ABSTRACT
Supply of autodisable (AD) syringes has been a key component of global COVID-19 vaccination campaigns, and it is critical to maintaining safe injection practices for routine immunization as well as pandemic response. AD syringe production increased significantly in response to demand, but distribution challenges have included the need to coordinate syringes to meet the specific delivery requirements of various COVID-19 vaccines, shipping bottlenecks, and syringe export restrictions. Stockpiling syringes, ensuring standardization of future vaccine dose volumes, and geographical diversification of syringe production would improve syringe logistics in the future. Balancing syringe supply and demand and stabilizing the market over the long term is essential to ensure that the world is prepared for possible new variants of COVID-19 or a new global outbreak. This will require concerted action on the part of public, nonprofit, and private partners.
COVID-19 pandemic response increased syringe needs
Within two years of the World Health Organization (WHO) declaring COVID-19 a global pandemic on 11 March 2020, more than 20 novel and effective vaccines have been developed and cleared for use by countries, and more than 4.8 billion people have received at least one vaccine dose.Citation1–4 Both are heroic achievements. And yet, there are still large inequities in access to COVID-19 vaccines globally. For instance, just 15% of the 1.2 billion people living in Africa have received a COVID-19 vaccine.Citation3
If COVID-19 vaccination campaigns accelerated to the pace needed to hit the WHO 70% global immunization coverage target by mid-2022, access to syringes for vaccine delivery could easily become a bottleneck.Citation5 PATH’s syringe gap models from November 2021 estimated that a shortage of more than a billion syringe units could occur if the WHO target is reached on time.Citation6 However, recently the Africa Centres for Disease Control and Prevention (Africa CDC) has highlighted challenges and requested that shipments of donated COVID-19 vaccines be paused to the second half of the year, due to multiple factors, including logistical challenges and vaccine hesitancy.Citation7 This pause will potentially enable syringe supply to better match demand but delay immunization coverage.
One logistical complication facing countries is pairing the different available COVID-19 vaccines with their specific syringe types/sizes at the point of use. For instance, Nepal, the Philippines, Rwanda, South Africa, Kenya, and Japan have reported stockouts of specific types of syringes causing short-term delays to COVID-19 immunization campaigns.Citation8–11 Lack of reporting may make the real number of countries experiencing shortages much higher.
Autodisable syringes are a key tool for safe injection
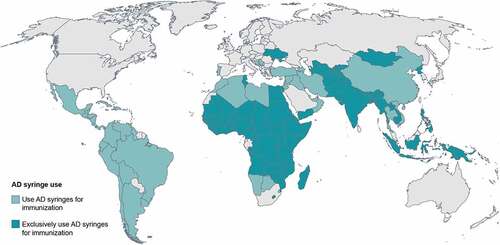
Due to its unique safety features, the most common safe injection device used for vaccination in low- and middle-income countries over the past two decades is the autodisable (AD) syringe.Citation12 A low-cost (typically 0.03–0.04 USD each) AD syringe has a fixed needle and locking mechanism to prevent reuse and is fixed-dose, meaning it holds the exact amount for one vaccine dose and does not require measurement of the dose, reducing the risk of error. In 1999, and again in 2019, WHO, the United Nations Children’s Fund (UNICEF), and the United Nations Population Fund issued a joint statement on injection safety strongly recommending exclusive use of AD syringes for immunization globally because they “present the lowest risk of person-to-person transmission of blood-borne pathogens such as hepatitis B or HIV.”Citation13 The introduction of these safety syringes, paired with sharps disposal technologies and initiatives to promote safe injection practices, resulted in an 86% reduction in the rate of unsafe injections between 2000 and 2010.Citation14 Nearly 100 different countries have used AD syringes for immunization since the late 1990s, supplied mainly by UNICEF and Pan American Health Organization (PAHO) (). If syringe scarcity leads to vaccines being delivered without safe injection devices and practices, a resurgence of blood-borne infections such as HIV and hepatitis could be an unintended consequence of this step backward, eroding decades of progress by countries’ health systems and health organizations.
To procure and improve access to appropriate devices for the lower-income countries receiving vaccines, millions of dollars have been mobilized through COVID-19 Vaccines Global Access (COVAX). At the same time, organizations such as UNICEF, PAHO, PATH, and the Bill & Melinda Gates Foundation have worked with injection device manufacturers to bolster the supply to meet increasing demand. And to address the increased need for AD syringes, in the short term, UNICEF has filled gaps by drawing down on its stockpile of 0.5-mL AD syringes, which are the most common dose size used for COVID-19 and routine immunizations. Unfortunately, not all COVID-19 vaccines use the standard 0.5-mL dose volume, limiting their current utility and complicating future stockpile strategies.
Autodisable syringe manufacturing and distribution
In preparation for an unprecedented surge in syringe demand, device manufacturers have made concerted efforts and investments to increase AD syringe production more than threefold pre-pandemic production levels. However, expanding capacity further would take time; building a new production line can take at minimum 8 months. Additionally, manufacturers have had to respond to the specialty device demands of some COVID-19 vaccines. For example, the Pfizer vaccine required the development of new low-dead-space 0.3-mL AD syringes to accommodate its atypical dose volume and reduced wastage requirements.Citation15 Custom syringes are made on the same manufacturing lines as the 0.5-mL AD syringes needed for most childhood immunizations, which can delay or impact production of syringes for these essential vaccines. Additionally, having multiple COVID-19 vaccine dose volumes has created market coordination challenges, requiring the matching of vaccine and syringe shipments. Recently, pediatric and booster vaccines have been developed with different dose volumes (0.2 mL and 0.25 mL), further complicating sourcing and distribution of appropriate syringes for countries that use fixed-dose AD syringes for immunization. Health care workers, medical trainers, and country procurement staff will have to carefully coordinate distribution and use of different syringes for each vaccine with an alternative dose volume. Ensuring standardization of future vaccine dose volumes would streamline syringe supply chains and production, help to ease the burden on health workers and systems, and maintain achievements in injection safety through the continued use of AD syringes.
Donated vaccine doses have been essential in moving toward equitable vaccination coverage but can exacerbate shortages when not provided with syringes. A joint statement released by the African Vaccine Acquisition Trust, Africa CDC, and COVAX urged organizations and countries that are generously donating vaccines through agencies such as COVAX to include all essential ancillaries (e.g., syringes and diluent) bundled along with the doses.Citation16 However, in the event the donating countries only have supply of non-AD syringes, careful attention must be given to ensure devices are appropriate for the recipient context and policies, and that supplemental training can be provided to health workers. If AD syringes are unavailable, the most appropriate substitute safety syringe options are reuse prevention (RUP) syringes, which have a feature to prevent reuse but may not have fixed-dose settings or fixed needles and would require additional training in some health systems.Citation17
Supply chain complications
Syringe supply chain challenges have also created logistical problems for the uptake of COVID-19 vaccines. Unlike most vaccines, which are frequently transported via airplanes, syringes are typically transported via container ship, and shipments can be disrupted by port and logistical delays, as reported in Asia where AD syringe manufacturing is concentrated.Citation18 To expedite delivery times, syringes have needed to be shipped by air during the pandemic, significantly increasing transport costs. A recent investment from the Bill & Melinda Gates Foundation announced in November 2021 seeks to increase AD syringe production on the African continent, with the stated aim of creating more equitable access and reducing cost and timelines for syringe shipments to a region that predominantly uses these devices for immunization.Citation19
Recommendations to ensure future autodisable syringe supply availability
Balancing syringe supply and demand and stabilizing the market over the longer term is essential to ensure that the world is well prepared for possible new variants of COVID-19 or a new global outbreak. This will require additional tactics and concerted action on the part of public, nonprofit, and private partners. Manufacturers are not likely to invest in multiyear expansions or multistep regulatory processes without incentives given the uncertainty of long-term demand in the market. Donors, investors, and governments can draw on the tool kit used to incentivize vaccine suppliers, including grants, no or low-interest loans, and volume guarantees to offset some of the suppliers’ risk and expand manufacturing capacity. Building sustainable supply may also require supporting a wider geographical diversity of suppliers, especially in regions like Africa and Latin America with a limited supply base, which could decrease shipping delays in these regions from months to weeks, or even days. Additionally, syringes have the advantage over vaccines of being shelf-stable, and therefore they can be stockpiled or strategically pre-positioned in advance of potential demand surges. Since these syringes are also needed for routine immunizations, stockpile wastage risks are mitigated.
Of course, for these mechanisms to be effective, national syringe export restrictions must be avoided so suppliers can meet demand in all countries, and future vaccines must be formulated for delivery with existing AD syringe sizes, or else stockpiles will be rendered useless. Currently, the supply of AD syringes, like that of many essential goods, has been further limited by syringe nationalism and hoarding of the needed commodities since the start of the pandemic. Mitigating the risk of export restrictions and ensuring the right syringes are in place when vaccine shipments arrive will continue to be critical for achieving COVID-19 and routine immunization targets.
As COVID-19 continues to spread, the importance of timely, effective vaccinations is clearer than ever, and safe injection practices must not be overlooked in this pursuit. But AD syringe shortages pose a risk beyond the current COVID-19 vaccination campaign: Families around the world rely upon essential vaccines to protect their children from life-threatening diseases like measles and polio. Key childhood vaccinations are estimated to prevent over 2 million deaths per year, saving approximately four lives per minute.Citation20 These routine—but essential—immunizations, along with protection against emerging COVID-19 variants and future pandemics, will all be endangered without adequate supply of AD syringes. However, these grim scenarios can be avoided if global partners take coordinated and sustainable actions to prepare the AD syringe market to meet fluctuating long-term demand.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Randall T, Sam C, Tartar A, Murray P, Cannon C More than 11.1 billion shots given: Covid-19 tracker. Bloomberg. Updated 2022 March 11 [accessed 2022 Mar 11]. https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/ .
- Kreier F. Ten Billion COVID vaccinations: world hits new milestone. Nature. 2022 Jan 31 [accessed 2022 Mar 11]. doi:10.1038/d41586-022-00285-2 .
- Holder J Tracking coronavirus vaccinations around the world. New York Times; 2022 March 11 [accessed 2022 Mar 11]. https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html .
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020 Mar 19;91(1):1–4. doi:10.23750/abm.v91i1.9397. PMID: 32191675; PMCID: PMC7569573.
- Independent Allocation of Vaccines Group (IAVG) of COVAX. Achieving 70% COVID-19 immunization coverage by mid-2022. World Health Organization [accessed 2022 Mar 11]. https://www.who.int/news/item/23-12-2021-achieving-70-covid-19-immunization-coverage-by-mid-2022 .
- PATH. Autodisable syringe gap analysis. Seattle: PATH; 2021 [accessed 2022 Mar 11] .
- Payne D. Africa CDC to ask world to pause Covid-19 vaccine donations. Politico; 2022 February 22 [accessed 2022 Mar 11]. https://www.politico.com/news/2022/02/22/africa-asks-covid-vaccine-donation-pause-00010667 .
- Sharma B. Nepal halts Covid vaccinations in children because of a lack of syringes. New York Times; 2021 Dec 27 [accessed 2022 Mar 11]. https://www.nytimes.com/2021/12/27/world/nepal-halts-covid-vaccinations-in-children-because-of-a-lack-of-syringes.html .
- Javier P. DOH, NTF assure enough supply of syringes for COVID-19 vaccination. CNN Philippines; 2021 Dec 12 [accessed 2022 Mar 11]. https://www.cnnphilippines.com/news/2021/12/12/syringe-supply-covid19-vaccine.html .
- Takenaka K. Syringe shortage hampers Japan’s COVID-19 vaccination roll out. Reuters; 2021 February 15 [accessed 2022 Mar 11]. https://www.reuters.com/business/healthcare-pharmaceuticals/syringe-shortage-hampers-japans-covid-19-vaccination-roll-out-2021-02-16/ .
- Khan A. Syringe shortages could be yet another obstacle in Africa’s Covid vaccination efforts, the W.H.O. warns. New York Times; 2021 October 28 [accessed 2022 Mar 11]. https://www.nytimes.com/2021/10/28/world/africa/africa-syringe-shortages-who.html .
- UNICEF Supply. UNICEF price data overview. Tableau Public [accessed 2022 Mar 11]. https://public.tableau.com/app/profile/supply.division/viz/UNICEFPricedataoverviewforvaccines/Fulldashboard .
- World Health Organization, UNICEF. Joint policy statement: promoting the exclusive use of injection safety devices for all immunization activities; 2016 February [accessed 2022 Mar 11]. https://www.technet-21.org/media/com_resources/trl/5051/multi_upload/RUP_WU_JointStatement_FINAL_13Feb2019.pdf .
- Pépin J, Abou Chakra CN, Pépin E, Nault V. Evolution of the global use of unsafe medical injections, 2000–2010. PLoS One. 2013;8(12):e80948 .
- Pfizer. Emergency use authorization (EUA) fact sheets for vaccination providers. [accessed 2022 Mar 11]. https://www.cvdvaccine-us.com/ .
- Gavi. The vaccine alliance. Joint statement on dose donations of COVID-19 vaccines to African countries; 2021 November 29 [accessed 2022 Mar 11]. https://www.gavi.org/news/media-room/joint-statement-dose-donations-covid-19-vaccines-african-countries .
- World Health Organization. Injection safety in the context of coronavirus disease (COVID-19) vaccination: policy brief; 2021 November 5 [accessed 2022 Mar 11]. https://www.who.int/publications/i/item/WHO-2019-nCoV-Policy-brief-Vaccination-Injection-safety .
- World Health Organization. Injection devices for immunization [accessed 2022 Mar 11]. https://extranet.who.int/pqweb/immunization-devices/category-information/injection-devices-immunization.
- Bill & Melinda Gates Foundation. Amid surging demand for syringes, a new investment supports long-term supply on the African continent [accessed 2022 Mar 11]. https://www.gatesfoundation.org/ideas/articles/syringe-vaccine-distribution-in-africa .
- Li X, Mukandavire C, Cucunubá ZM, Echeverria Londono S, Abbas K, Clapham HE, Jit M, Johnson HL, Papadopoulos T, Vynnycky E, et al. For the vaccine impact modelling consortium. Estimating the health impact of vaccination against ten pathogens in 98 low-income and middle-income countries from 2000 to 2030: a modelling study. Lancet. 2021 ;397(10272):398–408. Erratum in: Lancet. 2021 Feb 20;397(10275):670. PMID: 33516338; PMCID: PMC7846814. [accessed 2022 Mar 11]. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32657-X/fulltext .