ABSTRACT
The 4-valent human papillomavirus (HPV) vaccine (4vHPV vaccine), Gardasil®, is indicated for the prevention of several HPV-related diseases. The objective was to assess the safety of 4vHPV vaccine administered to males as part of routine care. The study used a US health insurance claims database, and included males, age 9 to 26 years, who initiated 4vHPV between October 2009 and December 2016. General safety outcomes were identified using ICD diagnosis codes associated with emergency room visits and hospitalizations in the claims database in risk periods (Days 1–60 and Days 1–14 following vaccine administration) and self-comparison periods (Days 91–150 and 91–104 for the Days 1–60 and Days 1–14 analysis, respectively). Incidence rates (IRs) and relative rates (RRs) with 95% confidence intervals (CIs) were calculated comparing the risk and self-comparison periods. In this study, 114,035 males initiated 4vHPV vaccine and received 202,737 doses. Using the 60-day time window, 5 outcomes had significantly elevated RRs after accounting for multiple comparisons: ear conditions (RR 1.28, 95% CI 1.03–1.59); otitis media and related conditions (RR 1.65, 95% CI 1.09–2.54); cellulitis and abscess of arm (RR 2.17, 95% CI 1.06–4.72); intracranial injury (RR 1.23, 95% CI 1.01–1.50); and concussion (RR 1.29, 95% CI 1.05–1.59). A higher rate of allergic reactions was noted on the day of 4vHPV vaccine receipt compared to other vaccines (21.07 events per 10,000 doses, 95% CI 18.89–23.44 versus 11.44 per 10,000 doses, 95% CI 9.84–13.22). A higher incidence rate of VTE was observed following vaccination but this association was not significant (RR 2.17, 95% CI 0.35–22.74). The 4vHPV vaccine was associated with same-day allergic reactions as well as ear infections, intracranial injury, cellulitis, and concussion within 2 months after vaccination. While allergic reaction and cellulitis are consistent with the known safety profile of 4vHPV vaccine, the association of the other outcomes were determined by an independent Safety Review Committee to be most likely a result of activities common in adolescent males that coincide with the timing of vaccination and not directly related to vaccination itself.
Implications and Contributions: The study results support the general safety of routine immunization with 4vHPV vaccine among males to prevent HPV-related diseases and cancers.
Introduction
Human Papillomavirus (HPV) infection is responsible for nearly all cervical cancers among women as well as a significant fraction of anogenital and oropharyngeal cancers in both men and women resulting in >500,000 annual cancer cases globally. Among men globally, HPV has been attributed to nearly 70,000 cases of penile, anal, oropharyngeal, and other head and neck cancers.Citation1,Citation2 In the United States (US), the most common HPV-related cancer between 2008–2012 was HPV-related oropharyngeal cancer, with 80% of cases occurring in men.Citation3 Projections from the US Surveillance, Epidemiology, and End Results Program (SEER) registry have estimated that the annual burden of oropharyngeal cancer will increase from 20,000 cases in 2016 to 30,000 in 2029, with the majority of this increase driven by men.Citation4
The 4-valent human papillomavirus (HPV) vaccine (4vHPV vaccine), Gardasil®, was first approved in the US by the Food and Drug Administration (FDA) for use in females ages 9 to 26 years in June 2006 as a 3-dose vaccine, with an expansion of the indication to include males of the same ages in October 2009 for the prevention of HPV-related external genital warts. However, while the US Advisory Committee on Immunization Practices (ACIP) provided guidance that 4vHPV vaccine may be given to males it did not add the vaccine to the male routine immunization schedule.Citation5 An additional indication was approved for the 4vHPV vaccine in December 2010: use in males and females, ages 9–26 years, for the prevention of anal intraepithelial neoplasia (AIN) and anal cancer caused by HPV.Citation6 In October 2011, ACIP revised their recommendation to include routine use in males aged 11 to 12 years, along with routine use in older males (13 to 21 years) to initiate or complete the regimen.Citation7 A 9-valent HPV (9vHPV) vaccine was approved by the FDA in December 2014, and has been the only vaccine available in the US since May 2017, but remains available in other countries.
Information on the safety profile of 4vHPV vaccination among males is somewhat limited with most data generated from pre-licensure trials, post-licensure safety surveillance, and specific post-marketing studies conducted among females.Citation8–13 These studies have identified primarily injection-site pain as well as syncope as the main safety events following vaccination.Citation11 A pre-licensure clinical efficacy trial of 4vHPV vaccine among over 4,000 males 16 to 26 years of age demonstrated a similar safety profile.Citation13 The current study was initiated following the approval of the 4vHPV vaccine among males in the US. We undertook the current study of 4vHPV with the primary aim to evaluate the safety of 4vHPV administered to males as part of routine healthcare.
Methods
Data source
This study utilized the Optum Research Database (ORD), which includes patient demographics, insurance enrollment dates, reimbursement claims for physician and hospital services, and pharmacy records for members of a US commercial health insurer. For 2017, there were approximately 14.6 million individuals within the database for whom both medical and pharmacy benefit coverage were available. The patient population in the database is geographically diverse across the US and corresponds well to the US population with respect to gender, age distribution less than 65 years of age, and census region. Reimbursement claims include diagnoses recorded using the International Classification of Diseases, Ninth Revision (ICD-9) (through September 2015) and Tenth Revision (ICD-10) (since October 2015) along with procedures coded using ICD-9/ICD-10 procedure, Common Procedural Terminology (CPT), and the Healthcare Common Procedure Coding Systems. The database is geographically diverse across the US and corresponds well to the US population with respect to gender, age (for those less than 65 years), and census region.
Source population
In this self-controlled risk interval cohort study, we identified male recipients of the 4vHPV vaccine using the specific procedure code (CPT 90,649) for this vaccine. Accrual of patients began on 16 October 2009 (day of US approval in males) and continued through 31 December 2016, with follow-up extending through 31 May 2017. The first dose of 4vHPV vaccine administration observed in the data source defined initiation. The regimen complete cohort was a subset of the regimen initiator cohort of males who received 3 doses of 4vHPV vaccine and were 9 to 26 years old at the date of first dose of and received the 3 doses according to the recommended schedule (0, 2, and 6 months).
Safety outcomes
General safety outcomes were identified by claims corresponding to an ER visit or hospitalization to identify serious events and the associated ICD-9 (prior to 1 October 2015) and ICD-10 diagnosis codes (starting on 1 October 2015). The specific diagnosis codes from these claims were grouped according to hierarchically organized, clinically meaningful categories developed by the Healthcare Cost Utilization Project (HCUP).Citation14 The ICD-9 and ICD-10 codes from ER visit and hospitalization claims were mapped to 139 pre-specified HCUP categories. Health outcomes in level 4 are the most specific, whereas levels 3, 2, and 1 are increasingly less specific; health outcomes represented in level 4 are also represented in levels 3, 2, and 1. Analyses were conducted using all level 1 and 2 (i.e., high level) HCUP categories, and the following select lower level categories: ‘otitis media and related conditions’ (HCUP 6.8.1); ‘cellulitis and abscess of arm’ (HCUP 12.1.1.3); and ‘concussion’ (HCUP 16.4.1).
The occurrence of venous thromboembolism (VTE), including deep vein and superficial thrombosis as well as pulmonary embolism, was identified using diagnosis codes associated with a hospitalization, ER visit, or outpatient visit. Medical chart review by physician internists was conducted to confirm case status and timing of event onset.
Pre-specified events (syncope, epilepsy/convulsions, head trauma, and allergic events) that occurred on the day of vaccination (referred to as Day 0) were identified by specific diagnosis codes associated with an outpatient visit, ER visit, or hospitalization.
Death outcomes among vaccinated males were identified in the study databases as well as through external sources, including the National Death Index.
Statistical methods
General safety and VTE
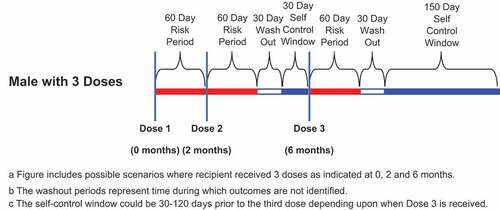
The study used a self-controlled risk-interval design to assess differences in the incidence of general safety and VTE outcomes in the time period immediately following vaccination (risk period) relative to a later time period (self-comparison period) (). For each HCUP category and VTE outcome, the incidence of events occurring in the risk period was compared to that in the self-comparison period. Risk periods were defined as Days 1–60 and 1–14 following vaccination. There was a 30-day washout period (time during which outcomes are not identified) following the risk period, and the self-comparison period began 91 days following vaccination with a similar duration to the risk period. For the 3-dose population, the self-comparison period was 180-day period that began 91 days after the last 4vHPV dose. The 180-day self-comparison period was selected, because it is equivalent to the sum of the 3 postvaccination 60-day risk intervals. The self-comparison period was 60 days for males who received one 4vHPV vaccine, 120 days for those who received 2 doses, and 180 days for those who received 3 doses. In the Dose 1 analysis the risk period was 60 days in length and the self-comparison periods started 91 days following Dose 1. Censoring of risk periods and comparison periods were independent of each other. The censor date for these periods was the earliest of death, disenrollment from the health plan, calendar day before receipt of subsequent dose, calendar day before receipt of 9vHPV vaccine, or the end of the risk period or self- comparison period.
For VTE outcomes, the first code within the risk or self-comparison period was selected as a potential event. Repeat occurrences of VTE codes within 7 days of one another were assumed to represent continuing care for the same event. VTE codes occurring more than 7 days apart were considered to represent potentially separate events.
Incidence rates (IRs) and 95% confidence intervals (CIs) for each general safety and VTE outcome were based on first occurrence of the event in the risk and self-comparison periods and calculated as the number of events divided by the person-time in the period in which the event occurred. Analyses were conducted after combining risk periods for all doses received by an individual (all dose analysis) and for the 1st dose only (Dose 1 analysis). Separate analyses were conducted excluding males with codes for the same HCUP category up to 12 months prior to receipt of their first dose in any healthcare setting (outpatient, ER, or hospitalization) (no prior codes analysis). For VTE outcomes, chart-confirmed cases were used in the calculation of IRs (events divided by person-time). Potential VTE cases with insufficient medical chart information to confirm the event were excluded from analyses.
Relative rates (RRs) were calculated as the ratio of the incidence of the health outcome in the risk and self-comparison periods for each general safety HCUP category and VTE outcome. For general safety outcomes, RRs were adjusted for multiple-comparisons using the double false discovery rate (DFDR) method.Citation15 Groupings for application of the DFDR were made at HCUP levels 1 and 2.
Day 0 outcomes
For the Day 0 analysis, counts of the pre-specified events of interest were assessed in contrast to a concurrent, matched (age within 1.5 years and calendar time within 1.5 months) comparison cohort of males with a physician office visit during which a different vaccine (Td/Tdap, meningococcal, or influenza (injectable)) was administered. Analyses were repeated after excluding from each outcome category (syncope, epilepsy/convulsions, head trauma, and allergic reactions) those individuals who had codes for the same outcome in the 30 days prior to Day 0. Incidence was calculated as the number of events per 10,000 doses.
Death outcomes
Death outcomes among all males vaccinated with 4vHPV vaccine were identified using patient discharge status codes, ICD-9 and ICD-10 diagnosis codes, as well as the Death Master File (DMF) from the Social Security Administration (SSA) from the date of receipt of Dose 1 of 4vHPV vaccine through 60 days after the last vaccine dose.Citation16 Medical records associated with death outcomes were reviewed by a clinician to identify date and cause of death. Searches were conducted in the National Death Index (NDI) to identify potential additional deaths and to confirm deaths identified through other means.Citation17
All data analyses were performed using SAS version 9.4.
Safety review committee
An external Safety Review Committee (SRC) comprised of 4 experts in adolescent medicine, vaccine safety, autoimmune conditions, and pharmacoepidemiology was established prior to the start of the study. The SRC’s primary responsibility was to review and evaluate the safety data emerging from the study. The SRC also reviewed and approved the study protocol, the data analysis plan, and the methods used to conduct the study.
Privacy and confidentiality
This study was conducted in a manner consistent with the Guidelines for Good Pharmacoepidemiology Practices,Citation18 and was approved by the New England Institutional Review Board (IRB) and Privacy Board.
Results
Between 16 October 2009 and 31 December 2016, a total of 114,035 males received at least one dose, 58,552 males received at least 2 doses, and 30,150 males received 3 doses of 4vHPV vaccine (). Ninety-nine percent (99%) of the regimen initiators were in the age range of 9 to 26 years, and 83% of those initiators received the vaccine between age 9–17 years of age. Vaccination rates were much higher in the July-September period than in other calendar periods ().Citation19 General Safety
Table 1. Summary of the age distribution of 4vhpv vaccine recipients, October 2009 to December 2016.
Nine HCUP categories had significantly elevated RRs (lower bound of the 95% CI for the RR > 1.00) associated with an ER visit or hospitalization in at least one of the analyses, i.e., one risk interval or dose level, and/or without prior codes for the event (). These HCUP categories included: ear conditions; otitis media and related conditions; skin and subcutaneous tissue infections; cellulitis and abscess of arm; other inflammatory condition of skin; injury and poisoning; intracranial injury; concussion; and external causes of injury.
Table 2. Summary of the Incidence Rates (IR) and Relative Rates (RR) for health outcomes significantly increased among 4vhpv vaccine recipients (N = 114,035) (All doses combined).
Following multiple-comparison adjustment, 5 HCUP category elevations remained statistically significant in the Days 1 to 60 analysis all doses combined: ear conditions (RR 1.28, 95% CI 1.03–1.59); otitis media and related conditions (RR 1.65, 95% CI 1.09–2.54); cellulitis and abscess of arm (RR 2.17, 95% CI 1.06–4.72); intracranial injury (RR 1.23, 95% CI 1.01–1.50); and concussion (RR 1.29, 95% CI 1.05–1.59). All outcomes remained significant in the no prior codes analysis except for ear conditions.
To investigate the observed seasonal pattern of 4vHPV administration may be responsible for these observed associations, additional analyses were performed to create visual displays of the number of 4vHPV vaccine administrations by month, which demonstrated a substantial increase in vaccine administration in the summer months (June through September) for both first and second doses (). This pattern of vaccine administration was accompanied by a similar distribution of follow-up time by calendar month for the risk and self-comparison period. Subsequently, given the self-controlled design of the study, follow-up time in the risk period showed a clustering in the summer months while a cluster of follow-up time for the self-comparison period was observed in the late fall/early winter.
In Dose 1 analysis, one HCUP category, skin and subcutaneous tissue infections, remained statistically significant, only in the Days 1 to 14 (RR 2.35 95% CI 1.15–5.11). () There were no HCUP categories that had significantly elevated RRs after multiple-comparison adjustment in Days 1 to 60 and Days 1 to 14 risk intervals.
Table 3. Summary of the Incidence Rates (IR) and Relative Rates (RR) for health outcomes significantly increased among 4vhpv vaccine recipients (N = 114,035) (Dose 1 only).
Three HCUP categories had RRs that were significantly decreased after multiple-comparison adjustment: mental illness, diseases of musculoskeletal system and connective tissue, and concussion (). These 3 HCUP categories remained statistically significant in the no prior codes analysis.
In analyses looking at hospitalization only, there were no outcomes with a significantly increased RR after multiple-comparison adjustment in any risk interval or dose level (). One HCUP category had a significantly decreased RR associated with a hospitalization: mental illness in the Days 1–14 and 1–60 all doses, no prior codes analysis (RR [Day 1-14] 0.41, 95% CI 0.24–0.70; RR [Day 1-60] 0.62, 95% CI 0.47–0.81). The incidence of general safety outcomes within Days 1–14 post-vaccination as compared to the self-comparison period did not differ by vaccine dose number (Dose 1 vs. Dose 2+).
VTE
There were 33 and 17 potential VTE events identified within the 60-day period following vaccination (risk period) and in the self-comparison period, respectively. Among these potential events in the risk period, medical charts were obtained for 18 and 12 were confirmed. In the self-comparison period, medical charts were obtained for 9 and 4 were confirmed. The incidence of VTE was 1.6 (95% CI: 0.5–3.7) and 0.7 (95% CI: 0.1–2.6) per 10,000 person-years among the risk and self-comparison periods (). The unadjusted RR was 2.17 (0.35–22.74).
Table 4. Rate of Venous Thromboembolism (VTE) Outcomes (per 10,000 Person-Years) among 4vhpv vaccine recipients in Risk Period versus Post-Vaccination Self-Control Period by Follow-up Window: All Doses Combined.
Day 0
Among 4vHPV vaccine recipients (n = 160,867 doses) with all doses combined (), the incidence of emergent allergic reaction outcomes was higher than in the comparison cohort that received another vaccine (21.07 per 10,000 doses, 95% CI 18.89–23.44 versus 11.44 per 10,000 doses, 95% CI 9.84–13.22). Conversely, the incidence of emergent epilepsy/convulsion outcomes was lower among 4vHPV vaccine recipients than in the comparison cohort (5.66 per 10,000 doses, 95% CI 4.55–6.95 versus 7.58 per 10,000 doses, 95% CI 6.30–9.06). The incidence of syncope (5.47 per 10,000 doses, 95% CI 4.39–6.74 versus 5.41 per 10,000 doses, 95% CI 4.33–6.67) and head trauma (7.83 per 10,000 doses, 95% CI 6.52–9.33 versus 7.71 per 10,000 doses, 95% CI 6.41–9.19) were similar among 4vHPV vaccine recipients and comparators.
Table 5. Rates of Emergent Outpatient, Emergency Room Visit, and Hospitalization Day 0 Outcomes among 4vhpv vaccine recipients and Concurrent Controls with Td/Tdap, Meningococcal, or Influenza Vaccine.
Deaths
There were 10 deaths confirmed by medical record review and/or the NDI and/or the SSA DMF. The causes of death included heart failure (n = 1), motor vehicle collision (n = 1), suicide/self-inflicted injury (n = 7), and unknown cause of death (n = 1). The 10 confirmed deaths were compared to the expected number of deaths based on age-, sex-, and race-specific mortality rates from the US National Vital Statistics Report applied to the person-time available among the 4vHPV male vaccinated study cohort, which was 9.8 deaths.Citation20
Discussion
Using a US commercial claims database, we assessed the safety of 202,737 4vHPV vaccine doses administered during routine clinical care to 114,035 males. We evaluated all post-vaccination ER and hospitalization events and identified significantly elevated RRs in 9 HCUP categories. Five categories (intracranial injury, concussion, ear conditions, otitis media and related conditions, cellulitis and abscess of arm) remained significantly elevated after multiple-comparison adjustment in the Days 1 to 60 analysis. Abscess of the arm and cellulitis have been reported previously in studies of 4vHPV vaccine in males and females.Citation6,Citation8 Additional analyses of the timing of intracranial injury, concussion, ear conditions, otitis media and related conditions relative to calendar time and time since vaccination seem to suggest that these outcomes are related to activities and behaviors among adolescent males that coincide with vaccination and not related to the 4vHPV vaccination itself. A higher incidence of nonspecific allergic reactions on the day of vaccination was observed among 4vHPV vaccines as compared to individuals receiving other vaccines. This health outcome has been observed in both pre- and post-licensure studies of the 4vHPV vaccine primarily conducted among females. Overall, the study’s independent safety review committee determined that the results from this study do not raise any new safety concerns among males receiving the 4vHPV vaccine.
The associations of 4vHPV vaccine administration with intracranial injuries and concussion may be due to confounding by calendar time. Upon assessment of these findings by the study’s independent safety review committee, additional analyses were conducted to assess the distribution of follow-up time and the timing of the occurrence of these outcomes by calendar time and time since vaccination. Given the observed seasonal pattern of increased vaccine administration in the summer months (June through September), a similar increase in follow-up time in the risk period was observed in these specific months. The increase in vaccine administration during the summer months (July to September) among adolescent males in the US coincides with summer activities such as summer camp/school attendance and/or participation in school or collegiate sports which may put these healthy individuals at risk for such outcomes. Lastly, the distribution of the reported occurrence of these specific health outcomes in the 14- and 60-day time period following vaccination was generally uniform with no evidence of clustering closer to the time of vaccination. These additional analyses led to the determination by the study’s independent safety review committee that the observed associations are most likely a result of confounding by calendar time in this self-controlled study.
The association between ear conditions, including otitis media, and 4vHPV vaccination has been reported previously. A safety study of 4vHPV vaccine among females initially identified an association of vaccination with ear conditions but this association did not remain significant after adjustment for multiple comparisons and outcome confirmation by medical record review.Citation8 Given this prior work, the association observed in this study is most likely explained by unmeasured potential confounding.
The associations between 4vHPV vaccine administration and skin infections, cellulitis and abscess of the arm and other inflammatory conditions of the skin are consistent with clinical trial and other observational studies. These may represent local skin reactions at the vaccine site that are misdiagnosed as cellulitis or abscess of the arm. Local injection site reactions are known adverse events following the vaccine observed in clinical trials in both males and females.Citation6 Our findings are consistent with a previous post-licensure safety study of 4vHPV vaccine among over 150,000 US adolescent females that found vaccination to be associated with skin infections during the two weeks following vaccination.Citation8
The absence of association of VTE following vaccination observed in this study is in agreement with other studies of VTE risk among male vaccine recipients that did not find an association. Two studies conducted in the US Vaccine Safety Data link and the Danish national registers among a large group adolescent males vaccinated with the 4vHPV vaccine observed RRs for VTE within 60–180 days post-vaccination of 0.92 (95% CI: 0.54–1.57) and 0.88 (95% CI: 0.33–2.35), respectively.Citation21,Citation22 The small number of confirmed VTE cases identified in our study limits the generalizability of our findings.
The elevated incidence of allergic reactions, including anaphylaxis, on the day of vaccination among 4vHPV vaccine recipients observed in this study is consistent with other studies. An Institute of Medicine (IOM) report in 2011 identified adverse events, including anaphylaxis, associated with 4vHPV vaccine.Citation23 The incidence of these allergic outcomes among 4vHPV vaccines in our study using claims data is higher than what has been reported previously in another study conducted among women 12 to 26 years of age from Australia, in which allergic reactions were identified and evaluated using expert panel reviews.Citation24 A post-licensure safety study conducted among more than 189,000 females aged 9 to 26 from the United States who received the 4vHPV vaccine initially identified an elevated incidence rate of allergic/anaphylactic reactions on the day of vaccination. However, upon review of medical records of the 3 allergic/anaphylactic events, the study’s independent safety review committee determined that these events were not associated with 4vHPV vaccination.Citation8 Similar, medical record review of select Day 0 outcomes in this study was conducted to confirm the occurrence, timing, and nature of the Day 0 events identified using claims data. A majority of the day 0 events identified were events that occurred prior to the day of vaccination and were recorded in the database on that day. This chart review also demonstrated that the codes identifying Day 0 outcomes in this claims-based study lack the specificity available from other data sources. Therefore, similar to prior studies, the elevated incidence of allergic reactions in this study of males is most likely not associated with 4vHPV vaccination.
The identification of study exposures and outcomes was based on health insurer claims and primarily reflects interactions related to billing. Therefore, some of the study information may have been influenced by the nature of these interactions. The described diagnoses were based on claims that could include a combination of actual individual characteristics, billing diagnoses that were submitted to the insurer in order to justify a service, and other uncertainties about what aspects of a healthcare service are coded when a claim for reimbursement is submitted. While the administrative capture of routine healthcare from a broad-based population provides a level of external validity to health outcomes related to the routine use of 4vHPV vaccine among males, it does result in challenges, such as ascertaining the certainty of the accuracy and timing of health outcomes based on diagnostic codes as well as incomplete recording of medical services.Citation25–27 These uncertainties were addressed in several ways, including confirmation of both the diagnosis and timing of study outcomes through select profile review and medical record confirmation (e.g., for VTE and select Day 0 events) and a mix of self-controlled and concurrent matched cohort designs.
Conclusions
The safety findings of this large database study of males who received 4vHPV vaccine as part of routine care were consistent with the known safety profile of this vaccine developed based on a large number pre- and post-licensure studies of both the 4vHPV and 9vHPV vaccine conducted among females. The observed association of vaccination with ear infection, intracranial injury, and concussion may be a result of activities common in adolescent males, especially in the time period (summer and early fall) that follows the majority of 4vHPV vaccinations. The use of healthcare data from a large number of geographically dispersed males who received routine care from many different healthcare settings across the US, combined with the use of self-controlled matched study designs, helped reduce bias and contributed to the generalizability of the study findings. These results support the safety profile of routine vaccination with 4vHPV vaccine among males.
Abbreviations
| ACIP | = | Advisory Committee on Immunization Practices |
| CI | = | Confidence Interval |
| CPT | = | Common Procedural Terminology |
| DFDR | = | Double False Discovery Rate |
| DMF | = | Death Master File |
| ER | = | Emergency Room |
| FDA | = | Food and Drug Administration |
| HCUP | = | Healthcare Utilization Project |
| HPV | = | Human Papillomavirus Vaccine |
| ICD-9 | = | International Classification of Diseases, Ninth Revision |
| ICD-10 | = | International Classification of Disease, Tenth Revision |
| IR | = | Incidence Rate |
| IRB | = | Institutional Review Board |
| NDI | = | National Death Index |
| ORD | = | Optum Research Database |
| RR | = | Relative Rate |
| SRC | = | Safety Review Committee |
| SSA | = | Social Security Administration |
| TD | = | Tetanus, Diphtheria |
| TDAP | = | Tetanus, Diphtheria and Acellular Pertussis |
| US | = | United States |
| 4vHPV | = | 4-valent human papillomavirus |
| 9vHPV | = | 9-valent human papillomavirus |
Acknowledgement
The authors would like to acknowledge the members of the Safety Review Committee: Dr. Neal Halsey (Chair), Director, Institute for Vaccine Safety, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD; Dr. Andrew Eichenfield, Columbia University Medical Center, New York, NY; Dr. Robert Jacobson, Director of Clinical Studies, Vaccine Research Group, Mayo Clinic, Rochester, MN; Dr. Alec Walker, World Health Information Science Consultants, Newton, MA.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–18. doi:10.1016/S2214-109X(19)30488-7.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. doi:10.1002/ijc.30716.
- Viens LJ, Henley SJ, Watson M, Markowitz LE, Thomas CC, Thompson TD, Razzaghi H, Saraiya M. Human papillomavirus–Associated Cancers — United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661–66. doi:10.15585/mmwr.mm6526a1.
- Tota JE, Best AF, Zumsteg ZS, Gillison ML, Rosenberg PS, Chaturvedi AK. Evolution of the oropharynx cancer epidemic in the United States: moderation of increasing incidence in younger individuals and shift in the burden to older individuals. J Clin Oncol. 2019;37(18):1538–46. doi:10.1200/JCO.19.00370.
- Centers for Disease Control. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010;59:630–32. [accessed 2020 Jan 30].
- Food and Drug Administration. Highlights of prescribing information. Gardasil (human papillomavirus quadrivalent [types 6, 11, 16 and 18]). Silver Spring, MD: Food and Drug Administration; 2011. accessed 2020 Jan 30.
- Centers for Disease Control. Recommendations on the use of quadrivalent human papillomavirus vaccine in males – advisory committee on immunization practices, 2011. MMWR Morb Mortal Wkly Rep 2011;60:1705–08. [accessed 2020 Jan 30].
- Klein NP, Hansen J, Chao C, Velicer C, Emery M, Slezak J, Lewis N, Deosaransingh K, Sy L, Ackerson B, et al. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch Pediatr Adolesc Med. 2012;166(12):1140–48. doi:10.1001/archpediatrics.2012.1451.
- Gee J, Naleway A, Shui I, Baggs J, Yin R, Li R, Kulldorff M, Lewis E, Fireman B, Daley M, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the vaccine safety datalink. Vaccine. 2011;29(46):8279–84. doi:10.1016/j.vaccine.2011.08.106.
- Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, Izurieta HS, Ball R, Miller N, Braun M, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. Jama. 2009;302(7):750–57. doi:10.1001/jama.2009.1201.
- Vichnin M, Bonanni P, Klein NP, Garland SM, Block SL, Kjaer SK, Sings HL, Perez G, Haupt RM, Saah AJ, et al. An overview of quadrivalent human papillomavirus vaccine safety: 2006 to 2015. Pediatr Infect Dis J. 2015;34(9):983–91. doi:10.1097/INF.0000000000000793.
- Stillo M, Carrillo Santisteve P, Lopalco PL. Safety of human papillomavirus vaccines: a review. Expert Opin Drug Saf. 2015;14(5):697–712. doi:10.1517/14740338.2015.1013532.
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, et al. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N Engl J Med. 2011;364(5):401–11. doi:10.1056/NEJMoa0909537.
- Agency for Healthcare Research and Quality. HCUP research tools and software. Healthcare Cost and Utilization Project (HCUP); 2022 [accessed 2022 April 8]. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/CCSCategoryNames_FullLabels.pdf .
- Mehrotra DV, Heyse JF. Use of the false discovery rate for evaluating clinical safety data. Stat Methods Med Res. 2004;13(3):227–38. doi:10.1191/0962280204sm363ra.
- National Technical Information Service. Social security administration death master file; 2020 [accessed 2020 Jan 29]. https://dmf.ntis.gov/ .
- Centers for Disease Control. National Death Index; 2017 [accessed 2020 Jan 29]. https://www.cdc.gov/nchs/ndi/index.htm .
- ISPE. Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf. 2008;17(2):200–08. doi:10.1002/pds.1471.
- Amend KL, Turnbull B, Zhou L, Marks MA, Velicer C, Saddier P, Seeger JD. Vaccine initiation and 3-dose series completion of 4vhpv vaccine among US insured males 2012-2016. Vaccine. 2022;40(4):682–88. doi:10.1016/j.vaccine.2021.10.070.
- Centers for Disease Control. Deaths_ final data for 2016. National Vital Statistics Reports (Vol 67, No 5). 2018 Jul 26.
- Naleway AL, Crane B, Smith N, Daley MF, Donahue J, Gee J, Greene SK, Harrington T, Jackson LA, Klein NP, et al. Absence of venous thromboembolism risk following quadrivalent human papillomavirus vaccination, vaccine safety datalink, 2008-2011. Vaccine. 2016;34(1):167–71. doi:10.1016/j.vaccine.2015.10.006.
- Frisch M, Besson A, Clemmensen KKB, Valentiner-Branth P, Mølbak K, Hviid A. Quadrivalent human papillomavirus vaccination in boys and risk of autoimmune diseases, neurological diseases and venous thromboembolism. Int J Epidemiol. 2018;47(2):634–41. doi:10.1093/ije/dyx273.
- Institute of Medicine.Adverse effects of vaccines. 2011.http://www.nationalacademies.org/hmd//media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf .
- Brotherton JM, Gold MS, Kemp AS, McIntyre PB, Burgess MA, Campbell-Lloyd S. New South wales health HPV adverse events panel. Anaphylaxis following quadrivalent human papillomavirus vaccination. CMAJ. 2008 Sep 9;179(6):525–33. doi:10.1503/cmaj.080916.
- Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–37. doi:10.1016/j.jclinepi.2004.10.012.
- Seeger JD. Commercial databases. In SB, editor. Pharmacoepidemiology. 5 ed. Chicester, UK: Wiley; 2012. pp. 189–208.
- Ferver K. The use of claims data in healthcare research. 2009 [accessed 2020 Jan 30]. https://openpublichealthjournal.com/contents/volumes/V2/TOPHJ-2-11/TOPHJ-2-11.pdf .
Appendix
Figure A1. 4vhpv doses by month* (2009–2016).
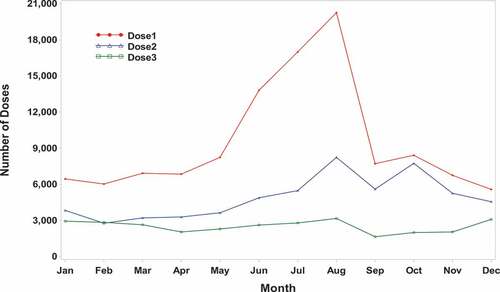
Table A1. Summary of the Incidence Rates (IR) and Relative Rates (RR) that are significantly decreased among regimen initiators (N = 114,035)a,b in a risk period compared to a post-vaccination self-comparison period for potential combined ER/Hospital general safety outcomes by analysis categoryc.
Table A2. Summary of the Incidence Rates (IR) and Relative Rates (RR) that are significantly decreased among regimen initiators (N = 114,035)a,b in a risk period compared to a post-vaccination self-comparison period for combined potential hospital only general safety outcomes by analysis categoryc.