ABSTRACT
Vaccination is a critical tool in protecting against COVID-19. It is essential to know the time for each activity in a COVID-19 vaccination process for better management, especially during a pandemic. Thus, we conducted a time-motion study to identify activities that led to delayed/increased waiting time in an urban primary health center in Bhubaneswar, India. We observed 196 COVID-19 vaccine beneficiaries over one month (June 2021) from when they arrived at the vaccination center until they left the center. A data collection form and a Stopwatch were used to estimate the time taken for various activities involved in COVID-19 vaccine delivery. The time taken was expressed in mean and median. We also compared the time taken during the first and second doses using the Mann-Whitney U test. The total mean time spent at the vaccination center was 40:56 ± 20:52 minutes. The activity that took the longest was ‘waiting time in queue before vaccination’, which was 34:22 ± 20:56 min constituting 82% of the total time. The activity that took longer for the second dose than the first was the beneficiary verification in the Co-WIN portal with a median of 27 seconds and 36 seconds, respectively (p < .001). This study will help program managers formulate better strategies to improve the vaccination process making it more efficient.
Introduction
It has been almost two years since COVID-19 was declared a pandemic.Citation1 It continues, with new variants emerging now and then.Citation2 Vaccination against COVID-19 and other COVID appropriate measures like social distancing, wearing masks, and hand washing are strategies to control the pandemic. The genomic sequence of SARS- CoV-2 was published in January 2020, followed by the development of multiple vaccines against COVID-19.Citation3,Citation4 There have been trials of re-purposeful drugs to treat COVID-19, but more emphasis was on vaccine development.Citation5 In India, two vaccine candidates, namely BBV152(COVAXIN) and AZD1222(COVISHIELD), received restricted emergency approval by the Drug Controller General of India (DCGI).Citation6 COVISHIELD was produced in collaboration with Oxford University, and COVAXIN is India’s indigenous COVID-19 vaccine developed by Bharat Biotech company Pvt Ltd.Citation7
India’s National expert group vaccination committee (NEGVAC) formulated the COVID-19 Vaccine strategy. Ministry of Health and Family Welfare (MOHFW) introduced a digital platform called Co-WIN for monitoring COVID-19 vaccine delivery. The platform uses a mobile application where users will be able to self-register to get vaccinated.Citation8 India vaccinated its population in phases. On 16th January 2021, the first phase of the world’s largest COVID-19 vaccination drive was started, where Healthcare workers (HCWs)were vaccinated.Citation9 In the second phase, the elderly above 60 years and people with comorbidities in the 45–60 years age group received the vaccine.Citation10 From 1st May 2021, COVID-19 vaccination was expanded to all individuals above 18 years.Citation11
India is one of the most populous countries, and vaccinating all its citizens involves vast logistics and human resources. It also led to huge crowds at the vaccination centers, as people flouted the social distancing norms.Citation12 A time-motion study determines the time required for a specific activity. It increases the performance by measuring and minimizing the time taken and movement needed to conduct various activities without compromising the quality of services.Citation13,Citation14
Hence, we conducted a time-motion study to estimate the time taken for various activities in the COVID-19 vaccination process at an urban primary health center (UPHC). The study results will help the policymakers and implementers to improve the vaccination process and make it more efficient.
Methods
We conducted a time-motion direct observational study in an urban primary health center, Odisha, India, for one month (June 2021). The vaccination center is state government-operated, similar to most centers in urban and rural areas. The Department of Community Medicine, All India Institute of Medical Sciences (AIIMS) provides the services at the center in collaboration with the state staff. Verbal permission was taken prior to the study from the medical officer in charge of the center. Assuming around 60% of people will spend about 60 minutes at the vaccination center, power of 80% and alpha error of 5%, the calculated sample size was 196. The Institute Ethics Committee, AIIMS, Bhubaneswar, granted ethical approval (Reference number: T/IM-NF/CM&FM/21/03). The HCWs involved in COVID-19 vaccination at the study center were state officials. We took verbal consent from them, which was approved by the ethics committee, and none of them denied consent.
Data collection
In June 2021, in India, those above 18 years were being vaccinated, and vaccine beneficiaries had to pre-register themselves in the Co-WIN portal and book the slot prior to vaccination. There were two vaccination booths at the study center, and we observed the vaccination process at only one booth. The total number of COVID-19 vaccination beneficiaries per day was around four hundred, including both sessions. We took verbal permission from the medical officer in charge of the center before starting the study.
Two observers conducted the study, one being the principal investigator and the other being an Intern trainee who was explained regarding study procedure and trained under supervision. Every fifth beneficiary attending the COVID-19 vaccination center was selected by systematic random sampling on the study day. The COVID-19 vaccination at the center was conducted in two sessions; a morning session between 9–12 PM and an afternoon session between 3–6 PM. The vaccine given at the center was COVAXIN. The first dose of the vaccine was given in the morning session and the second dose in the afternoon. The time-motion study was conducted during both sessions observing 196 COVID-19 vaccine beneficiaries. The vaccine beneficiaries were observed from when they arrived at the center until they left the center.
COVID-19 vaccination process
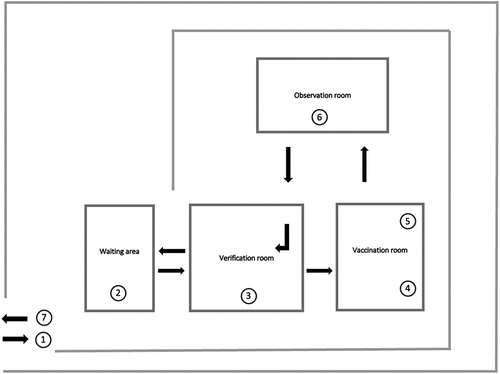
The flow of beneficiary movement starts with their arrival at the center and waiting for the vaccination process to start. Once the vaccination process starts, they move to the verification room, where their details are verified in the Co-WIN portal. After that, they enter another room to receive the vaccine against COVID-19. After vaccination, the beneficiary is sent to the observation room to observe for Adverse Events following Immunization (AEFI). Post observation, the beneficiary exits through the same verification room from where the beneficiary enters ().
Figure 1. The layout of the vaccination center, UPHC, Odisha.
We have assessed the COVID-19 vaccination process for five main activities:
The total waiting time in the queue
Time taken to verify beneficiary details in Co-WIN portal (All beneficiaries came pre-registered)
Time taken to enter the beneficiary details in offline records
Time taken to vaccinate the beneficiary
Total time spent in the observation room
On the days of data collection, we gave a token to every fifth beneficiary when they arrived at the UPHC for vaccination after taking informed consent. We explained the purpose of the study to the selected beneficiaries. The token contained the serial number, date, name, age, the dose of vaccine, entry time, and exit time. We entered the entry time in the token and asked them to return the token while they left the center. Twelve beneficiaries did not return the token. To know the beneficiaries’ experience of the COVID-19 vaccination process, a 5 point Likert scale was used, which included very unsatisfied, unsatisfied, neutral, satisfied, and very satisfied. The token accompanied the rating scale. We collected the tokens at the end and asked them to rate the vaccination process. If they were observed to be leaving earlier than prescribed, we also asked why. The study tools included a Mobile Stopwatch and a data collection sheet to enter the time taken for various activities. The data collected was captured using the Epicollect5 app and was exported to MS Excel.
Statistical analysis
Statistical analysis was done using SPSS Version 25. The time taken for various activities are expressed in mean and median. We have also compared the time taken during the first and second dose using the Mann Whitney U test.
Results
Socio-demographic details
Of 196 participants observed in the study, 111(56.6%) were males, and 85 (43.4%) were females. The mean age of the study participants was 28 ± 6 years. The number of participants who took the first dose was 90 (45.9%), and the second was 106 (54.1%).
The total mean time spent by the beneficiaries at the vaccination center was 40:56 ± 20:52 min, and the median was 38:59 (24:59- 54:59) min. The total mean time spent in the waiting queue was 34:22 ± 20:56 min, and the median was 32:00(17: 15 - 49:00) min. Waiting time constituted 82% of the total time spent at the vaccination center. Verification in the CoWin portal for both the first and second doses took 38.98 ± 37.62 seconds, and the median time was 31.50s. The mean time taken to enter beneficiary details into the register was 20.87 ± 9.17s, and the median time was 19.0 (14–27)s. The mean time taken for vaccination was 17.83 ± 8.18s, the median time was 17.0(12–21)s, and the mean time spent in the observation room post-vaccination was 3:54 ± 2:18 minutes, and the median time was 3:59(1:59-5:44) min ()
Table 1. Service delivery time at different activity points of COVID-19 vaccination.
The time difference in COVID-19 vaccination service delivery points between the first and second doses showed that except for verification in the Co-WIN portal and waiting time in queue, the time taken for all other activities was less for the second dose when compared to the first dose. For verification of beneficiary for the second dose, the time taken was more, which was statistically significant (p-value < .001) ()
Table 2. COVID-19 vaccination service delivery time in relation to the first and second dose.
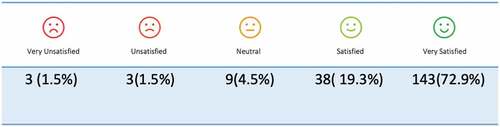
The rating of the process showed that 3 (1.5%) were very unsatisfied, 38(19.3%) were satisfied, and 143(72.9%) were very satisfied ().
Discussion
Vaccination against COVID-19 serves as a critical tool in combatting the pandemic. Time motion studies determine the time required for various activities and identify delays to improve the process efficiency. At the start of the present study, a literature search revealed no time-motion study on COVID-19 vaccination; time-motion studies existed only for routine childhood immunization services. They compared the time required for old and new registries, assessed vaccination costs at different adult provider practices, and also conducted to develop a model for evaluating the pediatric vaccine schedule efficiency.Citation15–19 Therefore, this would be one of the first motion studies on the COVID-19 vaccination process.
During our study period, India witnessed the dreadful second wave of the pandemic with high mortality and morbidity rates all over the country. So, vaccination against COVID-19 was an essential tool for control, and ensuring its efficient functioning and not letting it be the source of infection was crucial. Of the total time spent by the beneficiaries in the vaccination center, 82% constituted only waiting time in the queue, which was causing crowding, which could increase the chance of transmission of the virus. The beneficiaries were given time slots for vaccination when they registered online; each beneficiary was allotted a time slot of one hour. This fixed number of beneficiaries for each hour was an attempt by the government to prevent crowding at the centers. However, most of them did not adhere to the allotted time slots. They arrived at the center about one hour prior to the allotted time to be the first ones to get vaccinated. This early arrival led to the long waiting time and crowding at the vaccination center. Similarly, a time-motion study done at the immunization clinic attached to a rural health center in Delhi found that 64.1% of their study participants spent their time waiting.Citation17 However, they attributed the reason for the long waiting time to the lack of health care workers, whereas in the present study, it was due to the non-adherence of the beneficiaries to the allotted time.
As expected, the second dose administration was more efficient; most activities took a shorter time during the second dose than the first. However, it was not so for the verification process. Beneficiary verification took more time for the second dose. This was because, for the second dose, the beneficiary had to show a partial vaccination certificate (vaccinated with the first dose). Many of the beneficiaries were unaware of this; therefore, they had to download the partial vaccination certificate at the center resulting in more time for verification. During verification before the second dose, they also had to provide their registered mobile number, and many had more than one phone number and did not remember which they had used earlier, which led to further delay. These problems could be avoided by providing prior information to the beneficiaries during slot allotment for the second dose.
About 400 vaccine beneficiaries were being vaccinated per day at the study center. In our study, except for the observation time, the time taken for vaccination took the least time compared to other activities. It could indicate that the healthcare workers were efficient in the vaccinating process. This finding contrasts with a time-motion study in an immunization clinic of a tertiary care hospital in Kolkata, where time spent on vaccination activity was more when compared to other activities, with the median being 300s. However, their finding was probably due to the administration of multiple vaccines as per the routine childhood immunization schedule.Citation15
A critical finding in the present study is the mean time spent in the observation room post-vaccination to watch for Adverse events following immunization (AEFI), which was very short at only 3 minutes 54 seconds. This is far less than the recommended waiting time of 30 minutes. While collecting the tokens, the observer asked why the beneficiaries were leaving early; most of them mentioned that they had to leave early to attend to their duties. Leaving too early could have been risky if they had any AEFI outside the center. However, there was no record of any AEFI in the register maintained by the HCWs. One may assume that there were no serious AEFI after the COVID-19 vaccination at the UPHC as none of the beneficiaries reverted with any complaints but, non-reporting of AEFI cannot be the same as its absence. According to the Government of India’s policy, AEFI surveillance for the COVID-19 vaccine was only passive; therefore, the possibility of missing out on AEFIs remains. Hence, both the public and the health care workers at the centers need education about the importance of the observation time after vaccination, especially after the first dose. The employers could also be instructed to permit half an hour delay for work.
Another important observation was regarding the flow of beneficiaries; entry and exit points of the vaccination room were the same, resulting in the crisscross movement of beneficiaries which could increase the chance of infection. This arrangement was also against the guidelines for the conduct of COVID-19 vaccination process.Citation20 According to the guidelines, there should be a one-way flow of beneficiaries with separate entry and exit points, which would not allow for their intermingling.
Regarding the rating of the vaccination process, more than 90% of the beneficiaries were satisfied or very satisfied, though some of them mentioned the lack of proper signages at the center, which could have made the process easier for them. This high satisfaction rate maybe because people had been waiting for a vaccine against COVID-19 since the emergence of the COVID-19 pandemic, and it was provided at government centers free of cost; also, the vaccination process took a short time.
The present study reveals that most of the lacunae that increased various activity times at the vaccination centers were amenable to simple solutions. Beneficiaries can be informed to adhere to their allotted time using Information, Education and Communication through multiple information routes. Co-WIN portal itself can carry a short message before allotting the vaccination slot. The message could also be broadcasted through various mass media and social platforms such as Facebook, WhatsApp, Twitter, etc., regarding strict adherence to the allotted time slot for vaccination. Similarly, these media and the portal could also be used to provide information regarding pre-requisites of registered phone numbers and partial vaccination certificates before the second dose.
Another intervention could be at the level of slot allotment. Reducing the time slots duration to 15 minutes instead of an hour could result in fewer people turning up for the allotted slot. For the first dose, beneficiaries could comply with the allotted time. For the second dose, HCWs at the centers could also counsel the beneficiaries in the observation room about the second dose requirements and on adhering to the allotted time. The COVID-19 vaccination centers should ensure 30 minutes post-vaccination observation time after the first dose by monitoring and employing supportive supervision. The vaccination surveillance officers must ensure separate entry and exit points at all the COVID-19 vaccination centers.
Our study has a few limitations; we did not capture the proportion of beneficiaries who adhered to the allotted time slots and the factors that influenced the same. This study also represents only the primary level facility vaccination centers. Though fewer in number, secondary and tertiary level centers may deal with bigger crowds. Further research can be done at other centers, namely at secondary and tertiary care levels, for optimal functioning of COVID-19 vaccination.
Even though this study was conducted in one of the urban primary health care centers, the vaccination process and the layout would not be vastly different from other primary level vaccination centers in the urban or rural areas of the government sector. Hence, the recommendations based on the present study would benefit the overall vaccination drive in the state and the country.
Conclusion
Vaccination against COVID-19 is the first-ever mass adult vaccination program. Efficient implementation of COVID-19 vaccination will serve to fight against COVID-19. Our study gives useful insights into the COVID-19 vaccination process at a primary care level which is the first point of contact with the community. The study found that the longest time was spent waiting for vaccination, primarily due to the non-adherence of the beneficiaries to their allotted time. Secondly, a lack of awareness about pre-requisites before the second dose also caused delays. Post-vaccination observation time was grossly insufficient; however, no untoward event was recorded. These findings can provide an impetus for studying the vaccination process at the secondary and tertiary level centers to find ways to make them more effective. The findings of our study can also be useful for policymakers in planning future vaccination strategies.
Author’s contribution
PPG, SHS, and BKB conceptualized the study. AG and AMC have searched the literature. AG and AMC conducted and analyzed the study under the supervision of PPG, SHS, and BKB. AG wrote the manuscript with inputs from SHS, PPG, BKB, and AMC. SHS, PPG, BKB, and AMC reviewed the final manuscript. All authors approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic [Internet]. Acta Biomedica Mattioli. 2020 [accessed 2021 Mar 22]; 91(1885):157–6.
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Peacock SJ, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol [Internet]. 2021 Jun 1[accessed 2021 Dec 5];19(7):409–24. doi:10.1038/s41579-021-00573-0.
- Genomic sequencing of SARS-CoV-2: a guide to implementation for maximum impact on public health [Internet]. [accessed 2021 Dec 5]. https://www.who.int/publications/i/item/9789240018440 .
- Heaton PM. The Covid-19 vaccine-development multiverse. New Engl J Med [Internet]. 2020 [accessed 2021 Dec 5];383(20):1986–88. https://www.nejm.org/doi/full/10.1056/NEJMe2025111 .
- Repurposed antiviral drugs for COVID-19 –interim WHO SOLIDARITY trial results | medRxiv [Internet]. [accessed 2020 Nov 14]. https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1 .
- PRESS INFORMATION BUREAU GOVERNMENT of INDIA press statement by the Drugs Controller General of India (DCGI) on restricted emergency approval of COVID-19 virus vaccine. [accessed 2021 Dec 5]. https://www.pib.gov .
- Vaccine information, ICMR New Delhi - COVID-19 Vaccine [Internet]. [accessed 2021 Mar 28]. https://vaccine.icmr.org.in/covid-19-vaccine .
- COVID-19 Vaccine strategy has been formulated as per India’s National expert group vaccination committee (NEGVAC). Ministry of Health and Family Welfare has introduced a digital platform, namely, CoWIN, for monitoring COVID vaccine delivery - Google Search [Internet]. [accessed 2021 Dec 5]. https://www.mohfw.gov.in/pdf/COVID19VaccineOG111Chapter16.pdf .
- PM Modi to launch Covid vaccination drive with two-way live webcast on January 16 [Internet]. [accessed 2021 Mar 22]. https://theprint.in/health/pm-modi-to-launch-covid-vaccination-drive-with-two-way-live-webcast-on-16-january/584573/ .
- Coronavirus | Phase 2 of vaccination begins on March 1 amid a COVID-19 surge - the Hindu [Internet]. [accessed 2021 Mar 22]. https://www.thehindu.com/news/national/coronavirus-phase-2-of-vaccination-begins-on-march-1-amid-a-covid-19-surge/article33956115.ece .
- Jacob K. Vaccines for all above 18 from May 1; States can buy directly - the Hindu [Internet]. The Hindu. 2021 [accessed 2021 Nov 14]. https://www.thehindu.com/news/national/from-may-1-everyone-over-18-years-eligible-for-covid-19-vaccination-government/article34359940.ece .
- Nishtha P. COVID vaccination Hyderabad: “mismanagement, crowds at My Hyderabad COVID vaccine center, Now Shu” [Internet]. the quint. 2021 [accessed 2021 Nov 14]. https://www.thequint.com/my-report/rangareddy-hyderabad-covid-vaccination-center-mismanagement-no-social-distancing .
- Lopetegui M, Yen PY, Lai A, Jeffries J, Embi P, Payne P. Time motion studies in healthcare: What are we talking about? J Biomed Inform 2014 Jun 1;49:292–99. doi:10.1016/j.jbi.2014.02.017 .
- Finkler SA, Knickman JR, Hendrickson G, Lipkin M, Thompson WG A comparison of work-sampling and time-and-motion techniques for studies in health services research. Health Services Research [Internet]. 1993 Dec [accessed 2021 Dec 5];28(5):577. https://pmc/articles/PMC1069965/?report=abstract .
- Chattopadhyay A, Ghosh R, Maji S, Ray TG, Lahiri SK A time motion study in the immunization clinic of a tertiary care hospital of Kolkata, West Bengal. Indian J Community Med [Internet]. 2012 Jan [accessed 2021 Mar 28];37(1):30–33. https://pmc/articles/PMC3326804/ .
- Deepika S, Parande MA, Surwade J, Tapare V, Tambe M, Bhattacharya S. A time motion study in the immunization clinic of a tertiary care hospital, BJGMC Pune. Indian J Public Health Res Dev. 2017 Oct 1;8(4):892–96. doi:10.5958/0976-5506.2017.00447.8.
- Kumar V, Mangal A, Panesar S, Yadav G, Talwar R, Raut D, et al. Operational efficiency of an immunization clinic attached to rural health training center in Delhi, India: a time and motion study. Adv Prevent Med [Internet]. 2014 [accessed 2021 Mar 29];2014:1–5. https://pmc/articles/PMC4241324/ .
- Shen A, Khavjou O, King G, Bates L, Zhou F, Leidner AJ, et al. Provider time and costs to vaccinate adult patients: impact of time counseling without vaccination. Vaccine [Internet]. 2019 Feb 4[accessed 2021 Mar 28];37(6):792–97. https://pmc/articles/PMC6848970/ .
- Ciarametaro M, Bradshaw SE, Guiglotto J, Hahn B, Meier G. Hidden efficiencies: making completion of the pediatric vaccine schedule more efficient for physicians. Medicine (United States) [Internet]. 2015 Jan 1 [accessed 2021 Mar 29];94(4). https://pmc/articles/PMC4602983/ .
- Guidance on COVID-19 vaccination at work places (government & private). [accessed 2021 Dec 5]. https://www.mohfw.gov.in/pdf/GuidancedocCOWIN2.pdf .