ABSTRACT
Vaccination against hepatitis B (HepB) provides long-term protection against infection. This is despite a reduction in HepB surface antibody (anti-HBs) concentrations over time to levels below the well-accepted correlate of protection of ≥10 mIU/mL. Continued evidence of immune memory and protection despite declined anti-HBs concentrations can be demonstrated by HepB virus surface antigen challenge studies. Long-term immune memory and protection against HepB infection has not been demonstrated previously for the pediatric hexavalent vaccine DTaP5-IPV-HepB-Hib. This phase 3, multicenter, single-group, open-label challenge study (NCT04490499; EudraCT: 2020–000126–26) evaluated immune memory against HepB infection in children who had received DTaP5-IPV-HepB-Hib at 2, 4, and 11–12 months of age, or at 2, 3, 4, and 12 months of age. At age 8–9 years, they were each challenged with 5 μg of monovalent HepB vaccine. Anti-HBs levels were measured on pre-challenge day 1 and post-challenge day 30. At baseline, 45.4% (93 of 205) had anti-HBs levels ≥10 mIU/mL. On post-challenge day 30, 99.5% (201 of 202) had anti-HBs levels ≥10 mIU/mL, regardless of initial vaccination schedule. Post-challenge, geometric mean concentrations increased 71-fold over baseline and 96.0% of children had a ≥4-fold rise in anti-HBs concentrations with similar results across both dosing schedules. The challenge dose was well tolerated. The robust anti-HBs responses after a single 5-μg dose of HepB vaccine confirm the persistence of a HepB immune memory and demonstrate that DTaP5-IPV-HepB-Hib provides long-term protection against HepB.
Introduction
Globally, hepatitis B virus (HBV) causes significant morbidity and mortality.Citation1 Vaccination is the primary means for preventing HBV infection and its complications.Citation2,Citation3 In 2015, 84% of infants worldwide received 3 doses of hepatitis B (HepB) vaccine. Widespread HepB vaccination, particularly in newborns, has helped reduce the global prevalence of HBV infection at 5 years of age from 4.7% to 1.3%.Citation1
A HepB surface antibody (anti-HBs) concentration of ≥10 mIU/mL, measured 1–2 months after a primary HepB vaccination series, is considered a reliable marker of long-term protection against HBV infection. After a 3-dose primary series of HepB vaccine, >95% of healthy infants, children, and young adults have protective antibody concentrations.Citation4,Citation5 However, levels of anti-HBs wane over time, and 15% to 50% of children who responded to the initial vaccination series will have low or undetectable anti-HBs levels by 5 to 15 years postvaccination.Citation6 Despite reduced circulating anti-HBs levels, a high rate of protection is expected years after the primary series of HepB vaccinationsCitation7 owing to B- and T-cell immunologic memory.Citation6,Citation8–10 Immune memory is the key to ongoing protection against HBV infection, as booster vaccinations are not recommended for the general population.Citation4,Citation10,Citation11
Immunologic memory is commonly demonstrated through exposure to HepB surface antigen (ie, a challenge) using a HepB vaccine. This challenge serves as a surrogate for exposure to HBV. In the absence of pre-existing immune memory, a single 5 μg HepB vaccination would not be expected to elicit an anti-HBs of ≥10 mIU/mL.Citation12,Citation13 Conversely, a 5-μg HepB challenge dose in the presence of immune memory, should induce an anamnestic response, or rapid increase in antibody concentration to levels ≥10 mIU/mL within a short period (1–30 days). Long-term protective immunity against HepB has not heretofore been demonstrated after an infant-toddler vaccination series with DTaP5-IPV-HepB-Hib (Vaxelis™; MCM Vaccine B.V., Leiden, The Netherlands).
DTaP5-IPV-HepB-Hib is a pediatric hexavalent vaccine indicated for primary and booster vaccination against diphtheria, pertussis, tetanus, poliomyelitis, HepB, and invasive diseases caused by Haemophilus influenzae type b.Citation14,Citation15 It is the only pediatric hexavalent vaccine licensed in the USCitation14,Citation16 and is 1 of 3 approved in the EU.Citation17 In two European studies of DTaP5-IPV-HepB-Hib administered as a 2-dose infant series and toddler dose (2 + 1) in Protocol V419–008Citation18 or 3-dose infant series and toddler dose (3 + 1) in Protocol V419–007,Citation19 98.1% and 99.6% of participants, respectively, had anti-HBs concentrations ≥10 mIU/mL 1 month after receipt of the toddler dose.Citation18,Citation19 In a follow-up study 3 to 4 years after the toddler dose, 65.8% and 70.2% of participants previously receiving DTaP5-IPV-HepB-Hib on a 2 + 1 (Protocol V419–008) and 3 + 1 schedule (Protocol V419–007), respectively, had anti-HBs levels ≥10 mIU/mL.Citation20 However, that follow-up study did not include an assessment of immune memory and continued protective immunity.
This clinical study aimed to evaluate the long-term durability of immune protection against HBV infection in a subset of children aged 8–9 years who were vaccinated with DTaP5-IPV-HepB-Hib on either a 2 + 1 or 3 + 1 schedule in the two previous clinical studies (Protocols V419–008 and V419–007, respectively). Ongoing protection was evaluated via a challenge dose study design in which anti-HBs levels were measured before and 30 days after a 5-μg challenge dose of the monovalent HepB vaccine.
Methods
Study design
This study (Protocol V419–013; ClinicalTrials.gov identifier: NCT04490499; EudraCT: 2020–000126–26) was an open-label, phase 3, single-dose, challenge study conducted in 10 centers in Finland from 2 September 2020 to 29 December 2020 (Supplemental Table S1). This is an estimation study, and the sample size of approximately 200 participants was determined using the number of participants needed to allow the estimation of the 95% CI of the primary immunogenicity endpoint having a half width of 4.0 percentage points, assuming an underlying response rate of 95%. The calculation was based on the exact binomial method proposed by Clopper and Pearson.Citation21,Citation22 Eligible children were healthy, aged 8 to 10 years, who had previously received DTaP5-IPV-HepB-Hib administered as a 2 + 1 (aged 2, 4, and 11–12 months) or 3 + 1 (aged 2, 3, 4, and 12 months) infant series and toddler dose as part of participation in Protocol V419–008 and Protocol V419–007, respectively. Exclusion criteria included a diagnosis of HBV infection determined by clinical, serologic, or microbiologic methods; receipt of any dose of a HepB vaccine other than the vaccines used in the initial studies; receipt of blood transfusion or blood products, including immunoglobulins within 6 months of study participation; receipt of any licensed, non-live vaccine within the 14 days before receipt of the challenge vaccine; or scheduled to receive any licensed, non-live vaccine within 30 days after receipt of the challenge vaccine. Each child was administered a single 0.5-mL (5 μg) dose of monovalent HepB vaccine (HBVAXPRO™; MSD, B.V., Haarlem, The Netherlands) as the challenge dose.
Scientists from Merck & Co., Inc., Rahway, NJ, USA, from MCM Vaccine B. V., Leiden, The Netherlands, and from Sanofi Pasteur, Swiftwater, PA, USA, contributed to the development of the study protocol and formulation of the statistical analysis plan. The study was conducted in accordance with principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. Written informed consent was provided by a legally acceptable representative before study enrollment of each study participant.
Immunogenicity
Anti-HBs levels were analyzed on serum from blood samples taken on day 1, prior to challenge, and 30 days post-challenge. The primary endpoint was the proportion of children with anti-HBs levels ≥10 mIU/mL on day 30 post-challenge. The secondary immunogenicity endpoint was anti-HBs geometric mean concentrations (GMC) on day 1 pre-challenge and on day 30 post-challenge. Additional exploratory endpoints included the proportion of children with anti-HBs levels ≥10 mIU/mL on day 1 and the proportion of participants with a ≥4-fold rise in anti-HBs levels from day 1 to 30 days post-challenge. A ≥4-fold rise in anti-HBs levels is a commonly accepted marker of robust anamnestic response.Citation10 The immunogenicity analyses were conducted using the per-protocol population, which included all enrolled children without protocol deviations that could have substantially affected the results of the immunogenicity endpoints. For subgroup analyses, the immunogenicity endpoints were also estimated and descriptively summarized by prior 2 + 1 and 3 + 1 dosing schedules to confirm the overall immunogenicity response was consistent across the dosing schedules. Quantification of anti-HBs was performed using a HepB-enhanced chemiluminescence assay (Q2 Solutions Vaccines, San Juan Capistrano, CA), which is a solid phase antigen sandwich enzyme-labeled immunoassay. Three internally prepared control serum pools—consisting of a high-positive, low-positive, and negative control—were used to monitor the performance of the assay. These pools were each prepared from four individual human immune sera obtained from an external vendor (Valley Biomedical, Winchester, VA). The 10-mIU/mL World Health Organization international standard concentration was also run as a control. The lower limit of quantitation of the assay was 5 mIU/mL, and the upper limit of quantitation of the assay was 1000 mIU/mL.
Safety
As specified in the protocol, adverse events (AE) resulting in study discontinuation and serious AEs were collected. The safety analysis population included all children who received the monovalent HepB vaccine challenge dose.
Statistical analysis
No statistical hypothesis testing was performed for immunogenicity analyses in this study. The point estimates for the proportion of participants with an anti-HBs level of ≥10 mIU/mL on pre-challenge day 1 and 30 days post-challenge were calculated, along with their 95% CIs using the Clopper–Pearson method.Citation21 The point estimates for anti-HBs GMC pre-challenge on day 1 and post-challenge on day 30 were calculated by exponentiating the estimates of the mean of the natural log values; 95% CIs were derived by exponentiating the bounds of the CIs of the mean of the natural log values based on the t-distribution. Reverse cumulative distribution (RCD) curves for anti-HBs GMC on day 30 post-challenge were plotted for the per-protocol population and by prior 2 + 1 and 3 + 1 dosing schedules.Citation23 Displaying results by RCD curves allows for the visual assessment and rapid comparison between distributions.Citation23 The point estimates for the proportion of participants with a ≥4-fold rise in anti-HBs levels from day 1 to day 30 post-challenge were provided with 95% CIs calculated using the Clopper–Pearson method.Citation21 The proportion of children with AEs resulting in discontinuation from the study and any serious AEs were summarized descriptively. All analyses and results were generated with SAS version 9.4.
Results
Study participants
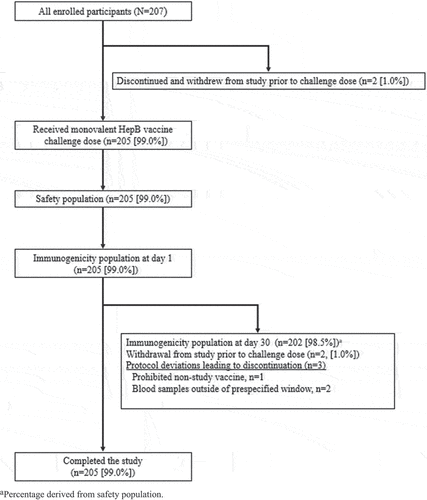
A total of 207 healthy children were enrolled in the study and 205 were vaccinated with monovalent HepB vaccine; 2 children withdrew from the study before receiving study vaccination (1 child experienced syncope after phlebotomy and 1 withdrew per a physician decision) (). Three protocol deviations were considered clinically important, and those data points (day 30 post-challenge immunogenicity) were excluded from the immunogenicity per-protocol analyses. These deviations were administration of prohibited non-study live or non-live vaccine during the 30 days after receipt of the study vaccine (n = 1) and blood samples drawn outside of the prespecified analysis window for the time point (n = 2). Eligible participants for this challenge-dose study were children 8–10 years of age. However, the age range of enrolled participants was 8–9 years, with a mean age of 8.4 years ().
Table 1. Demographics of the all enrolled population.
Immunogenicity
At baseline, 45.4% (n = 93/205) of children in the per-protocol study population had anti-HBs levels of ≥10 mIU/mL. These percentages were 40.9% and 49.1% among children who had received the DTaP5-IPV-HepB-Hib vaccine on a 2 + 1 and 3 + 1 schedule, respectively. Thirty days after receiving the monovalent HepB vaccine challenge dose, 99.5% (n = 201/202) of children in the immunogenicity analysis achieved anti-HBs levels of ≥10 mIU/mL (). When stratified according to a prior 2 + 1 and 3 + 1 dosing schedule, a similarly high percentage of children, 100% and 99.1%, respectively, achieved an anti-HBs level of ≥10 mIU/mL (), indicating similar responses across the two dosing schedules.
Table 2. Response rate at baseline and day 30 after the challenge dose with monovalent hepatitis B vaccine in the per-protocol population and in children stratified by 2 + 1 and 3 + 1 dosing DTaP5-IPV-HepB-Hib schedules.
A 71-fold increase in anti-HBs GMCs was observed from baseline to day 30 post-challenge in the per-protocol population, with increases observed when the population was stratified by prior 2 + 1 (83-fold increase) or 3 + 1 (63-fold increase) dosing of DTaP5-IPV-HepB-Hib (). In the per-protocol population, 96.0% (n = 194/202) of children had a ≥4-fold rise in anti-HBs levels from baseline to day 30 post-challenge, with similar results across the 2 + 1 and 3 + 1 dosing schedules ().
Table 3. Geometric mean concentrations (Anti-HBs) at baseline and day 30 after monovalent hepatitis B vaccine challenge in the per-protocol population and in children stratified by prior 2 + 1 and 3 + 1 dosing DTaP5-IPV-HepB-Hib schedules.
Table 4. Percentage of children with at least a 4-fold rise in anti-HBs concentration after monovalent hepatitis B vaccine challenge in the per-protocol population and in children stratified by Prior 2 + 1 and 3 + 1 dosing DTaP5-IPV-HepB-Hib schedules.
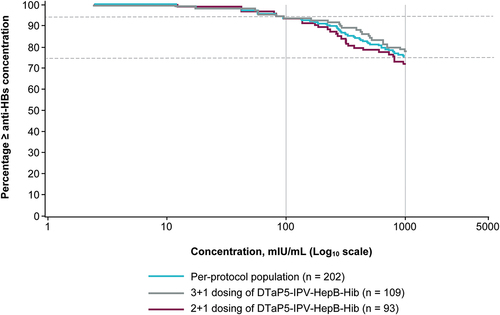
All but one child reached the seroprotective anti-HBs level of ≥10 mIU/mL at day 30 postchallenge in the per-protocol population and when stratified by 2 + 1 and 3 + 1 dosing of DTaP5-IPV-HepB-Hib (). As seen in the RCD curve for the per-protocol populations, a high percentage of children (94.0% [n = 189/202]) reached anti-HBs levels of ≥100 mIU/mL and many (75.0% [n = 152/202]) exceeded the upper limit of quantitation and reached anti-HBs levels ≥1000 mIU/mL at day 30 post-challenge; the distribution of anti-HBs was consistent across 2 + 1 and 3 + 1 dosing schedules ().
Figure 2. Reverse cumulative distribution curves of anti-HBs concentrations at day 30 post-challenge for the per-protocol population and for children stratified by a 2 + 1 dosing (V419–008) and 3 + 1 dosing (V419–007) of DTaP5-IPV-HepB-Hib. at day 30 post-challenge, anti-HBs levels of ≥ 100 mIu/ml (right solid vertical line) were achieved in 94% of children (upper dashed horizontal line) and anti-HBs levels of ≥ 1000 mIU/mL (left solid vertical line) were reached in 75% of children (lower dashed horizontal line).
Safety
No study discontinuations due to an AE or serious AEs were reported for children in the safety population. No deaths occurred during the study.
Discussion
This study demonstrated robust anti-HBs anamnestic responses after a monovalent HepB vaccine challenge dose in children 8–9 years who in the earlier studies had been vaccinated with DTaP5-IPV-HepB-Hib on either a 2 + 1 or 3 + 1 infant-toddler schedule. These findings confirm the presence of immune memory and durable protection against HepB among children who had received DTaP5-IPV-HepB-Hib.
The challenge dose elicited a 71-fold increase in anti-HBs GMCs, and this response was demonstrated in >99.0% of participating children, irrespective of anti-HBs levels at baseline. The percentage of responders after challenge was high for both dosing schedules (100% and 99.1%, respectively). The percentage of children with a ≥4-fold rise in anti-HBs was high (96.0%) and was similar across participants who had received 2 + 1 and 3 + 1 DTaP5-IPV-HepB-Hib schedules. Furthermore, a high percentage (94.0%) of children in the per-protocol population achieved an anti-HBs level ≥100 mIU/mL, and many (75.0%) achieved an anti-HBs level ≥1000 mIU/mL at day 30 after the challenge dose. These vigorous responses would not be expected from a single 5-μg dose of HepB vaccine challenge without the presence of immune memory.Citation12,Citation13
An anamnestic response after challenge with monovalent HepB vaccine has been observed in other studies of children previously vaccinated with a hexavalent vaccine during infancy. This anamnestic response has also been demonstrated in adults who received a series with the monovalent HepB vaccine component included in the multivalent vaccine evaluated in this study.Citation24 In a head-to-head study of DTaP2-IPV-HB-Hib (Hexyon®; Sanofi Pasteur, Lyon, France) and DTaP3-IPV-HB/Hib (Infanrix hexa™; GlaxoSmithKline, Rixensart, Belgium) in children aged 9–10 years, who had received a dose of HepB vaccine at birth, followed by the hexavalent vaccine at 2, 4, and 6 months of age; 92.8% and 98.7%, respectively, achieved anti-HBs levels ≥10 mIU/mL at day 30 post-challenge.Citation9 Other pediatric studies of DTaP3-IPV-HB/Hib vaccine were conducted and obtained similar results.Citation25–28 However, this is the first demonstration of ongoing immune memory and protection against HepB in children who received an infant-toddler series of DTaP5-IPV-HepB-Hib. The subgroup analyses showed that the 2 + 1 and 3 + 1 dosing schedules presented similar point estimates and clearly overlapping 95% CIs for the immunogenicity endpoints, and very similar distribution patterns on the RCD curves. This indicates an overall immune response that is consistent across the two dosing schedules, which is particularly pertinent in Western Europe where 2 + 1 dosing has become the dominant schedule. It is noteworthy that DTaP5-IPV-HepB-Hib administered with a 2 + 1 dosing schedule at 2, 4, and 11–12 months of age provided ongoing excellent responses despite the 2-month age at first vaccine administration and the 2 dose infant series.
Although the study demonstrated the long-term protection provided by DTaP5-IPV-HepB-Hib through a challenge study, certain limitations should be considered when evaluating these results. In addition to being an open-label study, the study was limited to children in Finland, who had participated in the initial infant studies. T-cell responses were not directly analyzed because of substantial heterogeneity and lack of standardization in the methods used for assessment; therefore, no direct observations can be made about the T-cell functions in this study. The measurement of antibody concentrations, however, does provide a well-established standardized platform for the assessment of B-cell function. Furthermore, T-cell activity and support are required to enhance B-cell function and subsequent antibody production; thus, the high IgG concentration in this setting of remote infant-toddler vaccination does reflect the complex interactions that occur between B and T cells.Citation8,Citation10,Citation29,Citation30 Total hepatitis B core antibody and hepatitis B surface antigen levels were not tested prior to study entry and, given the time between the toddler booster dose and the challenge dose, it is not possible to rule out natural exposure to HBV. However, children with a history of a clinical or serologic diagnosis of HBV were excluded from study participation. Furthermore, a 2017 publication showed a low annual rate of acute HBV infection in Finland (1.67 per 100,000 per year), and the 2 most common routes of transmission are sexual contact and intravenous drug use.Citation31 Children 1–11 years of age diagnosed with acute HBV were all foreign born, and the estimated incidence of acute infection in this age group was 0.55 per 100,000 per year.Citation31 Our study participants were composed of preadolescent children who were previously enrolled as infants in Finland in the V419–007 and V419–008 studies, both of which excluded participants born to mothers with evidence or history of chronic HBV infection. Therefore, the participants in the current study are considered extremely low risk for undiagnosed HBV infection.Citation31
Despite these limitations, our study clearly demonstrated long-term immune memory and impressive rebounds in anti-HBs in children who had received DTaP5-IPV-HepB-Hib for their infant-toddler doses.
Contributors/authorship
All authors are responsible for the work described in this paper. All authors were involved in at least one of the following: [conception, design of work or acquisition, analysis, interpretation of data] and [drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content]. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supplemental Material
Download MS Word (43 KB)Acknowledgments
Medical writing and/or editorial assistance was provided by Susan E. DeRocco, PhD, CMPP, and Madiha Khan, PharmD, of The Lockwood Group, Stamford, CT, USA. This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and MCM Vaccine B. V., Leiden, The Netherlands. We would also like to thank Michelle Goveia, MD, MPH, of Merck & Co., Inc., Kenilworth, NJ, USA, for her contributions to this manuscript.
Disclosure statement
DG, JL, TM, MBW, and YZ are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who may own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. DRJ is an employee of Sanofi Pasteur, Swiftwater, PA, USA, who may own stock and/or stock options from Sanofi S.A., Paris, France. AA has nothing to disclose.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2073747.
Additional information
Funding
References
- World Health Organization. Global hepatitis report, 2017. 2017 [accessed 2021 May 25]. http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1.
- Schillie S, Vellozzi C, Reingold A, Harris A, Haber P, Ward JW, Nelson NP. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Recomm Rep. 2018;67(1):1–7. doi:10.15585/mmwr.rr6701a1.
- Whitford K, Liu B, Micallef J, Yin JK, Macartney K, Van Damme P, Kaldor JM. Long-Term impact of infant immunization on hepatitis B prevalence: a systematic review and meta-analysis. Bull World Health Organ. 2018;96(7):484–97. doi:10.2471/BLT.17.205153.
- World Health Organization. Hepatitis B vaccines: WHO position paper – July 2017. 2017 [accessed 2021 May 10]. https://apps.who.int/iris/bitstream/handle/10665/255841/WER9227.pdf;jsessionid=6475C9E555C20312F6326A7E2B3ED1DD?sequence=1.
- Immunization Practices Advisory Committee. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1991;40(RR–13):1–25.
- Van Damme P, Ward JW, Shouval D, Zanetti A. Hepatitis B vaccines. In: Plotlin S; Offit P; Orenstein W, and Edwards K, editors. Plotkin’s vaccines. Philadelphia (PA): Elsevier; 2018. p. 342–74.
- Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis. 2016;214(1):16–22. doi:10.1093/infdis/jiv748.
- Banatvala J, Van Damme P, Oehen S. Lifelong protection against hepatitis B: the role of vaccine immunogenicity in immune memory. Vaccine. 2000;19(7–8):877–85. doi:10.1016/S0264-410X(00)00224-3.
- Kosalaraksa P, Chokephaibulkit K, Benjaponpitak S, Pancharoen C, Chuenkitmongkol S, B’-Chir S, Da Costa X, Vidor E. Persistence of hepatitis B immune memory until 9-10 years of age following hepatitis B vaccination at birth and DTaP-IPV-HB-PRP∼T vaccination at 2, 4 and 6 months. Hum Vaccin Immunother. 2018;14(5):1257–65. doi:10.1080/21645515.2018.1426418.
- Van Damme P, Dionne M, Leroux-Roels G, Van Der Meeren O, Di Paolo E, Salaun B, Surya Kiran P, Folschweiller N. Persistence of HBsAg-specific antibodies and immune memory two to three decades after hepatitis B vaccination in adults. J Viral Hepat. 2019;26(9):1066–75. doi:10.1111/jvh.13125.
- Poorolajal J, Mahmoodi M, Majdzadeh R, Nasseri-Moghaddam S, Haghdoost A, Fotouhi A. Long-Term protection provided by hepatitis B vaccine and need for booster dose: a meta-analysis. Vaccine. 2010;28(3):623–31. doi:10.1016/j.vaccine.2009.10.068.
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two- and three-dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics. 2001;107(4):626–31. doi:10.1542/peds.107.4.626.
- Diez-Domingo J, Flores SA, Martin JC, Klopfer SO, Schödel FP, Bhuyan PK. A randomized, multicenter, open-label clinical trial to assess the anamnestic immune response 4 to 8 years after a primary hepatitis B vaccination series. Pediatr Infect Dis J. 2010;29:972–74.
- United States Food & Drug Administration. VAXELIS™ [prescribing information]. Swiftwater (PA): MSP Vaccine Company; 2020 [accessed 2022 May 10]. https://www.fda.gov/vaccines-blood-biologics/vaxelis.
- European Medicines Agency. VAXELIS™ [summary of product characteristics]. Leiden (The Netherlands): MCM Vaccine B.V; 2020 [accessed 2022 May 10].https://www.ema.europa.eu/en/medicines/human/EPAR/vaxelis.
- Merck & Co., Inc. Merck and Sanofi’s first and only six-in-one pediatric combination vaccine now available in the United States. 2021 [accessed 2022 January 5]. https://www.merck.com/news/merck-and-sanofis-first-and-only-six-in-one-pediatric-combination-vaccine-now-available-in-the-united-states/.
- Knuf M, Haas H, Garcia-Corbeira P, Turriani E, Mukherjee P, Janssens W, Berlaimont V. Hexavalent vaccines: what can we learn from head-to-head studies? Vaccine. 2021;39(41):6025–36. doi:10.1016/j.vaccine.2021.08.086.
- Silfverdal SA, Icardi G, Vesikari T, Flores SA, Pagnoni MF, Xu J, Liu GF, Stek JE, Boisnard F, Thomas S, et al. A phase III randomized, double-blind, clinical trial of an investigational hexavalent vaccine given at 2, 4, and 11-12 months. Vaccine. 2016;34(33):3810–16. doi:10.1016/j.vaccine.2016.05.054.
- Vesikari T, Becker T, Vertruyen AF, Poschet K, Flores SA, Pagnoni MF, Xu J, Liu GF, Stek JE, Boisnard F, et al. A phase III randomized, double-blind, clinical trial of an investigational hexavalent vaccine given at two, three, four and twelve months. Pediatr Infect Dis J. 2017;36(2):209–15. doi:10.1097/INF.0000000000001406.
- Vesikari T, Xu J, Johnson DR, Hall J, Marček T, Goveia MG, Acosta CJ, Lee AW. Hepatitis B and pertussis antibodies in 4- to 5-year-old children previously vaccinated with different hexavalent vaccines. Hum Vaccin Immunother. 2020;16(4):867–74. doi:10.1080/21645515.2019.1673119.
- Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(4):404–13. doi:10.1093/biomet/26.4.404.
- Collett D, Stepniewska K. Some practical issues in binary data analysis. Stat Med. 1999;18(17–18):2209–21. doi:10.1002/(SICI)1097-0258(19990915/30)18:17/18<2209:AID-SIM250>3.0.CO;2-U.
- Reed GF, Meade BD, Steinhoff MC. The reverse cumulative distribution plot: a graphic method for exploratory analysis of antibody data. Pediatrics. 1995;96(3 Pt 2):600–03. doi:10.1542/peds.96.3.600.
- Sharma R, Ahlm C, Ostergaard L, Dowell A, Tran C, Thomas S, Eymin C. Persistence of immunity in healthy adults aged ≥ 50 years primed with a hepatitis B vaccine 3 years previously. Hum Vaccin Immunother. 2015;11(7):1709–16. doi:10.1080/21645515.2015.1019187.
- Behre U, Van Der Meeren O, Crasta P, Hanssens L, Mesaros N. Lasting immune memory against hepatitis B in 12-13-year-old adolescents previously vaccinated with 4 doses of hexavalent DTPa-HBV-IPV/Hib vaccine in infancy. Hum Vaccin Immunother. 2016;12(11):2916–20. doi:10.1080/21645515.2016.1202388.
- Schwarz TF, Behre U, Adelt T, Donner M, Suryakiran PV, Janssens W, Mesaros N, Panzer F. Long-Term antibody persistence against hepatitis B in adolescents 14-15-years of age vaccinated with 4 doses of hexavalent DTPa-HBV-IPV/Hib vaccine in infancy. Hum Vaccin Immunother. 2019;15(1):235–41. doi:10.1080/21645515.2018.1509658.
- Steiner M, Ramakrishnan G, Gartner B, Van Der Meeren O, Jacquet JM, Schuster V. Lasting immune memory against hepatitis B in children after primary immunization with 4 doses of DTPa-HBV-IPV/Hib in the first and 2nd year of life. BMC Infect Dis. 2010;10:9. doi:10.1186/1471-2334-10-9.
- Van Der Meeren O, Bleckmann G, Crasta PD. Immune memory to hepatitis B persists in children aged 7-8 years, who were vaccinated in infancy with 4 doses of hexavalent DTPa-HBV-IPV/Hib (Infanrix hexa) vaccine. Hum Vaccin Immunother. 2014;10(6):1682–87. doi:10.4161/hv.28480.
- Simons BC, Spradling PR, Bruden DJ, Zanis C, Case S, Choromanski TL, Apodaca M, Brogdon HD, Dwyer G, Snowball M, et al. A longitudinal hepatitis B vaccine cohort demonstrates long-lasting hepatitis B virus (HBV) cellular immunity despite loss of antibody against HBV surface antigen. J Infect Dis. 2016;214(2):273–80. doi:10.1093/infdis/jiw142.
- Zinkernagel RM, Bachmann MF, Kündig TM, Oehen S, Pirchet H, Hengartner H. On immunological memory. Annu Rev Immunol. 1996;14:333–67. doi:10.1146/annurev.immunol.14.1.333.
- Karvonen T, Auranen K, Kuusi M, Leino T. Epidemiology of hepatitis B infection in Finland: implications for immunisation policy. Vaccine. 2017;35(3):412–18. doi:10.1016/j.vaccine.2016.11.090.