ABSTRACT
Yearly administration of influenza vaccine with recommendations can help control seasonal influenza epidemics in adults aged ≥60 years. Here, we describe the results of a prospective study observing the immunogenicity and persistence of induced immunity of a trivalent inactivated split-virion influenza vaccine (TIV) in adults aged ≥60 years during the 2018–2019 season in Taizhou City, Zhejiang Province in China. A total of 422 participants completed the study period. Vaccinated participants (284) received a single dose of TIV, but unvaccinated participants (138) didn’t receive any vaccine. Study participants vaccinated with TIV had significantly higher GMTs of Hemagglutination Inhibition (HI) antibodies against AH1N1, AH2N3, and B/Victoria strains (all p < .0001) at day 30 post-vaccination compared with unvaccinated participants, but the antibody response to the B/Victoria strain was the weakest. Rates of seroprotection and seroconversion were generally higher in the TIV-vaccinated group. At day 180 post-vaccination, the seroconversion rates (95%CI) in the vaccinated group were 99.6% (99.0%–100.3%), 97.9% (96.2%–99.6%), and 68.3% (62.9%–73.8%) for antibodies against three influenza strains, respectively; these rates were significantly different compared with unvaccinated group only for strains AH3N2 and B/Victoria (p = .002 and p < .0001, respectively). These results confirm that in adults aged ≥60 years, a single dose of TIV can induce a protective immune response against influenza, but the protective HI antibody levels induced against strain B/Victoria do not persist through 6 months.
Introduction
Influenza is a contagious, acute respiratory disease that is caused by influenza A and influenza B viruses in humans.Citation1 During seasonal influenza infections, this disease can lead to numerous complications, hospitalization, and even death.Citation2 The World Health Organization (WHO) estimates that there are one billion influenza cases worldwide each year, of which three to five million are severe cases, resulting in 290,000–650,000 deaths from influenza-related respiratory diseases.Citation3 Owing to the prevalence of comorbidities and immunocompromised state increasing with age, older individuals are more vulnerable to serious sequelae and mortality compared with younger people.Citation4 A model study on the global influenza excess mortality rate showed that the excess mortality rate in the 65–74-year-old age group is 2.9/10 million–44/10 million and that in the ≥75-years-old population is 17.9/10 million–223.5/10 million.Citation3
Influenza vaccination, which can induce specific antibody responses in the respiratory tract, is the most effective and inexpensive public health strategy for the prevention of influenza infection.Citation5 In many countries, adults aged ≥60 years are listed as the key recommended population for influenza vaccination.Citation6 There is evidence that influenza vaccine effectiveness is lower in adults aged ≥60 years than in those aged 18–64 years. According to meta-analysis results, vaccine effectiveness against influenza was estimated as being approximately 49% (95%CI: 33%–62%) in older adults but approximately 59% (95%CI: 51%–67%) in healthy young adults.Citation7 Regarding immunogenicity of influence vaccine, the influenza seroprotection induced by an influenza vaccine is only 29%–46% among those aged ≥75 years, compared with 41%–58% among those 60–74 years of age.Citation8 Some earlier studies also reported that the immune response induced by influenza vaccination and the associated antibody titers decrease over time in older adults because of age-associated decreased immune competence.Citation9 Limited data on antibody persistence after influenza infection is currently available. Another meta-analysis of evidence on the year-round persistence of vaccine-induced antibody in individuals aged ≥60 years following their vaccination with a trivalent, inactivated, seasonal influenza vaccine found a decline from days 21–42 to 360 post-vaccination in the geometric mean titers (GMTs) of specific antibodies and the proportion of seroprotected subjects, which suggests that clinical protection does not persist year-round in adults aged ≥60 years.Citation10
Influenza viruses are prone to immunogenic changes; thus, yearly administration of the influenza vaccine to those at greatest risk, as recommended by WHO, is the main public health strategy in China.Citation6 Currently, to improve vaccine accessibility among at-risk populations, new policies, such as free vaccination for adults aged ≥60 years, are being explored in China.Citation11 The trivalent inactivated split-virion influenza vaccine, which is generally well tolerated and highly immunogenic in Chinese individuals, is widely used for free annual vaccination of this population in China.Citation12 However, vaccination against matched seasonal influenza strains protects people only from the currently circulating influenza viruses. Therefore, it is necessary to evaluate the immune effect induced by the seasonal influenza vaccine. In this context, we performed a prospective cohort study based on free vaccination programs to evaluate the immunogenicity of the trivalent influenza vaccine and the persistence of its induced immunity in adults aged ≥60 years during the 2018–2019 season.
Materials and methods
Setting and subjects
This was a prospective study to evaluate the immunogenicity of trivalent influenza split-virion vaccine (TIV) and the persistence of induced immunity in adults aged ≥60 years. Participants were recruited from the community that relied on a free influenza vaccination program for individuals aged 60 years and above in Taizhou City, Zhejiang Province in China from September 2018 to April 2019.
Participants were enrolled in a community health service center in Taizhou city in September 2018. The trained medical staffs performed it. The enrolled participants had all lived in Taizhou City for a long time, which was convenient for follow-up. Eligible participants were individuals older than 60 years of age who had not received an influenza vaccine for the present season. They all signed an informed consent form and volunteered to participate in this study. Participants were excluded if they: (1) had a history of allergic reactions to egg, Guillain-Barre syndrome from previous TIV vaccination, or severe allergic reactions after vaccination; (2) took aspirin presently; (3) had any confirmed or suspected immunosuppressive or immunodeficiency disease; (4) suffered from heart disease, respiratory disease, liver or kidney disease, mental disorder, or chronic infection; (5) had used glucocorticoids in the past 3 months; or (6) had a history of major surgery.
Participants were separated into two groups, the vaccinated group and the unvaccinated group, according to whether they voluntarily choose to receive influenza vaccine. Those in the vaccinated group received one dose of the study vaccine. Those in the unvaccinated group didn’t receive any vaccine or placebo treatment.
Study vaccine
The study vaccine was trivalent inactivated split-virion influenza vaccine (TIV, HuaLan Biological Bacterin Inc.) containing HA for the following three influenza strains: A/Michigan/45/2015(H1N1)pdm09-like virus, A/Singapore/INFIMH-16-0019/2016(H3N2)-like virus, and B/Colorado/06/2017 (Victoria strain). For vaccine administration, a single dose of .5 ml of TIV was injected intramuscularly into the outer deltoid muscle of the upper arm. This vaccine was previously approved by the China Institute for Food and Drug Control.
Data collection
Individual-level information on participants, including their name, age, sex, and any chronic medical conditions, was collected via a face-to-face interview. Serum samples for use in serologic analysis were collected at baseline (before vaccination) and at 30 days and 180 days after vaccination for all participants to assess the immunogenicity of the vaccine and the persistence of induced immunity.
Serum test
Serum levels of HI antibody against AH1N1, AH3N2, and B/Victoria influenza strains were measured by performing a hemagglutination inhibition assay with 0.5% turkey erythrocytes following Standard Operating Procedures of National Influenza Center published by Chinese Center for Disease Control and Prevention. Serum were tested in duplicate and diluted 2-fold starting from 1:10. The HI titer was defined as the reciprocal of the maximum serum dilution with complete hemagglutination inhibition. The final HI titer was estimated as the geometric mean of duplicate samples; a value of 5 was used for HI < 10.
Main outcomes
The primary outcome of this study was influenza vaccine immunogenicity. Immune responses were followed through 180 days post-vaccination.
Immunogenicity end points included the seroconversion rates, seroprotection rates, and GMTs. In accordance with the European Medicines Agency (EMA) criteria, the seroconversion rate was defined as the percentage of people who went from a pre-immunization HI antibody titer of < 10 to a post-immunization one of ≥ 40 or who had a pre-immunization HI antibody titer of ≥ 10 and exhibited a ≥ 4-fold increase in this titer after vaccination. The seroprotection rate was defined as the percentage of patients with an HI antibody titer of at least 1:40.
Sample size calculation
The sample size calculation was performed by PASS15 software. The total power was set to be .85. Since there were three virus types, the probability of type II errors in each test should be (1–0.85)/3 = .05, that was, the power of each comparison was .95; the control group rate p2 was taken as .10, and the difference D1 of the positive conversion rate between the vaccinated group and the control group was taken as .40; The vaccinated group and the control group were designed according to 2:1; the test statistic was Z Test with Continuity Correction. 200 participants in the vaccinated group and 100 participants in the control group were calculated. The drop-off rate was estimated at 15%, then the vaccinated group and the control group needed 240 participants and 120 participants respectively. Based on the above results, 300 participants in the vaccinated group and 150 participants in the control group were determined.
Statistical analysis of data
The study data were entered in duplicate using EpiData3.2.2 Software and were organized and analyzed using Microsoft Excel 2010 and SPSS 24.0 statistical software. GraphPad Prism 9 was used to generate graphs.
The demographics of participants in the vaccinated and unvaccinated groups were compared. Chi-squared tests were used to analyze categorical outcomes. GMTs for HI antibodies against each influenza strain were calculated via a log10 transformation of the HI antibody titers. GMTs and their corresponding 95% confidence intervals (95%CIs) were calculated based on the standard normal distribution of log 10-transformed HI antibody titers. Seroconversion and seroprotection rates and their corresponding 95%CI derived from a binomial distribution were evaluated using a chi-squared test or Fisher’$3 test. All statistical tests were two-sided, and differences with a p-value of <.05 were considered significant.
Ethics approval
The study was approved by the ethics committee of the Zhejiang Provincial Center for Disease Control and Prevention (T-043-R). A signed informed consent was required from each participant.
Results
Subjects
A total of 461 participants aged ≥60 years were enrolled in the study, 309 (67.0%) of whom were given the influenza TIV (vaccinated group) and 152 (33.0%) who did not receive this vaccine (unvaccinated). Of the 461 enrolled participants, 422 completed the study (provided data on days 0–180); their mean age was 67.8 years old. The most common reason for withdrawal was participant unwillingness to continue ().
The two study groups differed in some of their baseline characteristics (). The sex distributions of the participants were very similar, with male patients accounting for approximately half of the participants in each group. In contrast, the mean ages and age distributions were not similar between the two groups; the unvaccinated group skewed older. Over half of the participants in the unvaccinated group had a chronic illness, whereas less than half of the participants in the vaccinated group had a chronic illness.
Table 1. Participants characteristics at baseline (n = 422).
Immunogenicity
The immunogenicity analysis was conducted on the 422 participants who completed the whole follow-up for the study.
shows the seroconversion proportions of the vaccinated and unvaccinated groups at the follow-up timepoints (). At 30 days after vaccination, the seroconversion rates (95%CI) for the vaccinated group were 72.5% (63.7%–77.9%), 71.1% (65.8%–76.4%), and 47.2% (41.3%–53.0%) for influenza strains AH1N1, AH3N2, and B/Victoria respectively, and the seroconversion rates (95%CI) for the unvaccinated group were 45.7% (37.2%–54.1%), 13.0% (7.4%–18.7%), and 11.6% (6.2%–17.0%); these seroconversion rates differed significantly between the vaccinated and unvaccinated groups for all three influenza strains (all p < .001). At 180 days after vaccination, the seroconversion rates (95%CI) for the vaccinated group were 69.4% (64.0%–74.8%), 71.1% (65.8%–76.4%), and 28.5% (23.2%–33.8%) for influenza strains AH1N1, AH3N2, and B/Victoria, respectively, and the corresponding seroconversion rates (95%CI) for the unvaccinated group were 65.2% (57.2%–73.3%), 63.8% (55.6%–71.9%), and 4.3% (.9%–7.8%). At this timepoint, only the seroconversion rate for strain B/Victoria was significantly different between the two groups (p < .001).
Table 2. Percentage of group with seroconversiona at each of two timepoints.
Most participants already met the criteria for seroprotection (i.e., an HI antibody titer of at least 1:40) at baseline (). At 30 days after vaccination, the seroprotection rates (95%CI) for the vaccinated group were 88.4% (84.6%–92.1%), 73.2% (68.1%–78.4%), and 47.9% (42.0%–53.7%) for influenza strains AH1N1, AH3N2, and B/Victoria, respectively, and the seroprotection rates (95%CI) for the unvaccinated group were 89.9% (84.8%–95.0%), 82.6% (76.2%–89.0%), and 43.5% (35.1%–51.9%). At 180 days after vaccination, the seroconversion rates (95%CI) for the vaccinated group were 99.6% (99.0%–100.3%), 97.9% (96.2%–99.6%), and 68.3% (62.9%–73.8%) for influenza strains AH1N1, AH3N2, and B/Victoria, respectively, and the seroconversion rates (95%CI) for the unvaccinated group were 100% (100%–100%), 91.3% (86.5%–96.1%), and 13.8% (7.9%–19.6%). The seroprotection rates differed significantly between the vaccinated and unvaccinated groups for influenza strains AH3N2 and B/Victoria at both 30 days and 180 days post-vaccination (all p < .005). In contrast, there was no significance in the seroprotection rate for strain AH1N1 between the vaccinated and unvaccinated groups at either of these timepoints.
Table 3. Percentage of group with seroprotectiona at each of three timepoints.
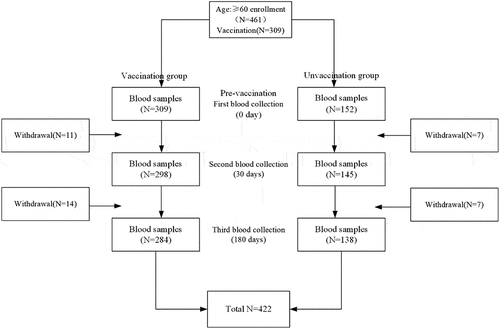
The trends in the levels of antibodies against seasonal influenza strains AH1N1, AH3N2, and B/Victoria, as assessed by GMT, were determined over three timepoints (). The HI antibody GMT at baseline was the lowest for strain B/Victoria, while the other two studied strains had similar HI antibody GMTs (). In the vaccinated group, immunization with a TIV induced the serum HI antibody GMT (95%CI) for all the vaccine component strains to peak at 30 days post-vaccination; the baseline GMTs of 360.7 (325.2–400.0) for AH1N1, 263.2 (231.7–299.0) for AH3N2, and 73.8 (68.2–79.9) for B/Victoria increased by approximately 5.4-fold, 5.4-fold, and 2.7-fold, respectively, at 30 days post-vaccination (). By 180 days post-vaccination, the HI antibody titer decreased to GMT (95%CI) of 324.7 (301.2–350.1) for AH1N1, 255.6 (229.3–285.0) for AH3N2, and 44.6 (40.4–49.4) for B/Victoria, which are reductions in the titer of approximately .9-fold, 1.0-fold, and .6-fold, respectively (). In the unvaccinated group, the HI antibody GMT increased at 30 days and 180 days post-vaccination by approximately 2.8-fold and 1.5-fold, respectively, against AH1N1 and by approximately 1.2-fold and 3.2-fold, respectively, against AH3N2, whereas the GMT for HI antibody against B/Victoria increased by approximately 1.3-fold at 30 days post-vaccination and then decreased by approximately .5-fold at 180 days post-vaccination. The HI antibody GMTs for all three influenza strains were all higher significantly in the vaccinated group than in the unvaccinated group at both 30 and 180 days post-vaccination (all p < .0001), except for the GMT of HI antibody against AH3N2, for which there was no difference between the vaccinated and unvaccinated groups at 180 days post-vaccination (p = .6444) ().
Figure 2. Time course of HI antibody GMTs in vaccinated and unvaccinated participants. Influenza hemagglutination inhibiting (HI) antibody geometric mean titers (GMTs) between the vaccinated and unvaccinated groups by strain at day 0, day 30, and day 180 post-vaccination. AH1N1 vac: GMT of HI antibody to AH1N1 in the vaccinated group; AH1N1 unvac: GMT of HI antibody to AH1N1 in the unvaccinated group; AH3N2 vac: GMT of HI antibody to AH3N2 in the vaccinated group; AH3N2 unvac: GMT of HI antibody to AH3N2 in the unvaccinated group; BV vac: GMT of HI antibody to B/Victoria in the vaccinated group; BV unvac: GMT of HI antibody to B/Victoria in the unvaccinated group.
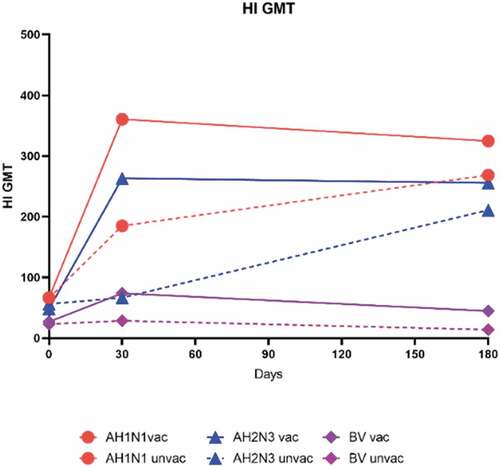
Figure 3. Influenza-Specific hemagglutination inhibiting antibody geometric mean titers (GMTs) between two vaccinated and unvaccinated groups by strain at day 0, day 30, and day 180 post-vaccination. GMT of HI antibody against AH1N1 (a), AH3N2 (b), or B/Victoria (BV) (c). GMT is shown above each bar. Error bars indicate 95% CIs. Unvaccinated participants did not receive the study vaccine (n = 138). Vaccinated participants received one dose of the study vaccine (n = 284). P-values result from a comparison between the two groups (vaccinated group versus unvaccinated group).
Table 4. Geometric mean titers (GMTs) of HI antibodies and geometric mean ratios among participants in the two groups by strain (N = 422).
Discussion
Infections with influenza virus occur annually worldwide, and these viruses are susceptible to mutation owing to their antigenicity. Mainly because of a waning immune system, individuals aged ≥60 years are among those at highest risk for serious complications. The WHO and European Center for Disease and Control agree that targeting those aged ≥60 years is a sound strategy for preventing adverse outcomes from influenza.Citation13,Citation14 Many studies have reported that an influenza vaccine can provide significant protection against influenza infection in individuals aged ≥60 years. Primary prevention via immunization is effective for reducing the burden of influenza illness among this population.Citation4 The immune effect induced by the influenza vaccine is also influenced by various factors. Therefore, it is necessary to carry out an evaluation of the immune effect induced by the annual administration of an influenza vaccine in each target population to provide a scientific basis for strategies to prevent and control influenza.
In the present study, we evaluated the immunogenicity of TIV influenza vaccine, which met the requirements for an influenza vaccine.Citation15 The short-term (day 0–day 30 post-vaccination) influenza-specific immune status of the vaccinated group was superior to that of the unvaccinated group for the three vaccine-matched influenza strains. After vaccination with TIV, the resulting seroconversion rates and seroprotection rates for each influenza strain are similar to those previously reported.Citation16,Citation17 However, the GMTs are much higher than those found in a previous study conducted in Shenzhen City and Changzhou City.Citation17 In terms of immunogenicity, the two influenza A strains outperformed the influenza B strain; in other words, the participants exhibited a lower antibody response to the B strain influenza.
In terms of immunity persistence, the GMTs of serum HI antibody against AH1N1 and AH3N2 increased with time (day 0–day 180) in both the vaccinated and unvaccinated groups. The observed increase in HI antibody GMTs in the unvaccinated group indicates that there may have been an influenza epidemic that occurred during our study period. Therefore, we unfortunately cannot draw conclusions about the persistence of immunity induced by the tested vaccine for influenza strains AH1N1 and AH3N2 from this study. However, the antibody response to influenza vaccination for the B/Victoria strain, which may not have been prevalent during the study period, was lower to as compared with that for the other two strains, and it declined by nearly half in the vaccinated group at 180 days post-vaccination. This means protective that the HI antibody levels induced by TIV against strain B/Victoria did not persist through 6 months in adults aged ≥60 years. Buxton et al.Citation18 reported that in a community-based study of 1-dose versus 2-dose vaccination of individuals aged ≥60 years, the GMTs against H3N2 and H1N1 at 6, 18, and 24 weeks after the administration of a single vaccine dose were >2-fold above baseline, whereas the GMTs against the influenza B component of the vaccine never rose by 2-fold, not even initially; these findings are similar to those of the present study.
Because of age-related declines in immune function, older adults exhibit lower antibody responses to influenza vaccination, especially for influenza B strains, compared with younger adults.Citation19,Citation20 A recent review of antibody responses in 31 influenza vaccine studies conducted from 1986 to 2002 concluded that adults aged ≥60 years were 2–4 times less likely to seroconvert or achieve protective HI antibody titers after influenza vaccination, compared with young adults.Citation21 Traditional inactivated influenza vaccines are often only modestly immunogenic. Therefore, individuals aged ≥60 years are recommended to be vaccinated annually or be vaccinated with high-dose or adjuvanted inactivated influenza vaccine to rapidly induce high titers of a broad range of anti-influenza antibodies.Citation22,Citation23
There are several limitations to our study. First of all, participants in our study had higher levels of preexisting antibody against the three tested influenza strains. The inclusion of non-susceptible participants may have affected the observed HI antibody seroconversion and seroprotection rates.Citation24 Owing to the geographical location and climate of Taizhou City, it has year-round circulation of seasonal influenza, which likely impacts the influenza exposure of residents; consequently, the high pre-vaccination HI antibody titers we observed in our study participants were likely due to natural exposure. Additionally, previous history of influenza vaccination, which also affects preexisting antibody levels, was probably high in our study population because of the free influenza vaccination policy that has been implemented in Taizhou City. But in this study, participants’ prior influenza infection and vaccination history was not collected. Second, mean ages and age distributions were not similar between the two groups and the unvaccinated group skewed older. In this study, age of subjects is an important factor. The unvaccinated group skewed older which might show lower immunogenicity. The difference of age in two groups may have skewed the results. Third, because this study took place during winter, it overlapped with the annual seasonal influenza epidemic, which may have affected the results of our immunogenicity evaluation; notably, we included a control group to exclude this interference. Moreover, the matching degree between vaccine strain and epidemic strains may not be high. A decrease in vaccine effectiveness during the winter season has been reported from surveillance studies in some countries, and the observed decline in effectiveness was most significant in adults aged ≥65 years.Citation25,Citation26 Therefore, the vaccine immunogenicity accessed here is not equivalent to “clinical protection”. Another limitation in the study was the lack of control adults aged <60 years old to compare the difference of immunogenicity and immune persistence in different age groups. The comparison of different age groups can provide a more comprehensive assessment of the immunogenicity of the vaccine. Lastly, we collected samples to measure the persistence of antibodies for only 6 months after vaccination, even though the influenza vaccine is administered annually; ideally, the observation period should have continued for a full year.
In summary, a single dose of inactivated influenza vaccine can induce a protective immune response against influenza in adults aged ≥60 years. A lower antibody response to the B strain influenza was observed. For immunity persistence, the protective HI antibody levels induced against strain B/Victoria do not persist through 6 months.
Author contributions
H. Lv contributed to study conception and design. J. Fu and Y. Jin recruited participants and conducted the study. Y. Liao contributed to statistical analysis. Y. Liao, H. Zhang and J. Yang participated in the drafting the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
We thank Baoping Yang, researcher of Hualan Biological Vaccine Co., Ltd., and all the investigators from The Center for Disease Control and Prevention of Zhejiang and The Center for Disease Control and Prevention of Taizhou for their support and help in this study. We would like to express our sincere gratitude to all participants in this study.
Disclosure statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Funding
References
- Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, et al. Influenza. Nat Rev Dis Primers. 2018;4:3. doi:10.1038/s41572-018-0002-y.
- Gaitonde DY, Moore FC, Morgan MK. Influenza: diagnosis and treatment. Am Fam Physician. 2019;100:751–10.
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–300. doi:10.1016/S0140-6736(17)33293-2.
- Smetana J, Chlibek R, Shaw J, Splino M, Prymula R. Influenza vaccination in the elderly. Hum Vaccines Immunother. 2018;14:540–49. doi:10.1080/21645515.2017.1343226.
- Tamura S, Ainai A, Suzuki T, Kurata T, Hasegawa H. Intranasal inactivated influenza vaccines: a reasonable approach to improve the efficacy of influenza vaccine? Jpn J Infect Dis. 2016;69:165–79. doi:10.7883/yoken.JJID.2015.560.
- National Immunization Advisory Committee Technical Working Group IVTWG. Technical guidelines for seasonal influenza vaccination in China (2021-2022). Zhonghua Liu Xing Bing Xue Za Zhi. 2021;42:1722–49. doi:10.3760/cma.j.cn112338-20210913-00732.
- Beyer WE, Nauta JJ, Palache AM, Giezeman KM, Osterhaus AD. Immunogenicity and safety of inactivated influenza vaccines in primed populations: a systematic literature review and meta-analysis. Vaccine. 2011;29:5785–92. doi:10.1016/j.vaccine.2011.05.040.
- Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46:1078–84. doi:10.1086/529197.
- Narang V, Lu Y, Tan C, Camous XFN, Nyunt SZ, Carre C, Mok EWH, Wong G, Maurer-Stroh S, Abel B, et al. Influenza vaccine-induced antibody responses are not impaired by frailty in the community-dwelling elderly with natural influenza exposure. Front Immunol. 2018;9:2465. doi:10.3389/fimmu.2018.02465.
- Young B, Zhao X, Cook AR, Parry CM, Wilder-Smith A, Ic MC. Do antibody responses to the influenza vaccine persist year-round in the elderly? a systematic review and meta-analysis. Vaccine. 2017;35:212–21. doi:10.1016/j.vaccine.2016.11.013.
- Zhang Y, Muscatello DJ, Wang Q, Yang P, Wu J, MacIntyre CR. Overview of influenza vaccination policy in Beijing, China: current status and future prospects. J Public Health Policy. 2017;38:366–79. doi:10.1057/s41271-017-0079-7.
- Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, Li R-C, Xia S-L, Zhao Y-L, Li F-J, et al. Safety and immunogenicity of 2009 pandemic influenza a H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi:10.1016/S0140-6736(09)62003-1.
- European Centre for Disease and Control (ECDC). AN, Tsolova S. ECDC, ed. Priority risk groups for influenza vaccination. 2008 [accessed 2017 Jul]. https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/0808_GUI_Priority_Risk_Groups_for_Influenza_Vaccination.pdf.
- Fleming DM, Elliot AJ. Estimating the risk population in relation to influenza vaccination policy. Vaccine. 2006;24:4378–85. doi:10.1016/j.vaccine.2006.02.053.
- Skowronski DM, Tweed SA, De Serres G. Rapid decline of influenza vaccine-induced antibody in the elderly: is it real, or is it relevant? J Infect Dis. 2008;197:490–502. doi:10.1086/524146.
- Brydak LB, Machala M, Mysliwska J, Mysliwski A, Trzonkowski P. Immune response to influenza vaccination in an elderly population. J Clin Immunol. 2003;23:214–22. doi:10.1023/A:1023314029788.
- Shu L, Zhang J, Huo X, Chen C, Fang S, Zong K, Guo Y, Zhao Y, Zhang J, Bao C, et al. Surveillance on the immune effectiveness of quadrivalent and trivalent split influenza vaccines — Shenzhen City and Changzhou City, China, 2018–2019. China CDC Wkly. 2020;2:370–75. doi:10.46234/ccdcw2020.095.
- Buxton JA, Skowronski DM, Ng H, Marion SA, Li Y, King A, Hockin J. Influenza revaccination of elderly travelers: antibody response to single influenza vaccination and revaccination at 12 weeks. J Infect Dis. 2001;184:188–91. doi:10.1086/322013.
- Fan R, Huang X, Nian X, Ou Z, Zhou J, Zhang J, Zeng P, Zhao W, Deng J, Chen W, et al. Safety and immunogenicity of a quadrivalent influenza vaccine in adults aged 60 years or above: a phase III randomized controlled clinical study. Hum Vaccines Immunother. 2021;18:1–9. doi:10.1080/21645515.2021.1967041.
- Greenberg DP, Robertson CA, Noss MJ, Blatter MM, Biedenbender R, Decker MD. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine compared to licensed trivalent inactivated influenza vaccines in adults. Vaccine. 2013;31:770–76. doi:10.1016/j.vaccine.2012.11.074.
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi:10.1016/j.vaccine.2005.08.105.
- Chahine EB. High-Dose inactivated influenza vaccine quadrivalent for older adults. Ann Pharmacother. 2021;55:89–97. doi:10.1177/1060028020935645.
- McElhaney JE, Beran J, Devaster JM, Esen M, Launay O, Leroux-Roels G, Ruiz-Palacios GM, van Essen GA, Caplanusi A, Claeys C, et al. AS03-Adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013;13:485–96. doi:10.1016/S1473-3099(13)70046-X.
- Roy S, Williams CM, Wijesundara DK, Furuya Y. Impact of pre-existing immunity to influenza on live-attenuated influenza vaccine (LAIV) immunogenicity. Vaccines (Basel). 2020;8(4):683. doi:10.3390/vaccines8040683.
- Castilla J, Martinez-Baz I, Martinez-Artola V, Reina G, Pozo F, Garcia Cenoz M, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5):20388. doi:10.2807/ese.18.05.20388-en.
- Pebody R, Andrews N, McMenamin J, Durnall H, Ellis J, Thompson CI, Robertson C, Cottrell S, Smyth B, Zambon M, et al. Vaccine effectiveness of 2011/12 trivalent seasonal influenza vaccine in preventing laboratory-confirmed influenza in primary care in the United Kingdom: evidence of waning intra-seasonal protection. Euro Surveill. 2013;18. doi:10.2807/ese.18.05.20389-en.