ABSTRACT
Among women aged 27–45 years, the quadrivalent human papillomavirus (qHPV; HPV6/11/16/18) vaccine was generally well tolerated, efficacious, and immunogenic in the placebo-controlled FUTURE III study (NCT00090220; n = 3253). The qHPV vaccine was also generally well tolerated and highly immunogenic in men aged 27–45 years who participated in the single-cohort mid-adult male (MAM) study (NCT01432574; n = 150). Here, we report results of a long-term follow up (LTFU) extension of FUTURE III with up to 10 years follow-up. To understand the relevance of the mid-adult women LTFU study in the context of mid-adult men vaccination, we report results from post-hoc, cross-study immunogenicity analyses conducted to compare immunogenicity (geometric mean titers; GMTs) at 1-month post-qHPV vaccine dose 3 in women and men aged 27–45 years versus women and men aged 16–26 years from prior efficacy studies. The qHPV vaccine demonstrated durable protection against the combined endpoint of HPV6/11/16/18-related high-grade cervical dysplasia and genital warts up to 10 years (median 8.9) post-dose 3 and sustained HPV6/11/16/18 antibody responses through approximately 10 years in women aged 27–45 years. Efficacy of qHPV vaccine in men aged 27–45 years was inferred based on the cross-study analysis of qHPV vaccine immunogenicity demonstrating non-inferior HPV6/11/16/18 antibody responses in men aged 27–45 years versus 16–26 years. In conclusion, durable effectiveness of the qHPV vaccine was demonstrated in women 27–45 years of age, and vaccine efficacy was inferred in men 27–45 years of age based on the serological results.
Introduction
The burden of human papillomavirus (HPV)-related disease remains substantial in adult women, with approximately 125,000 cases of high-grade cervical intraepithelial neoplasia (CIN) or adenocarcinoma in situ (AIS) diagnosed in US women aged 30 years or older in 2016 (of 196,000 US cases diagnosed overall).Citation1 Also, men do not appear to develop effective immunity following natural HPV infection and experience recurrent HPV infection and related disease throughout their lifetime as shown in several analyses from the HPV Infection in Men (HIM) study, a prospective natural history study of HPV infection in men.Citation2–5 Overall, adults remain at risk of acquisition of HPV infection and vaccination of this population remains a significant unmet medical need.
The quadrivalent (qHPV) vaccine demonstrated efficacy and immunogenicity in women aged 24–45 years in the Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) III study.Citation6,Citation7 To further the understanding of the applicability of these findings, we report long-term effectiveness and immunogenicity of the qHPV vaccine in adult women from FUTURE III after 10 years of follow-up. To understand the relevance of the mid-adult women LTFU study in the context of mid-adult men vaccination, we also report evidence of effectiveness against HPV-related disease in mid-adult men based on immunogenicity bridging analyses of historic data in younger (aged 16–26 years) clinical efficacy trial participants to mid-adult clinical trial participants (aged 27–45 years).
Methods
Study design and population
Two clinical studies were conducted in adults aged 27–45 years: one in adult women, the (FUTURE III study (Protocol V501–019; NCT00090220)Citation6,Citation7 and one among adult men, the Mid-Adult Males (MAM) study (Protocol V501–108; NCT01432574).Citation8
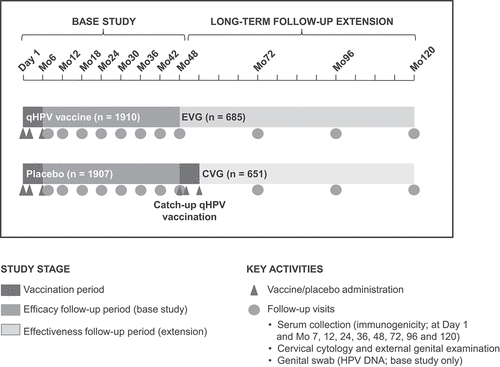
The FUTURE III study, a phase III, randomized, placebo-controlled, multinational, multicenter, double-blind, safety, immunogenicity, and efficacy study, enrolled 3819 women aged 24–45 years in seven countries (Colombia, France, Germany, Spain, the Philippines, Thailand, and the United States). Participants were equally randomized to receive three doses of qHPV vaccine or placebo (at Day 1, Month 2, and Month 6) and followed over approximately 4 years. The design and the results of the base study have been reported.Citation6,Citation7 Participants who had received placebo or an incomplete regimen of the qHPV vaccine were then offered vaccination with the qHPV vaccine. Participants from Colombia who received ≥1 dose of qHPV vaccine or placebo were eligible for enrollment in a 6-year, open-label LTFU extension. The study sites from Colombia were selected for LTFU because of the relatively large proportion of participants enrolled in this country in the base study (N = 1610; 42%) and willingness of the five investigators from Colombia to continue to follow the participants. Two cohorts were assessed: the early vaccination group (EVG), whose participants received qHPV vaccine during the base study, and the catch-up vaccination group (CVG), whose participants received placebo during the base study and qHPV vaccine once efficacy was demonstrated. EVG participants were followed for approximately 10 years after their first qHPV vaccine dose (i.e., approximately 4 years in the base study plus 6 years in LTFU). CVG participants received their first dose of qHPV vaccine more than 4 years after their base study enrollment. They were therefore older at vaccination (i.e., those aged 27–45 years at enrollment were vaccinated at age 32–50 years) and followed for approximately 4.5 years post-qHPV vaccine dose 1. No qHPV vaccination occurred during LTFU ( and S1).
Figure 1. Study design of FUTURE III LTFU. Catch-up vaccination was approximately 5 years after base study Day 1. Participant numbers (n) for the base study and LTFU period refer to participants vaccinated and entering LTFU, respectively (women aged 24–45 years).
In many countries, HPV vaccines are widely used in individuals aged 9–26 years.Citation9,Citation10 Therefore, in this analysis, we focus on the subset of participants aged 27–45 years at enrollment in FUTURE III to help inform public health decisions and vaccination recommendations.
The MAM study was a phase II, open-label, single-arm, immunogenicity, and safety study in men aged 27–45 years who received three doses of the qHPV vaccine. The study enrolled 150 men from two countries (Mexico and the United States) between February and October 2013 from a cohort of men who had completed a 4-year follow-up in the HIM study. The study design and the results have been reported.Citation8
The study protocols were approved by institutional review boards at each participating center. All participants provided informed consent prior to the FUTURE III base study and again before the LTFU as well as prior to the MAM study. Each study was conducted in conformance with applicable country or local requirements regarding ethical committee review, informed consent, and other statutes or regulations regarding the protection of the rights and welfare of human subjects participating in biomedical research.
Follow-up
During the FUTURE III LTFU, gynecological examinations were conducted to detect external genital lesions (EGLs) and cervical cytology samples were collected at Months 72, 96, and 120 using the same methodology as in the base study.Citation6,Citation7 New EGLs assessed to be possibly, probably, or definitely HPV-related, or of unknown etiology, were biopsied. Participants with cytological abnormalities were referred for colposcopy based on a protocol-specified triage algorithm. Tissue samples were adjudicated by a pathology panel and tested for HPV DNA by PCR as previously described.Citation11 Serum HPV antibodies were assessed at Months 72, 96, and 120 using competitive Luminex immunoassay (cLIA),Citation12–14 and at Month 120 using immunoglobulin G Luminex immunoassay (IgG LIA).Citation15 Deaths, serious AEs (SAEs) considered vaccine- or procedure-related, and pregnancy outcomes were collected during LTFU. A diagram of the entire study is shown in .
In the MAM study, all participants received three doses of the qHPV vaccine (at Day 1 and Months 2 and 6). Serum HPV antibodies were assessed at Day 1 and Month 7 using cLIA.Citation8
Statistical analysis
Efficacy/effectiveness
Vaccine efficacy was not assessed in the LTFU study because no placebo comparison group was available. Instead, vaccine effectiveness was assessed based on the incidence rate of the endpoint HPV6/11/16/18-related CIN and condyloma and interpreted in the context of the incidence rate of the same endpoint in the vaccine and placebo groups of the base study.
The per-protocol efficacy (PPE) population consisted of EVG participants who were seronegative at Day 1 and PCR-negative from Day 1 to Month 7 for the HPV type being analyzed, received all three doses of the correct clinical material within one year, had no protocol violations that could affect the evaluation of vaccine efficacy, and attended at least one LTFU visit. Cases were counted starting at Month 7. PPE analyses were not conducted for CVG participants because their last PCR and serology testing was at their last base study visit and the qHPV vaccine was given only after a lag (between the end of the base study and the start of LTFU) when they might have become PCR- and/or serology-positive to a new HPV type. Undetected HPV infection occurring between the last base study visit and the first dose of qHPV vaccine could potentially reduce the measured vaccine effectiveness in this population. An intention-to-prevent (ITP) population included participants who were seronegative and PCR-negative to the relevant HPV type prior to qHPV vaccination (at Day 1 for EVG participants; from Day 1 of the base study to the last follow-up visit prior to vaccination with qHPV vaccine for CVG participants), received ≥1 dose of qHPV vaccine or placebo, and had at least one LTFU visit.Citation16 Cases for the ITP analyses were counted starting at Day 1. A modified intention-to-prevent (mITP) population for analysis of incidence of cervical disease and genital warts caused by non-vaccine HPV types was defined similarly, except that prior to qHPV vaccination participants were seronegative to four HPV types (HPV6/11/16/18), PCR-negative to fourteen HPV types (HPV6/11/16/18/31/33/35/39/45/51/52/56/58/59), and had normal cervical cytology (defined as negative finding for squamous intraepithelial lesions). Counting of follow-up time for effectiveness of qHPV vaccination was done starting at post-dose 1 (relevant to the ITP analyses) and starting at post-dose 3 (relevant to the PPE analyses) through the last LTFU visit with assessment for cervical or external genital disease or cytological abnormalities.
Incidence of effectiveness endpoints per 10,000 person-years of follow-up and 95% exact Poisson confidence intervals (CIs) were reported based on: (1) total follow-up time within the LTFU period, and (2) total follow-up time during the base study period. When feasible, the percent reduction in the incidence rates in the EVG relative to those in the CVG were assessed and calculated as 100 × (1 – relative risk), where relative risk is the ratio of the incidence in the EVG relative to the CVG.
Immunogenicity
Immunogenicity was analyzed in the per-protocol immunogenicity (PPI) population. In FUTURE III, the PPI population included participants in the PPE population who received doses 2 and 3 within pre-specified day ranges and had an evaluable serology result within 14–49 days after dose 3. In the MAM study, PPI participants received all three qHPV vaccine doses and were seronegative and genital HPV-negative to the relevant HPV type prior to vaccination.Citation8 Geometric mean titers (GMTs) at 1 month post-dose 3 of qHPV vaccine were compared in participants aged 27–45 years versus participants aged 16–26 years. Women aged 27–45 years (Group A) were from FUTURE III; women aged 16–26 years (Group B) were from FUTURE I,Citation11 FUTURE II,Citation17 and FUTURE III (i.e., women aged 24–26 years at enrollment); men aged 27–45 years (Group C) and 16–26 years (Group D) were from the MAM study and Protocol V501–020,Citation18 respectively. The ratios of GMTs (women aged 27–45 years/women aged 16–26 years [Group A/Group B], and men aged 27–45 years/men aged 16–26 years [Group C/Group D]) and corresponding two-sided 95% CI were calculated. All comparisons were post-hoc. There was no pre-specified non-inferiority hypothesis testing.
Safety
Deaths and vaccine- and procedure-related SAEs were collected for the entire duration of the LTFU study. Safety results in the MAM study have been reported.Citation8
Results
Long term follow-up of adult women for effectiveness, immunogenicity, and safety in the FUTURE III study
The base study was conducted from 18 June 2004, to 30 April 2009, with LTFU conducted from 14 January 2011, to 12 November 2015. A total of 3253 base-study participants were aged 27–45 years (Figure S1). These included 1408 participants from Colombia, of whom 1189 (EVG = 600; CVG = 589) participated in LTFU. CVG participants received their qHPV vaccination dose 1 approximately 5 years (60 months) after the start of the base study (reflected in a difference of 5 years in median age at Day 1 in base study and at timepoint prior to catch-up vaccination, ). shows the baseline characteristics of EVG and CVG participants who continued into LTFU; Table S1 shows baseline characteristics of base study participants who did not continue into the LTFU extension. Baseline characteristics were generally similar among EVG and CVG participants.
Table 1. Baseline characteristics of LTFU participants aged 27–45 years in the FUTURE III studya.
The median follow-up time for effectiveness in the LTFU study in EVG participants aged 27–45 years was 9.6 years (range: 5.9–10.6 years) post-dose 1 or 9.1 years (range: 5.5–10.1 years) post-dose 3. The median follow-up time for effectiveness in the LTFU study in CVG participants was 4.5 years (range: 0.7–5.3 years) post-dose 1 or 4.0 years (range: 0.3–4.9 years) post-dose 3.
Among EVG participants in the PPE population, the incidence rates of HPV6/11/16/18-related CIN and condyloma, HPV16/18-related CIN2 or worse, and HPV6/11-related condyloma were similar during the LTFU period compared with the base study period (). Overall, there were no cases of HPV6/11/16/18-related CIN and condyloma during both the base study and LTFU period. Among EVG participants in the ITP population, the incidence rates of HPV6/11/16/18-related CIN and condyloma, HPV16/18-related CIN2 or worse, and HPV6/11-related condyloma were similar or lower during the LTFU period compared with the base study period ().
Table 2. Reduction in incidence of HPV-related cervical disease and genital warts in women 27–45 years of age who participated in the FUTURE III LTFU study.
Among CVG participants in the ITP population, the incidence rates of HPV6/11/16/18-related CIN and condyloma, HPV16/18-related CIN2 or worse, and HPV6/11-related condyloma were lower during the LTFU period (i.e., after qHPV vaccination) compared with rates during the base study period (i.e., placebo arm of the base study) ().
During the base study, the incidence rates of HPV6/11/16/18-related CIN and condyloma in the PPE and ITP populations were lower in the EVG compared with respective rates in the CVG (). The percent risk reduction (100% and 94.3%, respectively, in PPE and ITP analyses) was equivalent to and consistent with the statistically significant vaccine efficacy observed with qHPV vaccine in the full base study population.Citation6,Citation7 After CVG participants were administered the qHPV vaccine regimen, the incidence rate of HPV6/11/16/18-related CIN and condyloma in the ITP population of the CVG during LTFU was similar to the rate in the EVG during the base study and LTFU period (). This result indicates that the qHPV vaccine provided protection against HPV-related disease after qHPV vaccination in the CVG.
Among EVG participants, the incidence of HPV6/11/16/18-related disease endpoints during the base study was higher among those who did not continue in the LTFU study extension versus those who participated (Table S2). Vaccine efficacy against HPV6/11/16/18-related disease endpoints was similar or comparable in LTFU participants and non-participants (Table S2).
Incident cases of CIN and condyloma related to non-vaccine types (including HPV types 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) were observed during the base and LTFU study among EVG and CVG participants in the mITP population, indicating ongoing exposure to HPV and risk of acquiring new infections during both the base and LTFU study ().
Table 3. Incidence of nonvaccine HPV type-related cervical disease and genital warts in women 27–45 years of age who participated in the LTFU study (mITP populationa).
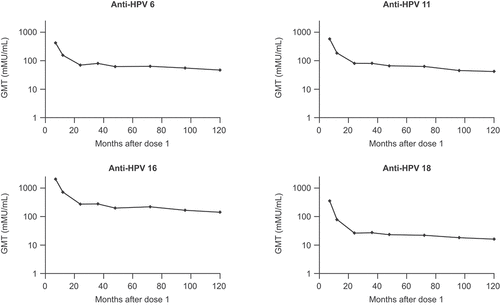
Regarding immunogenicity in EVG participants, GMTs for all vaccine HPV types peaked at Month 7, declined sharply up to Month 24 and more gradually thereafter, and persisted throughout the study (; Table S3). At Month 120, seropositivity rates for HPV6/11/16/18, respectively, were 78%, 85%, 94%, and 35% when assessed by cLIA, and 87%, 79%, 100%, and 84% when assessed by IgG LIA (Table S4). In the CVG participants, serum collections following catch-up vaccination at study Months 72, 96, and 120 () correspond to approximately 12, 36, and 60 months post-qHPV vaccination dose 1 in this group. GMTs and seropositivity rates at Months 72 and 96 in the CVG were comparable to those at Months 12 and 36 post-dose 1 in the EVG participants (Tables S3 and S4). Analyses of immunogenicity in the EVG by age strata (27–34 years and 35–45 years) and CVG participants (who received qHPV vaccine at age 32–50 years) showed that the vaccine was immunogenic across all ages studied (Tables S3 and S4).
Figure 2. Longitudinal anti-HPV6/11/16,/18 cLIA GMTs among LTFU-study participants who received qHPV vaccine at age 27–45 years in the FUTURE III Base study (PPI population).
No vaccine- or procedure-related SAEs were reported during LTFU. Two deaths (not vaccine-related) occurred during LTFU. Additional information about SAEs, deaths, and pregnancy outcomes during LTFU is presented in the online supplemental material.
Comparison of qHPV vaccine immunogenicity in women and men aged 27–45 years versus women and men aged 16–26 years
In the post-hoc, cross-study immunogenicity analyses in the PPI population, the point estimates of the GMT ratios for adult women/young women (Group A/Group B) ranged from 0.71 to 0.96, and for adult men/young men (Group C/Group D) from 0.74 to 0.91 (). The lower bound of the 95% CI of the GMT ratios was >0.5 for all comparisons.
Table 4. Comparison of anti-HPV GMTs at Month 7 (1 month post-dose 3) in women aged 27–45 years (FUTURE III study) versus women aged 16–26 years (FUTURE I, FUTURE II, and FUTURE III studies) and men aged 27–45 years (MAM study) versus men aged 16–26 years (V501–020 study) who received three doses of the qHPV vaccine (PPI population).
Discussion
Effectiveness analyses in the EVG showed no breakthrough cases of CIN or genital warts related to HPV6/11/16/18 for up to 10 years following qHPV vaccination of women aged 27–45 years. Among CVG participants, the incidence of HPV6/11/16/18-related CIN and EGL was lower post-vaccination (LTFU period) compared with the pre-vaccination incidence during the base study period, with no cases observed during LTFU. Although vaccination in the CVG was delayed 4 years such that participants were 32–50 years of age at the time of qHPV vaccination, it still provided effective protection against additional vaccine HPV type-related disease to which participants remained susceptible. Persistence of HPV6/11/16/18 antibody responses was observed through 10 years following vaccination. There were no vaccine-related SAEs during the LTFU study period.
In post-hoc, cross-study analyses, the point estimates of anti-HPV6/11/16/18 GMTs at Month 7 were lower in individuals aged 27–45 years compared with individuals aged 16–26 years; however, a greater than two-fold decrease in GMTs was ruled out. This criterion was used in previous studies of the qHPV vaccine program to demonstrate non-inferiority of HPV antibody responses and infer vaccine efficacy based on immunogenicity assessment and is an accepted regulatory pathway.Citation19,Citation20
Prophylactic HPV vaccination is ideally administered to young adolescents prior to exposure to HPV. However, optimal vaccine coverage has not yet been reached in adolescents in many countries.Citation21 Moreover, catch-up vaccination of unvaccinated adults has not been consistently implemented. As a result, most adult men and women are not protected against the HPV types targeted by HPV vaccines.
Adults remain susceptible to acquiring new HPV-vaccine-preventable infections. As previously reported,Citation22 at the baseline FUTURE III trial visit, most prevalent infections contained only one HPV type, and approximately 84% of participants were HPV DNA-negative to all nine HPV types covered by the 9vHPV vaccine, and thus would gain protection from vaccination. Additionally, qHPV vaccine prevents re-infection in women with serological evidence of previous HPV infection and who are PCR-negativeCitation7,Citation23 and provides protection from subsequent disease after surgical treatment for HPV-related disease.Citation24–26
Adult women in the control arms of the HPV vaccine trials continued to acquire new HPV infections over time.Citation7,Citation22,Citation27 In the placebo arm of FUTURE III, incident 6-month HPV6/11/16/18-related persistent infection was observed in 5% of the PPE population over a 4-year follow-up. In the HIM study, adult men were observed to remain at risk for acquiring new HPV infection throughout their lifespans.Citation28 HPV infections in adult women progress to high-grade CIN at rates similar to young women. In the placebo groups of qHPV vaccine efficacy studies in women, the rate of progression of HPV16/18‐related persistent infection to HPV16/18‐related CIN2 or worse in women aged 27–45 years (2.9/100 person‐years; 95% CI: 1.9; 4.3) was similar to that in women aged 16–26 years (2.6/100 person‐years; 95% CI: 2.0; 3.3).Citation29 Similarly, in the placebo arm of the bivalent HPV vaccine efficacy study in women ≥25 years (90% of whom were aged 25–45 years), persistent HPV infections lasting ≥6 months progressed within 48 months to low-grade and high‐grade cervical lesions at rates similar to young women.Citation30
A major strength of FUTURE III is to establish long-term effectiveness, using the same rigorous methodology in the base study and LTFU study. A similar methodology was also used in other LTFU studies of the qHPV vaccine in girls aged 9–15 yearsCitation31 and women aged 16–23 years.Citation32 Collectively, these studies established long-term effectiveness (≥10 years) in females aged 9–45 years. This LTFU study lacked a control group, since participants in the placebo arm were offered qHPV vaccination after the base study. Nonetheless, the absence of breakthrough cases and the continued exposure to non-vaccine HPV types during the LTFU study provide robust evidence of sustained vaccine effectiveness. The absence of breakthrough cases cannot be explained by herd protection since the national HPV vaccination program in Colombia was only initiated in 2012 and primarily targeted girls aged 9–18 years. No effectiveness assessment was conducted in men. However, efficacy results previously established in men aged 16–26 years can be extended to men aged 27–45 years based on the demonstration of non-inferior immunogenicity.
Overall, durable effectiveness of the qHPV vaccine was demonstrated in women aged 27–45 years and inferred in men aged 27–45 years. These results support catch-up vaccination programs of sexually active adults who have not been previously vaccinated as adolescents or young adults. Vaccination of adults may be important in obtaining rapid reductions in the incidence of all HPV-related cancers and diseases worldwide.Citation33
Supplemental Material
Download MS Word (355.6 KB)Acknowledgments
The authors would like to thank the study participants and investigators, and Robert Kurman for contributions serving on the pathology panel. Rodney Finalle, Barbara Kuter, Vimalanand Prabhu, Miriam Reuschenbach, Kunal Saxena, and Christine Velicer, all of Merck & Co., Inc., Rahway, New Jersey, USA, reviewed and provided feedback on a draft of this manuscript.
Disclosure statement
IM: Ivette Maldonado has received grants through her institutions for board membership and study conduct.
MP: Manuel Plata has received grants through his institutions for study conduct and support for travel. He has also received honoraria from the Fundación Cardioinfantil.
MG: Mauricio Gonzalez has grants via his institution to serve as an investigator and colposcopist of FUTURE III study (Protocol 019).
AC: Alfonso Correa has no interests to declare.
CN: Claudia Nossa has no interests to declare.
AG: Anna R. Giuliano reports grant support from, and is an advisory board member for, Merck & Co., Inc.
EJ: Elmar Joura reports grants from Merck during the conduct of the study and personal fees from Merck Sharp & Dohme (MSD) outside the submitted work.
AF: Alex Ferenczy received consultation fees from MSD as a member of the Pathology Panel.
BR: Brigitte M. Ronnett is a consultant for MSD as part of the Pathology Panel.
MS: Mark H. Stoler has received personal fees from MSD, Roche, Becton Dickinson, and Inovio Pharmaceuticals as a consultant, all outside of the submitted work.
HJZ: Hao Jin Zhou is a former employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock/stock options in the Company.
AJ: Amita Joshi is a former employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock/stock options in the Company.
RD: Rita Das is a former employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock/stock options in the Company.
OB: Oliver Bautista is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock/stock options in the Company.
TG: Thomas Group is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock/stock options in the Company.
AL: Alain Luxembourg is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock/stock options in the Company.
AS: Alfred Saah is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock/stock options in the Company.
UB: Ulrike Buchwald is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may own stock/stock options in the Company.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2078626
Additional information
Funding
References
- McClung NM, Gargano JW, Park IU, Whitney E, Abdullah N, Ehlers S, Bennett NM, Scahill M, Niccolai LM, Brackney M, et al. Estimated number of cases of high-grade cervical lesions diagnosed among women — United States, 2008 and 2016. MMWR Morb Mortal Wkly Rep. 2019;68:337–10. doi:10.15585/mmwr.mm6815a1.
- Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, Abrahamsen M, Salmeron J, Anic GM, Rollison DE, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi:10.1016/S0140-6736(10)62342-2.
- Lu B, Viscidi RP, Wu Y, Lee JH, Nyitray AG, Villa LL, Lazcano-Ponce E, da Silva RJC, Baggio ML, Quiterio M, et al. Prevalent serum antibody is not a marker of immune protection against acquisition of oncogenic HPV16 in men. Cancer Res. 2012;72:676–85. doi:10.1158/0008-5472.CAN-11-0751.
- Giuliano AR, Sirak B, Abrahamsen M, Silva RJC, Baggio ML, Galan L, Cintra RC, Lazcano-Ponce E, Villa LL. Genital wart recurrence among men residing in Brazil, Mexico, and the United States. J Infect Dis. 2019;219(5):703–10. doi:10.1093/infdis/jiy533.
- Beachler DC, Pinto LA, Kemp TJ, Nyitray AG, Hildesheim A, Viscidi R, Schussler J, Kreimer AR, Giuliano AR. An examination of HPV16 natural immunity in men who have sex with men (MSM) in the HPV in men (HIM) study. Cancer Epidemiol Biomarkers Prev. 2018;27:496–502. doi:10.1158/1055-9965.EPI-17-0853.
- Munoz N, Manalastas R Jr., Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, Clavel C, Luna J, Myers E, Hood S, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24–45 years: a randomised, double-blind trial. Lancet. 2009;373:1949–57. doi:10.1016/S0140-6736(09)60691-7.
- Castellsagué X, Muñoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, Luna J, Myers E, Mallary S, Bautista OM, et al. End-Of-Study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24–45 years of age. Br J Cancer. 2011;105:28–37. doi:10.1038/bjc.2011.185.
- Giuliano AR, Isaacs-Soriano K, Torres BN, Abrahamsen M, Ingles DJ, Sirak BA, Quiterio M, Lazcano-Ponce E. Immunogenicity and safety of Gardasil among mid-adult aged men (27–45 years)—the MAM study. Vaccine. 2015;33:5640–46. doi:10.1016/j.vaccine.2015.08.072.
- Bonanni P, Faivre P, Lopalco PL, Joura EA, Bergroth T, Varga S, Gemayel N, Drury R. The status of human papillomavirus vaccination recommendation, funding, and coverage in WHO Europe countries (2018–2019). Expert Rev Vaccines. 2020;19:1073–83. doi:10.1080/14760584.2020.1858057.
- Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: Updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68:698–702. doi:10.15585/mmwr.mm6832a3.
- Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, Tang GWK, Ferris DG, Steben M, Bryan J, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007;356:1928–43. doi:10.1056/NEJMoa061760.
- Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12:959–69. doi:10.1128/CDLI.12.8.959-969.2005.
- Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, et al. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10:108–15.
- Smith JF, Kowalski R, Esser MT, Brown MJ, Bryan JT. Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil: a vaccine for human papillomavirus types 16, 18, 6 and 11. Hum Vaccin. 2008;4:134–42. doi:10.4161/hv.4.2.5261.
- Opalka D, Matys K, Bojczuk P, Green T, Gesser R, Saah A, Haupt R, Dutko F, Esser MT. Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin Vaccine Immunol. 2010;17:818–27. doi:10.1128/CVI.00348-09.
- Saah A, Bautista O, Luxembourg A, Perez G. Intention-To-Prevent analyses for estimating human papillomavirus vaccine efficacy in clinical studies. Contemp Clin Trials Commun. 2017;7:189–93. doi:10.1016/j.conctc.2017.07.010.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi:10.1056/NEJMoa061741.
- Hillman RJ, Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr., Vardas E, Aranda C, Jessen H, Ferris DG, Coutlee F, et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin Vaccine Immunol. 2012;19:261–67. doi:10.1128/CVI.05208-11.
- Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsagueé X, Rusche SA, Lukac S, Bryan JT, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118(5):2135–45. doi:10.1542/peds.2006-0461.
- Dobson SRM, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–802. doi:10.1001/jama.2013.1625.
- Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, Afsar OZ, LaMontagne DS, Mosina L, Contreras M, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev Med. 2021;144:106399. doi:10.1016/j.ypmed.2020.106399.
- Ferris DG, Brown DR, Giuliano AR, Myers E, Joura EA, Garland SM, Kjaer SK, Perez G, Saah A, Luxembourg A, et al. Prevalence, incidence, and natural history of HPV infection in adult women ages 24 to 45 participating in a vaccine trial. Papillomavirus Res. 2020;10:100202. doi:10.1016/j.pvr.2020.100202.
- Olsson SE, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Hseon Tay E, et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum Vaccine. 2009;5:696–704. doi:10.4161/hv.5.10.9515.
- Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, Huh WK, Sings HL, James MK, Haupt RM, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ. 2012;344(mar27 3):e1401. doi:10.1136/bmj.e1401.
- Kang WD, Choi HS, Kim SM. Is vaccination with quadrivalent HPV vaccine after loop electrosurgical excision procedure effective in preventing recurrence in patients with high-grade cervical intraepithelial neoplasia (CIN2-3)? Gynecol Oncol. 2013;130:264–68. doi:10.1016/j.ygyno.2013.04.050.
- Garland SM, Paavonen J, Jaisamrarn U, Naud P, Salmeron J, Chow SN, Apter D, Castellsagué X, Teixeira JC, Skinner SR, et al. Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: post-hoc analysis from a randomized controlled trial. Int J Cancer. 2016;139:2812–26. doi:10.1002/ijc.30391.
- Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, Salmerón J, McNeil S, Stapleton JT, Bouchard C, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 AS04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled VIVIANE study. Lancet Infect Dis. 2016;16:1154–68. doi:10.1016/S1473-3099(16)30120-7.
- Ingles DJ, Lin HY, Fulp WJ, Sudenga SL, Lu B, Schabath MB, Papenfuss MR, Abrahamsen ME, Salmeron J, Villa LL, et al. An analysis of HPV infection incidence and clearance by genotype and age in men: the HPV infection in men (HIM) study. Papillomavirus Res. 2015;1:126–35. doi:10.1016/j.pvr.2015.09.001.
- Bautista OM, Saah A, Munoz NR. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2011;103:158–59.
- Skinner SR, Wheeler CM, Romanowski B, Castellsague X, Lazcano-Ponce E, Del Rosario-Raymundo MR, et al. Progression of HPV infection to detectable cervical lesions or clearance in adult women: analysis of the control arm of the VIVIANE study. Int J Cancer. 2016;138:2428–38.
- Ferris DG, Samakoses R, Block SL, Lazcano-Ponce E, Restrepo JA, Mehlsen J, et al. 4-Valent human papillomavirus (4vhpv) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:ii: e20163947.
- Kjaer SK, Nygård M, Sundström K, Dillner J, Tryggvadottir L, Munk C, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401.
- Bosch FX, Robles C, Diaz M, Arbyn M, Baussano I, Clavel C, et al. HPV-FASTER: broadening the scope for prevention of HPV-related cancer. Nat Rev Clin Oncol. 2016;13:119–32.
