ABSTRACT
This study aimed to investigate the public health and economic benefit of using a quadrivalent influenza vaccine (QIV) instead of a trivalent influenza vaccine (TIV) in past seasons in Paraguay. The budget impact of switching from TIV to QIV in the Immunization Program was also evaluated. The adapted model includes two modules. The first compared retrospectively Health and Economic outcomes resulting from the use of QIV instead of TIV. The second forecast the spending and savings that would be associated with the switch from TIV to QIV. Our findings estimate that the switch from TIV to QIV during the seasons 2012 to 2017 could have prevented around 2,600 influenza cases, 67 hospitalizations and 10 deaths. An alternative scenario using standardized estimates of the burden of influenza showed that 234 influenza-related hospitalizations and 29 deaths could have been prevented. The estimated annual budget impact of a full switch from TIV to QIV was around USD1,6 million both from the payer and societal perspectives. Those results are mainly driven by vaccine prices and coverage rate. In sum, this manuscript describes how the use of QIV instead of TIV could have prevented influenza cases and subsequent complications that led to hospitalizations and deaths. This could have generated savings for the health system and society, offsetting part of the additional investment needed to switch from TIV to QIV.
Introduction
Influenza is an acute respiratory infection caused by viruses that are members of the Orthomyxoviridae family. Four genera of influenza viruses exist (A, B, C and D); however, only types A and B, which co-circulate, cause seasonal epidemics in humans. The other two types of influenza viruses are clinically less important: type C can cause mild illness, while type D infects cattle but infections in humans have not been reported.Citation1,Citation2
The burden of seasonal influenza epidemics is still significant. On average, 389,000 deaths worldwide (CI [294,000;518,000]) are attributable to influenza annually (67% occurring in the elderly).Citation3 Another study estimated that, annually, the influenza infection is responsible for 5,678,000 hospitalizations (95% CI [3,205,000;9,432,000]).Citation4 In Paraguay, a study in 10 local hospitals from 2011 to 2015 reported that the incidence of influenza-related hospitalization was 20.3/100,000 and the associated mortality was 1/100,000. In the elderly, those estimates were 98.8/100,000 and 16.8/100,000, respectively.Citation4
Severe forms of influenza can lead to hospitalization and death, particularly in certain high-risk populations.Citation5 Populations at risk for severe complications of influenza include young children, the elderly and people with chronic comorbidities.Citation6 Therefore, the Paraguayan Ministry of Health offers free influenza vaccinations to individuals older than 60 years, children between 6 and 35 months old, and individuals within other age groups with comorbidities, as well as health care workers, pregnant women and other vulnerable groups.Citation7 Currently, trivalent influenza vaccines (TIVs) containing antigens derived from 2 influenza A virus subtypes (A/H1N1 and A/H3N2) and 1 influenza B virus lineage (either B/Victoria or B/Yamagata lineage, based on World Health Organization South Hemisphere annual recommendations) are available in Paraguay. Nevertheless, analysis of the distribution of influenza B/Victoria and B/Yamagata cases in Paraguay in the years 2010–2017 showed a high level of mismatch between circulating B viruses and the ones included in the TIV.Citation8 To offer broader protection against influenza B viruses, a quadrivalent influenza vaccine (QIV) containing both influenza B lineages has been developed.
This study aimed to assess the value of the use of a QIV instead of TIV investigating what would have been the public health and economic impacts of using QIV instead of TIV over six past influenza seasons (from 2012 to 2017) in Paraguay. The future budget impact of switching from TIV to QIV in the National Expanded Program Immunization was also estimated and presented.
Materials and methods
Model structural choices
Analysis
A model combining epidemiological and budget impact modules was used to:
compare the health and economic outcomes that would have occurred if the current population vaccinated with TIV would have received QIV in Paraguay and,
estimate the potential budget impact of the TIV switch with QIV.
To reflect the targeted population, it was stratified by age and risk status: 6 months to 3 years of age; 4 to 19 years of age with chronic comorbidities (referred to as high risk), 20 to 59 years of age at high risk, and 60 years of age and older.
The impact on the seasonal number of influenza cases, related consultations to the general practice, hospitalizations and deaths, as well as the associated costs were estimated. From the societal perspective, absenteeism due to sick leave was also considered. Long-term consequences of influenza infections, such as productivity losses due to premature death, were not taken into account.
The model included two modules: retrospective and prospective (i.e., epidemiological and budget impact).
Retrospective module
The retrospective epidemiological module () was based on the structure published by Reed et al. and adapted for several countries.Citation9–12 It assessed retrospectively how many influenza-related outcomes and associated expenditures would have been prevented by using QIV instead of TIV from 2012 to 2017 in Paraguay.
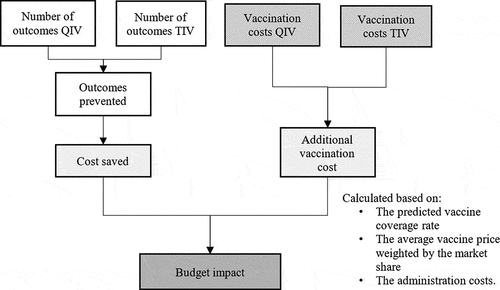
Figure 1. Diagram of the retrospective module. QIV = quadrivalent influenza vaccine; TIV = trivalent influenza.
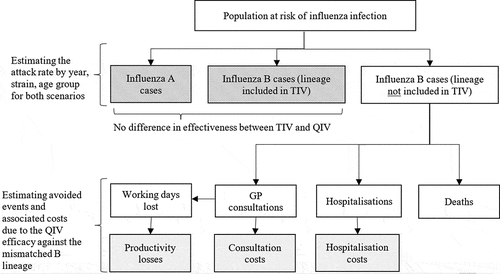
To simulate the number of cases in the vaccinated populations, the influenza-related attack, consultation, hospitalization and mortality rates from an unvaccinated population were adjusted by:
the vaccination coverage rate observed in the included populations,
the vaccine efficacy against the specific influenza virus type (A, B lineage included in the vaccine) and,
the percentage of cases due to each strain.
A level of cross-protection was assumed for the B lineage not included in the vaccine. The outcome rates being obtained from surveillance data in the general population were corrected to estimate the rates in a hypothetically unvaccinated population.
Prospective module
The prospective budget impact module () estimated the impact of switching from TIV to QIV on public spending over the subsequent five years. It compared the additional investment necessary to introduce QIV to the National Program Immunization to potential savings resulting from the use of QIV instead of TIV. Therefore, on top of the cost items previously cited, the model included the vaccination costs (i.e., prices of vaccine doses and administration costs). The model then projected the influenza-related outcome rates estimated in the retrospective module over the next five years (considering annual vaccine coverage rates and market share projections).
Population parameters
Local inputs were used when available. All inputs were automatically recalculated to match the age groups included in the model, assuming a uniform distribution of individuals in each year within an age group.
Population
The age-specific population size was derived from national statistics with projections for 2020 and served as a basis to calculate the percentages at high risk among individuals 36 months to 19 years of age and those 20 to 59 years of age.Citation13 The percentage of individuals categorized as “high risk” is the percentage of patients having at least one of the following conditions: HIV/AIDS, tuberculosis, cancers with direct immunosuppression, cancers with possible immunosuppression, cardiovascular diseases, chronic respiratory diseases, chronic liver diseases, obesity, diabetes mellitus, chronic kidney diseases, chronic neurological disorders and sickle cell disorders. This percentage was estimated for Paraguay in 2020 using the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) (2017) and UN population estimates for 2020 which were published by Clark et al. 2020.Citation14 This work was originally conducted for estimating the percentage of persons at increased risk of COVID-19. However, the conditions listed as risk factors are similar to those included in the recommendations for influenza vaccination.Citation15
Vaccination coverage rate
The vaccination coverage rate was calculated as the number of vaccinated individuals provided by the Ministry of Health divided by the population size. The average from 2006 to 2018 was considered. For infants (6 to 35 months old), with no prior vaccination, the recommendation is two doses, each of .25 mL, and for adults and children >4 years of age, the recommendation is one dose of .5 mL. Hence, for infants, the model included the price of two doses. However, no additional efficacy was considered for the second dose. This assumption was informed by the conclusions of a Cochrane review on vaccines for preventing influenza in healthy children, stating that there was no evidence of a difference in the vaccine effect between the group receiving two doses and the group receiving only one dose.Citation16 The same coverage was considered over the 5-year time horizon of the budget impact analysis.
Vaccine efficacy
The vaccine efficacy against influenza A and B, both for the strain included in the TIV and not (resulting from cross-protection), was derived from a cost-effectiveness analysis conducted by Clements et al.Citation17 The vaccine efficacy estimates used in the cost-effectiveness analysis were obtained from a meta-analysis of the published clinical data for TIV.Citation16,Citation18–20
Epidemiological data
The epidemiological data used in the reference case were obtained from surveillance data in the general population (i.e., in which a part of the population received the vaccine). Therefore, to be populated in the model, those rates were corrected by the observed vaccination coverage and TIV efficacy to estimate the rates that would have been obtained in a fully unvaccinated population.
Virus circulation
Unpublished virologic surveillance data provided by the Central Laboratory of Public Health (Laboratorio Central de Salud Pública, data on file) were considered.Citation21 However, the distribution of cases attributable to B/Yamagata and B/Victoria lineage before 2014 was not available; therefore, the average distribution over four seasons (from 2014 to 2017) was considered.
Influenza attack rates
The influenza attack rates considered in the reference cases were the number of influenza cases notified to the Directorate General for Health Surveillance (DGVS—Dirección General de Vigilancia de la Salud) and the influenza laboratory-confirmed cases reported by the Central Laboratory of Public Health (Laboratorio Central de Salud Pública—LCSP)”.Citation22
Consultations
Influenza-related consultation rates were calculated by applying to the influenza cases an average number of consultations per case (opinion from a panel of ten experts) and corrected by the probability of consultation per case.Citation23
Hospitalizations
Influenza-related hospitalization rates were obtained from the National Hospital Database (Sistema de Egresos Hospitalarios—SEGHOSP) provided by the Ministry of Public Health (Ministerio de Salud Pública y Bienestar Social—MSPyBS) and the General Directorate of Strategic Health Information (Dirección General de Información Estratégica en Salud—DIGIES).Citation24 As only the stays in hospitals depending on the MSPyBS were recorded, the population size used as the denominator was corrected to account only for the catchment population of these institutions (i.e., the population served by the facility). To do so, the general population size was corrected by the ratio of the total number of stays in the sentinel centers divided by the total number of stays in all establishments in Paraguay.
The influenza-related outcome rates used for the reference case are presented in Supplementary Table S1.
Mortality
The influenza-related mortality rates were calculated by applying to the hospitalization data the case-fatality ratio per influenza-related hospitalization published in the DGVS weekly epidemiological reports for the years 2010 to 2017.Citation22
Standardized approach
Local surveillance data, while being a valuable source of information, are subject to certain limitations. Firstly, the data are collected only if the patients enter the healthcare system, i.e., if they seek medical care. Influenza in its less severe form is well known to resolve without medical care. Therefore, an important proportion of patients choose not to seek medical care in case of influenza illness or influenza-like illness.Citation23,Citation25 In addition, even patients consulting medical staff (or hospitalized) for influenza are not guaranteed to receive a test to identify the pathogenic agent (depending on local practice), nor guaranteed to get positive test results (depending on the sensitivity of the test). In addition, severe forms of influenza usually aggravate existing comorbidities or become complicated by coinfections and the patient could die weeks after the primary infection, even after discharge. Hence, seasonal influenza is infrequently listed on death certificates of people who die from influenza-related complications. For these reasons, data collected through influenza surveillance and case finding usually represent only a fraction of persons infected with influenza.Citation26 Finally, to be able to extrapolate data (hospitalizations and mortality) we made assumptions (e.g. regarding catchment population and representativeness of the rates to the whole country) that could diminish the accuracy of our estimates.
To assess this uncertainty, a second scenario was conducted in which a standardized method was adapted for Paraguay. The age-specific attack rates in an unvaccinated population were obtained from pooled data within the control arms of several clinical trials.Citation27–29 As the effectiveness of the vaccines varies depending on the season (because of the strain circulation and especially, for TIV, the degree of match), the severity of each influenza season included was considered. To account for the seasonal heterogeneity in influenza infections circulation, the rates were distributed over the seasons using a severity coefficient. For each season, the coefficient was calculated as the percentage of positive influenza cases identified for the season divided by the average over the timeframe using the report of the FluNet network.Citation30 The same approach has already been reported in the literature.Citation10–12 To these attack rates, we applied probabilities of consultations, hospitalizations and deaths per case from the literature.Citation23 The alternative rates are presented in Supplementary Table S2.
Economic inputs
All the costs were converted into US dollars using the exchange rate of the appropriate year and normalized to 2020 using the annual consumer price indices when necessary.Citation31
Consultations and hospitalizations
Consultations and hospitalizations were based on a public survey that estimated the costs for the year 2009, including the human resource costs (based on the health care workers’ incomes) and the costs of supply and medicine in the public and private sector.Citation32
Administration
Costs of vaccine administration were based on the study reported by Peña Kieninger et al.Citation33
Vaccine price and market share
Those data were derived from the Pan American Health Organization (PAHO) in 2018.Citation34 A full switch from TIV to QIV is considered.
Productivity losses due to an acute influenza illness
The rate of absenteeism was informed by expert opinion. An average monthly wage for 2018 was obtained from the Encuesta Permanente de Hogares Continua (EPHC),Citation35 converted to a daily rate and adjusted by the employment rate.
The inputs considered in the model are summarized in .
Table 1. Parameters with intervals for the sensitivity analysis to inform the model.
Approach to uncertainty
We conducted the previously described scenario analysis using alternative influenza-related outcome rates based on international references.
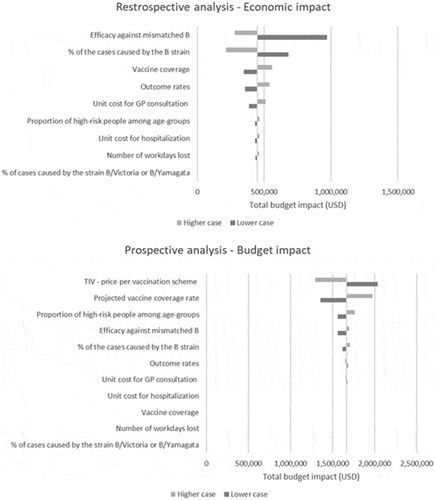
In addition, to assess the uncertainty around the parameters of the model, we conducted a deterministic sensitivity analysis. Input values were varied within the bounds of a plausible interval. The ranges of the results obtained using the extreme values of the parameter (either total costs that could have been prevented or total budget impact) were calculated and displayed on tornado diagrams.
Results
Public health and economic impact
Our simulation estimated that the greatest benefits were observed for the seasons 2016 and 2017, where the level of mismatch and disease severity (i.e., higher influenza-attributable hospitalization rates) were the highest. Our study estimated that from 2012 to 2017, the replacement of TIV with QIV would have resulted in preventing 67 additional hospitalizations and 10 deaths leading to a saving of almost USD400K from the third-party payer perspective and about USD450K from the societal perspective. When considering alternative influenza-related outcome rates, however, the use of QIV instead of TIV could have prevented 234 hospitalizations and 29 deaths attributable to influenza, resulting in a saving of almost USD850K from the third-party payer perspective and more than USD1 million from the societal perspective. The results per year and age groups are presented in .
Table 2. Costs and savings due to the replacement of TIV with QIV by seasons.
Budget impact
Changing from TIV to QIV would have required covering additional vaccination costs of almost USD1,8 million per year, to vaccinate 654,227 individuals (i.e., the estimated total addressable population). Meanwhile, we estimated that the switch would generate savings of around USD73K from a payer perspective and USD84K from a societal perspective. The annual budget impact of a full switch from TIV to QIV from year 1 (assuming an average influenza season, i.e., an average of the outcome rates, mismatch) was around USD1.6 million both from the payer and societal perspectives. The budget impact over five consecutive years was USD8.4 million from a payer perspective and USD8.3 million from a societal perspective. When considering an alternative set of inputs for the epidemiological parameter, the budget impact over five consecutive years was around USD7.6 million from a payer perspective and USD7.4 million from a societal perspective.
Deterministic sensitivity analysis
In the public health and economic impact module, the efficacy against the mismatched strain (i.e., resulting from cross-protection) and the percentage of cases due to the B strain were the most important drivers of the results. The total medical costs saved ranged from USD0 when assuming that the TIV offers complete protection against the mismatched B-strain, to USD3 million when assuming a lower efficacy against the B-strain not included in TIV. These conclusions are intuitive as the added value of QIV relies on the extended protection against an additional B lineage. The vaccination coverage rate, the variation in the influenza-related outcome rates, and the number of workdays lost (from the societal perspective) have less influence on the results.
The results of the budget impact module were driven mainly by vaccine prices and coverage rates. These parameters have an impact on the additional vaccination costs due to the switch from TIV to QIV, and thus the total budget impact. The budget impact ranged from USD6.5 million to USD10.6 million depending on the price of TIV, and from USD7.8 million to USD9.3 million, depending on the price of QIV. Other factors impacting the results are the TIV efficacy against the strain not included in the vaccine, the influenza-related outcome rates and the number of workdays lost (from the societal perspective; ).
Discussion
Influenza immunization has been available in Paraguay since 2006, and vaccination with TIV prevented many cases and associated deaths. However, the severity and relatively high level of mismatch observed during the recent seasons call for broader protection against the B strain.Citation37
The results of the reference case estimated that if QIV had been given instead of TIV between 2012 and 2017 in Paraguay, around 2,600 cases of influenza, 67 associated hospitalizations and 10 influenza-related deaths could have been prevented. At the same time, estimated savings on GP consultations and hospitalizations could have amounted to almost USD400K.
To address the possible underestimation of surveillance data, as revealed by a comparison with other data or outcomes of neighboring countries,Citation36 a complementary analysis was conducted, for which an alternative outcome rates data set was used. In this scenario, we estimated that the switch could have prevented around 13,000 influenza cases, 234 hospitalizations and 29 deaths, leading to almost USD850K in savings according to the payer perspective and more than USD1.1 million according to the societal perspective, over 6 past seasons. These results emphasize the advantage of opting for QIV instead of TIV in the immunization program in Paraguay. This complimentary analysis also highlights the importance of influenza surveillance to appreciate the burden of this particularly unpredictable disease.
However, switching from TIV to QIV would require additional investment to cover the additional vaccination costs of USD1,749,316 which could be offset in part by the savings generated from prevented outcomes. The budget impact over 5 subsequent years would have been ~USD8.4 million from the payer perspective and ~USD8.3 million from the societal perspective.
The parameter having the most important impact on the results was related to the vaccine efficacy against the influenza B virus, as the benefit of the QIV is driven by its ability to offer extended protection against the B strains. However, the estimates were derived from a robust primary source (i.e., meta-analysis), and a conservative approach pertaining to the cross-protection was adopted. While the topic of the existence of cross-protection has been discussed,Citation38–42 our analyses considered a cross-protection factor of 67%. Similarly, there is uncertainty regarding the percentage of cases attributable to the B strain in each season. However, this uncertainty is inherent in the influenza seasonality and its heterogeneity. The model accounted for this factor as adequately as possible by using season-specific epidemiological data to match the outcomes rates, circulation and the strain included in the vaccine.
The sensitivity and scenario analyses also highlight how the influenza-related outcome rates impact the results. We, nonetheless, used in the reference case the most robust data available for Paraguay. The rationale for conducting a complimentary scenario analysis was developed previously in the article and indicates that the results presented in the reference cases are conservative.
This study has generated results that provide clear information and elements of consideration for switching from one vaccine to another within the immunization program (public health and budget impacts); using a validated approach with a model structure published in the literature.Citation9,Citation10,Citation12 Another strength of this model is its adjustments to local characteristics. The model was mainly informed with high-quality local data. Furthermore, the model considers how seasonality, particularly the effect of seasonal severity and virus circulation, affects the vaccine’s effectiveness. This is more relevant now that the 2017 and 2018 seasons have shown a high degree of mismatch in Paraguay.
This analysis has several limitations. First, high-risk groups are more likely to develop influenza-related complications,Citation6,Citation43 but despite this, we applied influenza-related outcome rates characteristic for the overall population to the high-risk population. Such an assumption leads to an underestimation of the number of outcomes avoided for two main reasons: (1) it is reasonable to assume that the number of hospitalizations and deaths related to influenza used to derive the rates mainly occurred in the high-risk population; nonetheless, the whole population count was used to calculate them, leading to lower rates, and (2) these rates were applied not to the whole population but to the high-risk population only. This assumption is conservative, and it underestimates the potential impact of the use of QIV. Second, while we used the best epidemiological data available for Paraguay, the influenza burden reported by local surveillance data is arguably underestimated. The conducted scenario analysis informed that this bias would not favor QIV as it underestimates its additional benefit. Finally, certain outcomes were not included in the present model, e.g., no data were available regarding influenza-related admissions in the emergency department. Therefore, no potential savings associated with this outcome were observed.
The use of QIV instead of TIV could have prevented influenza cases and the subsequent complications, eventually leading to hospitalizations and deaths. This could have generated savings that could have offset, in part, the additional investment necessary to switch from TIV to QIV.
Supplemental Material
Download MS Word (44.5 KB)Acknowledgments
The data collection, model adaptation and scientific writing were funded by Sanofi Pasteur. The authors thank Małgorzata Biernikiewicz of Creativ-Ceutical, Kraków, Poland for providing medical writing support in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Disclosure statement
AA declares no conflict of interest. CMdC is employed in the Institute of Tropical Medicine, part of the National University of Paraguay, and received a grant from Sanofi Pasteur for the collection of the epidemiological data.
CV is employed in the Virology department of the Laboratorio Central de Salud Pública and received a grant from Sanofi Pasteur for the collection of the epidemiological data.CA, PMB, AP, HD, and JGL are employees Sanofi Pasteur, a company that manufactures and commercializes influenza vaccines.
LB is an employee of Creativ-Ceutical, which received funding from Sanofi Pasteur.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2069974.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Zambon MC. Epidemiology and pathogenesis of influenza. J Antimicrob Chemother. 1999;44(Suppl B):3–9. doi:10.1093/jac/44.suppl_2.3.
- Arbeitskreis blut, untergruppe «Bewertung Blutassoziierter Krankheitserreger» “Influenza Virus” Transfusion medicine and hemotherapy : offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie. 2009;36(1):32–39.
- Paget J, Spreeuwenberg P, Charu V, Taylor RJ, Iuliano AD, Bresee J, Simonsen L, Viboud C, Global Seasonal Influenza-associated Mortality Collaborator N, Teams* GLC. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR project. J Glob Health. 2019;9:020421. doi:10.7189/jogh.09.020421.
- Bataglia S, Von Horoch M, Cabello A, Penayo E, Vázquez C. Carga de Influenza asociadas a hospitalizaciones y muertes en Paraguay, 2011-2015. Revista Paraguaya de Epidemiologia. 2017;4:25–33.
- WHO SAGE seasonal influenza vaccination recommendations during the COVID-19 pandemic; 2020.
- Mertz D, Kim TH, Johnstone J, Lam PP, Science M, Kuster SP, Fadel SA, Tran D, Fernandez E, Bhatnagar N, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061. doi:10.1136/bmj.f5061.
- Ministerio de Salud Publica y Bienestar Social. Programa Ampliado de Inmunizaciones. Influenza. Accessed 25 April 2022. https://pai.mspbs.gov.py/influenza/
- Palekar R, Rodriguez A, Avila C, Barrera G, Barrera M, Brenes H, Bruno A, El Omeiri N, Fasce R, Ferreira de Almeida W, et al. Patterns of influenza B circulation in Latin America and the Caribbean, 2010-2017. PloS One. 2019;14:e0219595. doi:10.1371/journal.pone.0219595.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine. 2012;30:1993–98. doi:10.1016/j.vaccine.2011.12.098.
- Ruiz-Palacios GM, Beigel JH, Guerrero ML, Bellier L, Tamayo R, Cervantes P, Alvarez FP, Galindo-Fraga A, Aguilar-Ituarte F, Lopez JG. Public health and economic impact of switching from a trivalent to a quadrivalent inactivated influenza vaccine in Mexico. Hum Vaccin Immunother. 2020;16:827–35. doi:10.1080/21645515.2019.1678997.
- Uhart M, Bricout H, Clay E, Largeron N. Public health and economic impact of seasonal influenza vaccination with quadrivalent influenza vaccines compared to trivalent influenza vaccines in Europe. Human Vaccines Immunother. 2016;12:2259–68. doi:10.1080/21645515.2016.1180490.
- Jamotte A, Chong CF, Manton A, Macabeo B, Toumi M. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health. 2016;16(1):630. doi:10.1186/s12889-016-3297-1.
- Instituto Nacional de Estadística. Paraguay, Proyección de la población por sexo y edad, según distrito, 2000-2025. Revisión 2015”. Available at https://www.ine.gov.py/Publicaciones/Biblioteca/documento/7132_Proyeccion%20Distrital.pdf
- Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, Sanderson C, McKee M, Troeger C, Ong KL, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Global Health. 2020;8(8):e1003–e17. doi:10.1016/S2214-109X(20)30264-3.
- Piroth L, Cottenet J, Mariet A-S, Bonniaud P, Blot M, Tubert-Bitter P, Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Resp Med. 2021;9(3):251–59. doi:10.1016/S2213-2600(20)30527-0.
- Jefferson T, Di Pietrantonj C Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2014;3:CD004876. PMID: 20614424. doi: 10.1002/14651858.CD001269.pub4.
- Clements KM, Meier G, McGarry LJ, Pruttivarasin N, Misurski DA. Cost-Effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum Vaccin Immunother. 2014;10:1171–80. doi:10.4161/hv.28221.
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 20012;8:CD004879. PMID: 18425905. doi:10.1002/14651858.CD004879.pub3.
- Osterholm MT, Kelley NS, Sommer A, Belongia BA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet. 2011;12:36–44. doi:10.1016/S1473-3099(11)70295-X.
- Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med. 2013;11. doi:10.1186/1741-7015-11-153.
- Vazquez C, Ortega MJ, Villalba S, Horoch MV, Gamarra ML, Torales J, Gomez A, Chamorro G, Caello A. Detection of influenza b lineages from 2013 to 2015 at the sentinel surveillance of influenza in paraguay. Annual Meeting of the European Society for Paediatric Infectious Diseases, Brighton, UK; 2016:ESP16–1106.
- Boletines epidemiologico Semanal (2010-2017). Dirección General de Vigilencia de la Salud.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25(27):5086–96. doi:10.1016/j.vaccine.2007.03.046.
- Egresos Hospitalarios por años y Grupos de Edad, Según Regiones Sanitarias, Distritos y Establecimientos de Salud Del MSPyBS. 2019.
- Bayer C, Remschmidt C, An der Heiden M, Tolksdorf K, Herzhoff M, Kaersten S, Buda S, Haas W, Buchholz U. Internet-Based syndromic monitoring of acute respiratory illness in the general population of Germany, weeks 35/2011 to 34/2012. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. Jan 2014;19(4). PMID: 24507468. doi:10.2807/1560-7917.es2014.19.4.20684.
- Reed C, Chaves SS, Daily Kirley P, Emerson R, Aragon D, Hancock EB, Butler L, Baumbach J, Hollick G, Bennett NM, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PloS One. 2015;10(3):e0118369. doi:10.1371/journal.pone.0118369.
- Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E . Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. Feb 2018;1(2): CD004879. PMID: 22895945.
- Demicheli V, Jefferson T, Al-Ansary LA, Ferroni EAR, Di Pietrantonj C . Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. Feb 2018;1(2): CD001269. PMID: 24623315. doi:10.1002/14651858.CD001269.pub5.
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2014;3:CD001269. PMID: 20614424.
- Flunet. Influenza laboratory surveillance data. World health organization, 2012-2017.
- Development of inflation rates in Paraguay. WorldDatainfo; 2020. Available at https://www.worlddata.info/america/paraguay/inflation-rates.php
- Ministerio de Hacienda. Sistema Integrado de Contabilidad. Reportes de presupuesto y ejecución presupuestaria.
- Kieninger Martha Peña. et al.Cost-Effectiveness analysis of pneumococcal conjugate vaccine introduction in Paraguay. Vaccine. 2015;33(Suppl 1):A143–53.
- Panamerican health Organization. Revolving Fund Vaccine Prices 2018. Available at https://www.paho.org/en/documents/revolving-fund-vaccine-prices-2018
- Paraguay - Encuesta Permanente de Hogares Continua, I, II, III y IV Trimestres de 2018. In: La Dirección General de Estadística EyC-D, ed.; 2018.
- Tinoco YO, Azziz-Baumgartner E, Uyeki TM, Rázuri HR, Kasper MR, Romero C, Silva ME, Simons MP, Soto GM, Widdowson MA, et al. Burden of influenza in 4 ecologically distinct regions of Peru: household active surveillance of a community Cohort, 2009-2015. Clin Infect Dis. 2017;65:1532–41. doi:10.1093/cid/cix565.
- Flunet. Influenza laboratory surveillance information 2013 to 2018.
- García A, Aristegui J, Moreno D, Redondo E, Jimeno I, Cenoz MG, López Trigo JA. Documento de reflexión sobre la vacunación antigripal tetravalente. Asociación Españo la de Vacunología. 2019. https://vacunasaep.org/sites/vacunasaep.org/files/act_y_refl_vac_gripe_tetra._vfinal_2019.pdf
- Dos Santos G, Neumeier E, Bekkat-Berkani R. Influenza: can we cope better with the unpredictable? Hum Vaccin Immunother. 2016;12:699–708. doi:10.1080/21645515.2015.1086047.
- Barr IG, Jelley LL. The coming era of quadrivalent human influenza vaccines: who will benefit? Drugs. 2012;72(17):2177–85. doi:10.2165/11641110-000000000-00000.
- Dolin R. The quadrivalent approach to influenza vaccination. J Infect Dis. 2013;208(4):539–40. doi:10.1093/infdis/jit264.
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine. 2010;28(Suppl 4):D45–53. doi:10.1016/j.vaccine.2010.08.028.
- Coleman BL, Fadel SA, Fitzpatrick T, Thomas SM. Risk factors for serious outcomes associated with influenza illness in high- versus low- and middle-income countries: systematic literature review and meta-analysis. Influenza Other Respi Viruses. 2018;12:22–29. doi:10.1111/irv.12504.