82%, 1,485) were aware that vaccines were divided into NIP and non-NIP vaccines, 1,479 (87.46%%, 1,479) were aware that non-NIP vaccines were voluntary vaccines, which were administered at their own cost, and 1,010 (91.0%) believed that non-NIP vaccines were helpful in the prevention of related diseases. A total of 868 (78.2%) participants agreed on the safety guaranteed by the non-NIP vaccines, and only 648 (58.4%) believed that fever or redness and pain at the vaccination site were usual adverse effects of vaccination. The respondents stated that the primary factors influencing their vaccine selection were safety (612, 55.1%) and effectiveness of vaccines (292, 26.3%). Further, 420 (37.8%) reported receiving information about the vaccines from the Internet and 418 (37.7%) from doctors. Several variables were correlated with the acceptability of childhood non-NIP vaccines in the bivariate analysis described in .
Table 3. Bivariate correlates of childhood non-NIP vaccines acceptability for continuous variables.
Factors associated with non-NIP vaccines acceptance
Through the multivariable analysis, it was found that the respondents were more willingly get non-NIP vaccines for their children in the following cases:
If they had an income of 10000–20000 CNY (OR = 1.65, 95%CI: 1.21–2.26) or more (OR = 2.23, 95%CI: 1.27–3.90)
An educational level of college or above (OR = 2.36, 95%CI:1.73-3.23)
Had a moderate (OR = 1.45, 95%CI: 1.01–2.11) or convenient (OR = 4.68, 95%CI: 2.91–7.52) of immunization
Had awareness about non-NIP vaccines (OR = 1.77, 95%CI: 1.51–2.07)
Perceived non-NIP vaccines as safe (OR = 1.20, 95%CI: 1.05–1.38)
Consider the lower effect of vaccine price on vaccination intention (OR = 1.43, 95%CI:1.27–1.62)
However, the respondents were less willing if they were in the 30–39 age group (OR = 0.25, 95%CI: 0.14–.45), and had moderate (OR = 0.55, 95%CI: 0.37–.82) or bad immunization experience (OR = 0.22, 95%CI:0.09–.51) ().
Table 4. Multivariable correlates of non-NIP vaccine acceptability.
Discussion
Vaccination is considered to be one of the most remarkable public health interventions. The number of infectious diseases has been greatly reduced through vaccination,Citation13 and diseases, such as measles, rubella, and other respiratory diseases, have been effectively controlled. The COVID-19 pandemic might change people’s perception of vaccines. This study examined acceptability and hesitancy toward non-NIP vaccines among parents of children (0–6 years of age) and the factors associated with parents’ intention to vaccinate their children with non-NIP vaccines. Understanding these factors is significant for providing a scientific basis and theory for promoting the use of vaccines and strengthening the prevention and control of the infectious diseases.
We found that, in Shanghai, nearly 70% of parents would like to opt for non-NIP vaccines for their children at their own expense against COVID-19, and if non-NIP vaccines were covered by health insurance, the acceptability would increase to 86.3%. Considering China’s current budget, it is impossible to include all the non-NIP vaccines in NIP. To improve the coverage of non-NIP vaccines, a part of them can be included under residents’ health insurance. Previous research in other countries has also shown that incorporating vaccines into a publicly-funded program can significantly increase vaccination coverage,Citation14 and thus, it is necessary to explore the use of health insurance to pay for non-NIP vaccines in the future.
This study demonstrated that some demographic factors, such as age, educational level, immigration status, household income, were associated with parents’ vaccination intention. The multivariate analysis showed that the household income level was significantly correlated with the willingness to get non-NIP vaccines for children, and parents with a higher household income had higher acceptance of non-NIP vaccines for their children, which was consistent with other studies conducted in the provinces of the eastern, central and western part of China. The result showed that cost is an important barrier to the accessibility to self-paid vaccination in China.Citation15–17 Studies have shown a higher coverage rate of non-NIP vaccines associated with household income level and higher economic development.Citation18 Some non-NIP vaccines were expensive, therefore, family with lower income may be restricted the access to these self-paid vaccines. Immigration status influenced parents’ acceptability of non-NIP vaccines in this study. Immigration is thus another determinant of who is more likely to get their children vaccinated with non-NIP vaccines. Therefore, policymakers should communicate about vaccinations and health promotion among the immigrant population. In this study, parents with a college-level education or above were associated with greater acceptance of non-NIP vaccines. Studies have also shown is a positive correlation between the educational level and acceptability of non-NIP vaccines.Citation19–21 Parents’ education level may influence their knowledge, attitude and behavior, making them take advantage of vaccination services for their child.
Our analysis revealed that a greater price barrier perceived by parents was associated with lower acceptance of non-NIP vaccines for children. Economic income and price are important hindrance factors in the acceptance of non-NIP vaccines. Lack of confidence in the safety and knowledge of non-NIP vaccines directly lead to low acceptance, which is similar to previous studies on some non-NIP vaccines.Citation22
Further, our study found that recognition of the safety, effectiveness, and necessity of non-NIP vaccines was associated with acceptance from parents. Parents were often decided whether their children should receive non-NIP vaccines. Therefore, their understanding and knowledge of non-NIP vaccines were significantly related to their acceptability.Citation23,Citation24 We will expand publicity and education in the future. By publicizing non-NIP vaccines and generating awareness in community health service stations, community publicity boards, neighborhood committees, village committees and other places, the public’s vaccine awareness rate can be improved, further enhancing their willingness to vaccinate. Media channels can also be used as a further push to enhance the public’s knowledge of vaccination.
Studies have shown low public acceptance due to negative perceptions of the safety (19.2%) and effectiveness (42.9%) of the vaccines.Citation25 Contrastingly, in our study, the perceived safety and effectiveness of non-NIP vaccines were relatively high. It may be due to the following factors: first, the COVID-19 pandemic has changed people’s perception of vaccines across the country. Public acceptance of vaccines is at an unprecedented level, based on expectations for the novel coronavirus vaccine. This has greatly contributed to the promotion of immunization programs. Second, vaccination rates are high in economically developed areas where people’s health literacy is higher.
There were several limitations of this study. First, although our samples were from four different economically developing districts of Shanghai, the convenient sampling of parents who visited clinics to vaccinate their children during the proposed study period may not adequately represent Shanghai’s population. Second, the cross-sectional nature of this study limits the extent to which we can evaluate changes in the acceptance of vaccines over time. Third, the study design utilized face-to-face interviews. Thus, potential recall and social desirability bias could not be avoided, which could have affected our results.
In conclusion, nearly 70% of the surveyed parents were willing to get non-NIP vaccines for their children in the era of COVID-19. The acceptance would increase to 86.3% if non-NIP vaccines were covered by health insurance. Our results highlight that non-NIP vaccines acceptability may differ according to demographic characteristics, as well as the important role that convenience and satisfaction of vaccination play in acceptability of non-NIP vaccines. Furthermore, a lack of confidence in the safety and necessity and knowledge of non-NIP vaccines directly lead to low acceptance. These findings can guide the planning and development of future public health efforts to increase acceptability of non-NIP vaccines.
Ethics considerations
This study was approved by the ethical review board of Shanghai Municipal Center for Disease control and prevention. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from each participants.
Acknowledgments
The authors would like to thank the vaccination staff from the four districts CDCs for their help with the investigation and data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. 10 Facts on immunization [EB/OL]. Available from: http://www.who.int/features/factfiles/immunization/en
- Cao X. Immunology in China: the past, present and future. Nat Immunol. 2008;9(4):339–6. doi:10.1038/ni0408-339. PMID: 18349809.
- Standing Committee of the National People’s Congress, Standing Committee of the National People’s Congress. Vaccine administration law of the People’s Republic of China. 2019 Jan 12.
- Cao L, Wang HQ, Zheng JS, Yuan P, Cao LS, Zhang GM, Jiang KY. National immunization coverage survey in China after integrated more vaccines into EPI since 2008. Chin J Vaccines Immunization. 2012;05:40–45. Available from: http://hdl.handle.net/10393/30220
- Hu Y, Luo S, Tang X, Lou L, Chen Y, Guo J. Comparative assessment of immunization coverage of migrant children between national immunization program vaccines and non-national immunization program vaccines in East China. Hum Vaccin Immunother. 2015;11(3):761–68. [ Epub]. doi:10.1080/21645515.2015.1012015.
- Liu ZQ, Bai YH, Zheng DY. Introduction and application recommendations of National Immunization Program (NIP) and non-NIP vaccine in China. Zhonghua Er Ke Za Zhi. 2020;58(6):524–26. doi:10.3760/cma.j.cn112140-20200309-00202.
- Mahoney RT, Krattiger A, Clemens JD, Curtiss R. The introduction of new vaccines into developing countries IV: global access strategies. Vaccine. 2007;25(20):4003–11. doi:10.1016/j.vaccine.2007.02.047.
- Zhang XH, Li N, Zhang SF, Xia SC, Zhang RJ. Research progress on the status quo and influencing factors of the second type of vaccination in China. China Prev Med. 2018;07:548–52. doi:10.16506/j.1009-6639.2018.07.015.
- Wang J, Lu X, Lai X, Lyu Y, Zhang H, Fenghuang Y, Jing R, Li L, Yu W, Fang H. The changing acceptance of COVID-19 vaccination in different epidemic phases in China: a longitudinal study. Vaccines (Basel). 2021;9(3). Advance online publication. doi:10.3390/vaccines9030191.
- Shen HB, Qi XY. Epidemiology. 9th ed. Beijing: People’s Medical Publishing House; 18; 2018.
- Dror AA, Eisenbach N, Taiber S, Morozov NG, Mizrachi M, Zigron A, Srouji S, Sela E. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur J Epidemiol. 2020;35(8):775–79. doi:http://dx.doi.org/10.1007/s10654-020-00671-y.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi:http://dx.doi.org/10.1016/S0140-6736(20)30566-3.
- Greenwood B. The contribution of vaccination to global health: past, present and future. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645):20130433. doi:10.1098/rstb.2013.0433.
- Andrews RM. Assessment of vaccine coverage following the introduction of a publicly funded pneumococcal vaccine program for the elderly in Victoria, Australia. Vaccine. 2005;23(21):2756–61. doi:10.1016/j.vaccine.2004.11.039.
- Chang J, Hou ZY, Yue DH, Wu Q, Fang H, Meng QY. Factors related to self-paid vaccination and its related factors among children aged 0–3 years in China. Chin J Public Health. 2014;5:579–82.
- Wang ZB, Wang W, Guo WS, Xu J, Li J. Survey on vaccination rate of second class vaccine and influential factors in children in rural areas of nine counties in Henan province. Prog Microbiol Immunol. 2013;41(3):53–61. doi:10.3969/j.issn.1005-5673.2013.03.012.
- Liu Y, Lu HM. Data analysis of second type vaccine inoculation situation in Shanghai Songjiang district. World Clin Drugs. 2017;38:421–23.
- Zheng JS, Cao L, Guo SC, Kz A, Wang L, Yu WZ, Yuan P, Jiang KY, Zhang GM, Cao LS, et al. Survey on the immunization status of category B vaccine among children aged 1 to 2 years in China. Chinese Journal of Vaccines and Immunization. 2012;18(3):233–37. doi:10.13683/j.wph.2017.06.014.
- Hu HS, Wei XL, Ren ZF, Zeng XM, Zhao FH, Qiao YL. Investigation on acceptance of HPV vaccination and its determinants among the parents of junior high school students in Guangzhou city. Chin J Dis Control Prev. 2014;18:659–62.
- Jiang Y, Yin H, Shi YH, Yuan YF, Cao WN, Zeng QQ, Chang C. Immunization status of extra EPI vaccines and its influencing factors among children aged 1–6 years in Chongqing. Chin J Health Educ. 2013;7:605–7, 630.
- Ganczak M, Dmytrzyk-Daniłów G, Karakiewicz B, Korzeń M, Szych Z. Determinants influencing self-paid vaccination coverage, in 0–5 years old polish children. Vaccine. 2013;31(48):5687–92. doi:10.1016/j.vaccine.2013.09.056.
- Zhang J, While AE, Norman IJ. Seasonal influenza vaccination knowledge, risk perception, health beliefs and vaccination behaviours of nurses. Epidemiol Infect. 2012;140(9):1569–77. doi:10.1017/S0950268811002214.
- Bakhache P, Rodrigo C, Davie S, Ahuja A, Sudovar B, Crudup T, Rose M. Health care providers’ and parents’ attitudes toward administration of new infant vaccines–a multinational survey. Eur J Pediatr. 2013;172(4):485–92. doi:10.1007/s00431-012-1904-4.
- Poland GA, Jacobson RM, Tilburt J, Nichol K. The social, political, ethical, and economic aspects of biodefense vaccines. Vaccine. 2009 Suppl;27(4):D23–7. doi:10.1016/j.vaccine.2009.08.054.
- Wang XQ, Lu Q, Hou ZY. Vaccine confidence and vaccination attitude and willingness among Chinese residents: a systematic review. Chin J Public Health. 2020;36(12):1832–37. doi:10.11847/zgggws1126270.