ABSTRACT
Respiratory syncytial virus (RSV) is a highly contagious seasonal virus and the leading cause of Lower Respiratory Tract Infections (LRTI), including pneumonia and bronchiolitis in children. RSV-related LRTI cause approximately 3 million hospitalizations and 120,000 deaths annually among children <5 years of age. The majority of the burden of RSV occurs in previously healthy infants. Only a monoclonal antibody (mAb) has been approved against RSV infections in a restricted group, leaving an urgent unmet need for a large number of children potentially benefiting from preventive measures. Approaches under development include maternal vaccines to protect newborns, extended half-life monoclonal antibodies to provide rapid long-lasting protection, and pediatric vaccines. RSV has been identified as a major global priority but a solution to tackle this unmet need for all children has yet to be implemented. New technologies represent the avenue for effectively addressing the leading-cause of hospitalization in children <1 years old.
Introduction
The present study provides a narrative review of the recently published data on Human Respiratory Syncytial Virus (RSV) epidemiology and preventive interventions. RSV is a seasonal, highly contagious pathogen belonging to the Pneumoviridae (genus orthopneumovirus), a family of negative-strand RNA viruses.Citation1–7 RSV is a common cause of respiratory illness throughout life, but particularly in infants and older adults it is associated with significant morbidity and mortality.Citation8–10 For instance, around 90% of all children will be infected with RSV in their first two years of life, and up to 40% of these will develop a Lower Respiratory Tract Infection (LRTI) with the initial episode.Citation10–14
RSV-associated LRTI are characterized predominantly as bronchiolitis or pneumonia, and can manifest with both acute and long-term consequences to the developing lungs.Citation15 On the one hand, RSV infections account for around 60% to 80% of infant bronchiolitis and up to 40% of pediatric pneumonias.Citation16,Citation17 On the other hand, infants who have RSV bronchiolitis in early life may be at increased risk of developing asthma later in childhood, and at increased risk of recurrent wheezing.Citation18,Citation19
Management of RSV infection is mainly supportive and aims at both maintaining adequate oxygenation and hydration. No specific drugs are currently available. Hospitalization is required when either oxygen supplementation, or rehydration, or both are needed. Oxygen supplementation may be delivered via nasal continuous positive airway pressure (CPAP), or high flow nasal cannula (HFNC) therapy, or mechanical ventilation, depending on the severity of disease (eg, hypoxemia, respiratory failure).Citation20,Citation21 Bronchodilators and corticosteroids have not shown a benefit for the management of RSV bronchiolitis and, therefore, are not recommended.Citation22–24
Prevention of RSV illnesses in all infants is a major public health priority,Citation25 however, despite more than 60 years of attempted vaccine development,Citation26 there are no licensed vaccines or preventative options for all infants. The only currently approved prophylaxis for RSV is palivizumab (SYNAGIS®; USA approval 1998, EU approval 1999),Citation27 with license limited to infants who are either born at ≤35 weeks of Gestational Age (wGA) or children <2 years of age with chronic lung disease of prematurity [CLD] or hemodynamically significant congenital heart disease [CHD] (Synagis PI 2020, Synagis SmPC 2021). The group of infants eligible for this preventative may be further limited by local recommending bodies.
Close examination of the burden of RSV disease below demonstrates that in order to mitigate this impact significantly, protection from RSV would need to begin at the same time as the epidemic season, should last throughout the whole season and cover the entire cohort of infants entering their first epidemic season.Citation1–28–Citation30
Epidemiology and burden of disease
Before exploring the burden of RSV illness in a given birth cohort, analyses should take into account a number of factors, including: the age of the infant at the time of illness (which determines whether or not they are considered to be born in the season or out of season), gestational age at birth and preexisting conditions.Citation7–9−Citation17–27–Citation31–35
RSV is a major global health threat.Citation3,Citation36 A recent systematic review estimated a global burden of disease equal to 33.1 million clinical cases in children less than 5 years of age in 2015.Citation36 Interestingly, around 90% of cases did occur in low- to middle-income countries, with 2.8 million cases (95% confidence intervals 1.1 to 6.1) in high-income countries.Citation36 Although RSV infections occur throughout the year, RSV causes disease in clearly defined and somewhat predictable seasonal epidemics. In much of the Northern Hemisphere (including the USA, the UK, France, and Germany), RSV outbreaks usually begin in November or December, reach their peak during the winter month of January or February, and end in March or April. There are usually no outbreaks during the warm, summer season. Instead, new infections occur at low rates. In contrast, in humid tropical locations, RSV outbreaks peak closer to the rainy season.Citation7,Citation17,Citation32 To summarize, the annual epidemics of RSV seemingly follow a “climate-driven” trend, with correlations between several annually averaged climate variables and timing and amplitude of the RSV cycle.Citation37,Citation38
The rate of hospitalization due to RSV has been conservatively estimated between 1.2%Citation39 and 1.6%Citation36 at global level, equal to 3.2 million episodes in 2015 alone,Citation36 45% of them occurring in children aged 6 months or younger.Citation36 In other words, RSV represents a main cause of hospitalization, as stressed by US-based data reported in (Top five primary diagnoses in hospitalized infants <1 year of age in the USA).Citation3–36–Citation40–42 The drivers for RSV hospitalizations are complex, and the outcome remains highly unpredictable, particularly in the first year of life,Citation43–45 and cannot be explained simply by preexisting conditions or infant age at the time of first RSV exposure. First of all, up to 75% of infants hospitalized in a given season are otherwise healthy and full-term children.Citation17,Citation46,Citation47 Pre-existing risk factors are recognized in only 22 to 32% of total admissions, with prematurity being diffusely recognized as the most significant predictor of RSV hospitalization,Citation47 and 23% of RSV deaths associated with infants lacking additional risk factors.Citation17,Citation47,Citation48 In a recent study from Arriola et al.Citation47 encompassing the RSV season 2014–2015, 65.8% of 336 ICU admission for RSV occurred in full-term children, and 59.8% of infants requiring mechanical ventilation were children without any risk factors.
Figure 1. Top 5 primary diagnoses in hospitalized infants <1 year of age in the USA.Citation3,Citation36,Citation49–51
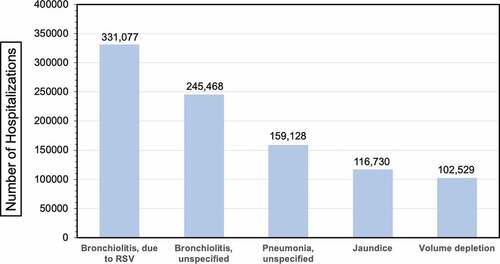
The risk for RSV-associated hospitalization is usually acknowledged as higher for infants born “in season” compared to those born outside it. For example, in a recent retrospective study from France including a total of 407,025 RSV-associated hospitalizations in children aged <5 years (2010–2018), 13.9% of all patients were born between September and November.Citation8 However, the raw figures (96,466 RSV infections for the time period October to March, vs. 85,292 for April to September) and the monthly proportions were comparable (1.3% to 4.1% vs. 5.2% to 0.1%, p value = 0.937).Citation8
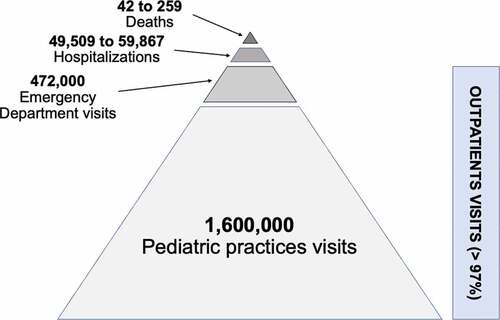
The burden of RSV infections on primary care is also considerable. Although it is hard to measure due to a relative paucity of viral testing in the community, health-care utilization is estimated in around 97% of cases resulting in outpatient visits . (Estimation of healthcare Utilization Related to RSV in Children <2 Year.Citation41,Citation46,Citation52). For instance, between 2004 and 2009 RSV caused around 1.6 million outpatient visits every year in the US alone.Citation47,Citation53 In England, it was estimated that 20% of children under 6 months of age will attend an outpatient health-care setting with an RSV-related LRTI.Citation33 The magnitude of the burden of RSV-related illness on outpatient settings remains significant throughout the first years of life.Citation53,Citation54 Among Italian subjects with influenza-like illness or severe-acute respiratory illness during five recent, consecutive influenza seasons, RSV was prevalent (17.6–19.1%) in children <5 years of age. Although this epidemiological observation could be interpreted to suggest that RSV is more frequently spread among children of this age group, it is possible that school-age children are bringing RSV home and infecting the 12- to-23-month-old infants who have the highest rate of infection.Citation55 In other words, RSV infections nearly double the number of primary care consultation compared with influenza, resulting in a time- and resource-consuming issue, not only for medical professionals, but also for parents caring for their children.Citation33
Figure 2. Estimation of healthcare utilization related to RSV in children <2 year.Citation41,Citation46,Citation52
Virology
RSV is an enveloped, pleomorphic virus with a diameter of approximately 150 nm (range 120–300 nm).Citation56 Its genome is represented by a single-stranded, non-segmented negative-sense RNA molecule with a total length of approximately 15.2 kb,Citation1,Citation56,Citation57 that encodes a total of 11 proteinsCitation56–58–Citation60 (See Annex. Protein encoded by viral genome of RSV). Among them, two surface glycoproteins are crucial for infectivity and pathogenesis: G (attachment protein), and F (fusion protein) ( Schematic representation of RSV and RSV genome (Adapted from Tognarelli et al., 2019).Citation56,Citation57
Figure 3. Schematic representation of RSV and RSV genome (Adapted from Tognarelli et al., 2019).Citation61
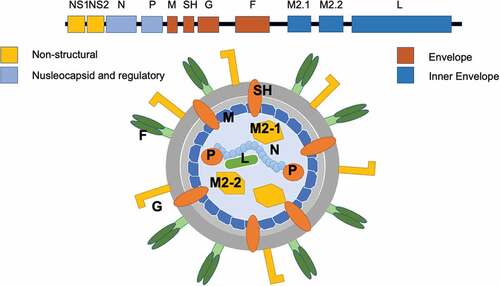
G mediates RSV attachment to the host cells. Studies on immortal cell lines have initially highlighted the role of surface glycosaminoglycans (GAG) as main viral receptors, and particularly of heparan sulfate proteoglycans (HSGP).Citation62,Citation63 As HSGP is not expressed on the apical surface of ciliated epithelial cells,Citation63,Citation64 other receptors mediate the interaction of G with airways cells in vivo. For instance, studies on human epithelial bronchial cell lines have shown that in vivo G mainly interacts with C × 3C chemokine receptor 1 (CX3CR1), also known as the fractalkine receptor or G-protein coupled receptor 13 (GPR13), whose tropism in human airways matches that of RSV.Citation62–64
Moreover, G does not only represent an attachment factor, but also a substantial pathogenetic factor. On the one hand, when secreted from infected cells, it acts as a “decoy” for circulating antibodies.Citation56,Citation57 On the other hand, it contributes to the suppression of interferon type I, leading to the imbalance in the Th-1/Th-2 response with a shift toward the type 2 response that is associated with RSV infection.Citation62,Citation65,Citation66
Even though RSV is considered a single-serotype pathogen,Citation56 G is quite heterogeneous among circulating strains (RSV A vs. B),Citation56,Citation57,Citation67 with an even larger number of variants.Citation1,Citation57,Citation59,Citation67 The genetic drift of the G gene is considered the main driver for the emergence of local variants, which in turn would be the main cause of seasonal reemergence of the pathogen, with new epidemics.Citation57,Citation61,Citation67,Citation68
F protein is a metastable class I fusion protein that is necessary for cell infection and represents the main target for vaccines and mAbs.Citation25,Citation52,Citation69 It mediates the entry process by binding to the cell and enabling the fusion of the virus envelope with the host cell plasma membrane,Citation52,Citation56,Citation57 a process that not only allows the virus passage into the host cells,Citation56,Citation57,Citation68 but significantly contributes to the immune escape strategy of RSV.Citation52,Citation56,Citation57,Citation59,Citation70 On the one hand, due to its critical role, F is highly conserved (i.e. around 90% homology between lineage A and B), and elicits both neutralizing antibodies (NA) and cytotoxic T-lymphocyte responses, being therefore considered as a suitable target for vaccine development and mAbs.Citation69,Citation71,Citation72 In fact, there is some evidence that serum and breast milk antibodies targeting F protein are associated with reduced disease.Citation73,Citation74 On the other hand, RSV F protein is initially expressed as a single-chain precursor (F0) that becomes fusion competent after intracellular cleavage by a furin-like proteaseCitation75,Citation76 into two resulting peptides, the C terminal F1, and the N terminal F2. F2 represents the actual fusion peptide. Triggered by unknown factors, F1 transition from a pre-fusion (pre-F) to post-fusion (post-F) conformationCitation58,Citation72 by inserting its hydrophobic N terminus into a nearby membrane.Citation75 As a consequence, post-F conformation loses neutralization sensitive antigenic sites (Ø and V),Citation52,Citation58,Citation77 impairing the binding affinity for NA.Citation34,Citation75,Citation76,Citation78 Therefore, alternative strategies for vaccines and mAbs based on the G protein have been recently assessed, with promising results.Citation49,Citation79,Citation80
Clinical details
Pneumoviridae exhibit a large array of potential natural hosts, mostly cattle and rodents, but humans are the only known natural hosts for human RSV.Citation56,Citation57,Citation81 All new cases are therefore acquired through human contact, starting early in life, with infection beginning shortly after the waning of maternal antibodies.Citation3,Citation4 As the half-life of maternal RSV-NA usually ranges from 36 to 42 days,Citation82,Citation83 with even shorter estimates,Citation84 it is reasonable that all children become susceptible or even highly susceptible to RSV infection shortly after the first month of live.Citation1,Citation55,Citation85
Although epidemiological data on the transmission patterns of RSV is not widely captured, some studies have demonstrated that community contact, including childcare settings, is the biggest contributor to infection risk. Contrary to other respiratory pathogens, such as Bordetella pertussis, where the main driver for the infection of newborns and infants is represented by the waning immunity in older age groups,Citation86–89 RSV usually is acquired by children from other individuals during the pediatric age,Citation1,Citation56,Citation67,Citation85 and RSV in older siblings is an important source of infection in infants.Citation85 In a recent study from Kenya,Citation67 54.8% of new cases were acquired at the community level, mostly from other children (siblings and cousins, 23.8% of all cases), while older subjects and even parents played a more marginal role.
With a basic reproduction number (R0) estimated at 3.0 ± 0.6,Citation90,Citation91 RSV is a relatively contagious but highly circulating pathogen, and virtually all infants will be infected by RSV by 2 years of age.Citation41,Citation45,Citation53,Citation84,Citation85,Citation92,Citation93 Following the vanishing of maternal NA, seroprevalence rates for RSV-NA usually decrease from birth to the 10th month, with a subsequent and stark rebound in the following months that mirrors the natural infection rates.Citation3,Citation5,Citation82,Citation83,Citation94 For example, in a seroprevalence study, performed in Tuscany and North-Rhine Westfalia (Germany) during the winter season 2009/2010, seropositivity rates ranged between 72 and 68.8% in children aged 6 months to 5 years, compared to 95.5% to 100% in older children.Citation95 Similarly, in a study based on two Dutch cohorts (2006/2007 and 2016/2017), the probability of RSV infection was nearly 0 at 1 month of age, but evolved to a 100% at three years of age.Citation5 In other words, as a thumb-rule, around half of children are infected by RSV within 1 year after birth, and virtually all of them by the time they are 2 years old.Citation1–Citation29–56–Citation94–96–Citation98
As RSV does not elicit a long-lasting immunity, adults are constantly re-infected throughout life, with rates ranging from 2 to 12%, peaking in institutionalized elders.Citation99,Citation100 On the one hand, the high re-infection rates sustain the circulation of the pathogen, increasing its capability to reach naïve, more vulnerable hosts. On the other hand, the repeated infections maintain the immune competence among healthy adults, that therefore rarely develop LRTI.Citation3–Citation99–101
The incubation period for RSV usually lasts around six days, with primary infection occurring through the upper respiratory tract, with further spread to the lower respiratory epithelium.Citation16,Citation17,Citation56 One to three days after the onset of common cold symptoms, such as nasal congestion, mild cough, fever and reduced appetite, LRTI may develop in up to 40% of affected infants, with symptoms including rapid breathing, wheezing, persistent cough, and difficulty feeding, which in turn can result in dehydration.Citation17,Citation44,Citation56,Citation102,Citation103 Bronchiolitis, the most common LRTI caused by RSV, is associated with necrosis and sloughing of the epithelium of the small airways, with edema and increased secretion of mucus. In young children, this leads to obstruction of air flow and the typical clinical picture of hyperinflation, atelectasis, and wheezingCitation16,Citation104
Disease prevention
Nonpharmaceutical interventions (NPIs)
RSV and SARS-CoV-2 are distinct pathogens, but share several characteristics, most notably their spread through respiratory inoculation of the upper airways with respiratory secretions from infected individuals.Citation56,Citation68 Moreover, on surfaces, RSV can survive for several hours, particularly with low temperatures and high humidity, and remain viable for up to a half an hour on hands, emphasizing the role for NPIs, such as repeated hand washing, and social distancing.Citation16,Citation17
The first wave of the SARS-CoV-2 pandemic (March 2020) occurred during a typical Northern Hemisphere RSV season, and at the beginning of the Autumn in Southern Hemisphere, that is, when the number of RSV cases typically begin to rise. Available data from Italy, Finland, Belgium, UK and USA showed a sudden and earlier-than-expected end of the RSV epidemic season, with substantially no cases detected in the following months.Citation105–109 Similarly, in Western Australia detection of RSV in children <16 years of age was reduced by 94% compared with the previous years, and such reduction lasted well over the winter season.Citation109
The lifting of these NPI measures further changed transitorily RSV epidemiology. In Australia, NPI were initially relaxed in April 2020, and even though RSV notification rates remained scarcely appreciable until August 2020, a substantial resurgence of RSV infections began between May and July, with unprecedented peaks between November and December 2020.Citation110,Citation111
This change has been interpreted as a direct consequence of the lifting of NPI measures that were taken to mitigate the spread of SARS-CoV-2, and blocked the normal transmission of RSV to susceptible infants at the community level.Citation50–107,Citation108–110,Citation111–113 Their extensive implementation during the RSV seasons avoided natural infection in naïve infants, that aged out of their maternal antibody protection prior to RSV exposure. In other words, the pandemic NPIs prevented RSV exposure, generating a larger RSV-vulnerable population, and preserving susceptibility to the pathogen during a subsequent season.Citation1,Citation50,Citation110,Citation111,Citation114 Even though is reasonable that the progressive shift of SARS-CoV-2 infection from a pandemic to an endemic infection will remove the causes for the recent outburst of RSV, further studies are needed to verify the behavior of RSV infections in the next seasons. More precisely, we need to understand if the risk of LRTI following RSV infection is persistently becoming more or less severe, with the return to the common seasonal pattern previously observed.Citation51–Citation105–114–Citation117
Although on an individual level it is clear that there is a place for the maintenance of basic NPI hygiene measures, such as handwashing, the level of NPI implemented during the SARS-CoV-2 pandemic is not a sustainable long-term option. The speed of resurgence of RSV infections following the relaxation of measures demonstrates that only the most stringent measures are capable of interrupting transmission, and resurgence is very high after them. This reinforces the need for other methods of prevention, such as pharmaceutical interventions.
Pharmacological interventions
There is currently no preventative option that is available for all infants.Citation118 Palivizumab, a F-protein targeting mAb, is indicated only for a small subset of infants born at ≤35 weeks of gestation 10,34, 335,or in those with specific comorbidities, this is usually no more than 5–7% of newborns. It must be injected once each month during the RSV season, for a total of 5 subsequent weight-dependent doses (i.e. 15 mg/kg). Real-world evidence has shown palivizumab to be effective in reducing hospitalizations and preventing lower respiratory tract infections on this specific population.Citation119
In order to contribute to a significant reduction in the burden of infections and hospitalizations caused by RSV, future pharmacological interventions must be available to use on all infants and not to only restrictive groups.Citation49,Citation69,Citation120
New preventative strategies are in development, including; maternal vaccines to protect neonates during the first months of life;Citation30,Citation83 extended half-life mAbs to provide direct and rapid protection for at least 5 months after administration with a single dose;Citation83,Citation121 and pediatric vaccines.Citation69,Citation71,Citation122
The main approaches currently being investigated and developed are summarized below (. main immunization approaches for RSV in infants and children).
Table 1. Main immunization approaches for RSV in infants and children.
Maternal vaccines
In 2017, World Health Organization developed Preferred Product Characteristics (PPC) for RSV vaccines that, in terms of maternal immunization, could by summarized as guaranteeing an efficacy greater than 70% against confirmed severe RSV in the offspring, from birth to age 4 months.Citation123 To date, not only vaccines for RSV are not commercially available,Citation52,Citation69,Citation71,Citation124 but, while we are waiting for the results of two large trials specifically focusing on maternal vaccination,Citation125,Citation126 available studies have reported mixed and still unsatisfactory outcomes. On the one hand, pre-fusogenic F protein nanoparticle vaccine (NCT02624947) was initially reported as 53% effective in reducing LRTI-associated hospitalizations during the first 90 days of life,Citation127 but the subsequent peer-reviewed report substantially downgraded these results (i.e. 39.4%, 95%CI 5.3 to 61.2).Citation128 On the other hand, during the very same timeframe (i.e. first 90 days of life) infants received fewer antimicrobial prescription courses than infants of mothers assigned to placebo group, stressing the potential efficacy in avoiding bacterial complications of primary RSV infections.Citation129
Nonetheless, the potential referral to maternal vaccination programs represents a promising preventive strategy.Citation128–130–Citation132 Maternal vaccination is well established for other pediatric infections and often provides protection from pathogens to both mothers and infants, as demonstrated by maternal pertussis vaccination programs.Citation133–135 The rationale for maternal immunization programs targeting RSV is represented by the consolidated evidence that high titers of maternal antibodies reduce the risk of RSV infection and can delay the onset of severe illness in the first month of life,Citation84–138 even though disease severity is not regularly associated with differences in RSV-specific IgG titers, RSV-IgG avidity, or virus neutralization.Citation139–141
The efficacy of maternal vaccination strategies clearly depends on the achievement of high vaccination rates, correct timing, efficacy of vaccines and duration in time, and vaccination strategies targeting pregnant women must therefore take in account some specificities of RSV and the specificities of antibody passage from the mother.Citation124,Citation127
First of all, in a sharp contrast to pertussis, for which maternal vaccination program have been successfully established, the burden of RSV remains significant throughout the first year, and is not mitigated by existing pediatric vaccine programs.Citation9,Citation67,Citation142 On the other hand, it is often impossible to predict the start of the RSV season far enough ahead to vaccinate the pregnant women and the conditions of future the new-born (like prematurity) that will affect the correct passage of antibodies.Citation1,Citation28
Second, while the RSV antibody transfer from mother to infant appears quite efficient,Citation140,Citation141,Citation143,Citation144 Yildiz et al. have recently shown that it may be negatively affected in both small (weight <10th percentile) and large (weight >90th percentile) for gestational age infants.Citation143 In fact, there is a consolidated base of evidence that transplacental antibody transfer is generally impaired in pre-term and small for gestational age children, and that puts these children at increased risk for all infectious diseases, even for those that are vaccine preventable.Citation145–147
Third, the estimated half-life for maternal RSV neutralizing antibodies is variable in the various settings, ranging from 21 to 27 days,Citation84 to 38 days (95%CI of 36–42 days),Citation82,Citation83,Citation144 and their effectiveness in guaranteeing infant protection during the entire length of a typical RSV season also remains somewhat uncertain.Citation30,Citation82,Citation83,Citation144 In this regard, there is consolidated evidence that vaccines may be able to elicit higher titers of NA antibodies when compared to the natural infection, and transplacental transfer is in turn associated with higher concentration of antibodies in the recipient infant.Citation124,Citation127,Citation128,Citation130,Citation140,Citation143,Citation146 However, despite some promising results,Citation124,Citation148 the actual duration of maternal NA elicited by vaccination remains and likely will remain largely undefined until later stages of RSV vaccine development. Nevertheless, most cases of RSV occur in the first months of life, when the levels of maternal NA may be considered substantially higher.Citation82,Citation149,Citation150
In addition, compared to other infections like influenza or pertussis, the impact of maternal vaccination on RSV disease or carriage in pregnant women is unknown.Citation30,Citation151 Although some reports have suggested that RSV infection in pregnancy may increase the risk of early delivery by cesarean section,Citation124,Citation152,Citation153 as well as higher rates of adverse pregnancy outcomes,Citation124,Citation154,Citation155 RSV usually does not cause significant disease in healthy adults, and the perception of direct benefit of vaccination to the mother may be therefore questionable. Nonetheless, a series of studies targeting knowledge, attitudes, and practices of pregnant women have stressed the substantial underestimation of both RSV disease burden and the potential health consequences of natural infections.Citation151,Citation156 Unfortunately, diffuse uncertainties and misbeliefs are also shared by potential caregivers and achieving high vaccination rates will require the implementation of extensive educative interventions, particularly among professionals who are less familiar with potentially severe consequences of RSV infections.Citation151,Citation156
Extended half-life mAbs
Due to the mechanism of action, long-acting serum mAbs would allow for rapid and direct protection for infants.Citation1,Citation14,Citation29,Citation56 Nirsevimab is the front-runner of these new mAbs, and was optimized from the human IgG1 antibody D25 that targets antigenic site Ø on the pre-F conformation.Citation121,Citation157
Providing direct and rapid protection lasting at least 5 months after administration, it has been shown to provide on healthy newborns of at least 35 weeks gestational age a stark reduction of medically attended RSV-associated lower respiratory tract infection (LRTIs) with an efficacy of 74.5% (95% confidence interval [CI], 49.6 to 87.1; P < 0.001) and a reduction of hospitalizations for RSV-associated lower respiratory tract infection of an efficacy of 62.1%; (95% CI, −8.6 to 86.8; P = 0.07) from the start of the RSV season, including those born out of RSV season.Citation1,Citation121 A single injection administered before the RSV season protected healthy late-preterm (>35w) and term infants from medically attended RSV-associated lower respiratory tract infections without the requirements of serial, monthly injections.Citation83,Citation121 Similarly, designed extended half-life mAbs could therefore be administered through a more convenient approach.Citation83 Given that RSV seasons are characterized by sustained circulation of the pathogen for a usually predictable length of time, an extended half-life mAb could be administered to all infants at birth, as a pre-discharge intervention post-delivery, but also could be administered during routine visits, representing a seasonal immunoprophylaxis, and an interesting tool to protect before expected RSV epidemic waves.Citation1,Citation83 As vaccination schedules in infancy have become increasingly crowded of vaccines,Citation28 extended half-life serum mAbs administered shortly before the return to the community of the newborn could guarantee extended flexibility to the vaccination services.Citation28,Citation30
Obviously, also mAbs are not deprived of possible drawbacks that must be considered when tailoring their role in the forthcoming preventive strategies.Citation158,Citation159 First of all, despite their obvious convenience advantage in being delivered as a single dose, extended half-life mAbs could share some of the limits of conventional mAbs likewise Palivizumab, for example, in terms of efficacy being limited to only small high-risk groups, requiring several dosages or having high costs.Citation158–160 In fact, as recently stressed by Li et al.,Citation160 cost-effectiveness of programs with extended half-life mAbs strictly depends on the characteristics of RSV circulation, being appropriate for seasonal interventions, and in order to be cost effective an appropriate price needs to be set similar to vaccine strategies.Citation160,Citation161 Similarly to the recommended PPC for RSV vaccines and maternal immunization, WHO has also issued PPC for mAbs,Citation162 stating that promising products like a single dose extended half-life mAbs need to have simplified delivery requirements and to be less costly than traditional mAbs, making them potentially suitable for use in all infants and not just those at high-risk.Citation160,Citation161 Moreover, WHO recommends that extended half-life mAbs will target all infants at least for their first 6 months of life, through a one dose regime that could be given from birth. They ask for a targeted safety and reactogenicity to be comparable to PPC for vaccines and ideally with at least 70% efficacy against RSV confirmed severe disease for a duration of five months following administration. Second, extended half-life mAbs still require injections to the newborn, representing at the very same time a further logistic issue, and a potential ethical one. This could represent a high level of complexity in which countries, based on their current vaccination strategies and public health ecosystems, will need to analyze the best strategy possible to implement a preventative strategy. From an acceptance perspective, issues could also arise, similar with Palivizumab today, both in health-care professionals,Citation163 and among parents and caregivers,Citation164 and proper educational strategies should be implemented eventually. By expanding the potential targets of RSV preventive strategies, mAbs could be offered to otherwise healthy infants, and not only to those affected by potential risk factors.Citation128,Citation158,Citation161 As a consequence, we cannot rule out that parents may exhibit increasing resistance toward interventions felt as “unnecessary,” particularly when tailored to cope with an often underscored pathogen as RSV.Citation156,Citation164
Despite the substantial progress seen today, as it will most probably be available before other strategies, even for extended half-life mAbs several further questions remain to be answered before their extensive recommendation.Citation121,Citation157 First, as mAbs target-specific epitopes on the RSV F protein, there is the potential for emergence of resistant strains, and this will need to carefully monitored.Citation62,Citation66,Citation165,Citation166 Second, mAbs specifically target F protein, with no effect on G, whose role in vivo has been increasingly stressed in more recent studies.Citation62,Citation66,Citation166 Moreover, also the protective potential of mAbs like Nirsevimab in the prevention of the development from RSV infection of asthma and chronic wheeze would need to be monitored over multi-year studies.Citation157,Citation165
Pediatric vaccines (older infants and children)
To date, no vaccine against RSV has been licensed. A prototype formalin-inactivated RSV vaccine was evaluated in the 1960s, but testing was discontinued due to lack of efficacy and resulting enhanced disease (i.e. enhanced RSV disease or ERD) in children following exposure to wild-type RSV leading to the death of several subjects.Citation52,Citation69,Citation72
One of the challenges associated with vaccination in infants relates to the immaturity of the immune system of newborns limiting their response to antigens. Infants under 4–6 months have an impaired ability to generate effective, long-lived adaptive immune responses following immunization. Consequently, it has been shown that natural RSV infection produces a low immune response in young children <18 months old.Citation167 Risk of RSV disease for infants born during the season starts at birth, which makes active vaccination unfeasible in this cohort because of their relatively immature immune systems and the time required for an immune response to mount, potentially leaving them vulnerable at a crucial time.Citation17 Pediatric RSV vaccines would therefore be best suited to infants older than 6 months, or toddlers entering their second RSV season. At present, several vaccine strategies for protection against RSV in infants are being investigated and none to our knowledge is targeting new borns.Citation25,Citation52,Citation69,Citation71
Vaccines under development and receiving preliminary assessment by regulatory agencies include protein vaccines that use stabilized pre-F protein subunits or virus-like particles, and live vaccines that include attenuated RSV strains, or virus vectors expressing RSV proteins.Citation17,Citation168 Briefly, particle-based vaccines are synthesized by self-assembling nanoparticles that express multiple copies of a selected antigen on their surface and mimic the native virions. Thanks to the high copy number of the selected antigen and the immune-boosting properties of the particulate matrix, these vaccines elicit strong humoral and cellular immune responses. The lack of the viral genome required for replication increases their safety, with a reportedly scarce risk for developing ERD.Citation69,Citation71,Citation122 On the contrary, subunit vaccines are created with RSV protein fragments. They are poorly immunogenic due to their non-replicating nature and their limited components. Booster doses and adjuvants are often necessary to make them effective. Moreover, they are at high risk for eliciting ERD because of their limited effect on CD4+ T cells.Citation69 Vector-based vaccines use a carrier vector to deliver RSV antigens and induce an immune response against RSV components exploiting the adjuvant effect of the vector.Citation69 Due to the chimeric nature of the vectors, there is no risk of reversion to wild-type RSV and of ERD. However, similar to other vaccines based on adenoviral vectors, the presence of preexisting anti-vector immunity or its potential development may challenge the clinical use of these vaccines.Citation69,Citation120,Citation122 Live-attenuated RSV vaccines (RSV-LAVs) are produced with versions of RSV that are able to replicate but have been modified to reduce disease induction. They can be created by traditional techniques (i.e., temperature or chemical sensitivity) or, thanks to an improved understanding of the RSV viral genome, by reverse genetics to create an attenuated replication-competent vaccine. ERD has not been observed with RSV-LAVs or replicating vaccine vectors.Citation69,Citation169,Citation181 Because these vaccines are typically administered intranasally, they generate superior mucosal immune responses and therefore are more likely to prevent upper respiratory infections as well as LRTI. More recently, promising results from a phase 1 study on a mRNA based, pre-F protein vaccine have been published, but further research is needed.Citation170
Conclusions
RSV is a pathogen associated with a significant global burden.Citation7,Citation8,Citation17,Citation32 As all infants are at risk of RSV infection and it is often impossible to ascertain which infants will develop serious disease, it is imperative to address as a public health problem RSV infections in all infants and to provide solutions that will protect them in an easy and effective manner. As up to 75% of hospitalization and around ¼ of RSV-associated deaths occur in otherwise healthy newborns, protection needs to extend beyond those currently protected by today’s available mAb (palivizumab).Citation17–46,Citation47,Citation48
A time- and resource-consuming health threat, RSV-related infections are difficult to prevent through common public health measures.Citation1,Citation55,Citation171,Citation172 Universal NPI are not sufficient to manage recurrent RSV epidemics as these would have to be stringent and permanent, which is clearly not sustainable, particularly at the household level.Citation50,Citation110,Citation111,Citation173 Less-than-universal NPI may fail to reduce circulation and spreading of RSV among susceptible individuals. In addition, the main target of preventive measures for RSV are children under 5 years of age, a group where the implementation and adherence to measures, such as the use of face masks, and improved hand hygiene may be particularly difficult.Citation174 Pharmacological prevention of RSV is therefore needed. To address the burden of RSV across health-care settings and to reduce impact of RSV on families, preventative measures would need to protect all infants.Citation8,Citation16,Citation17,Citation33,Citation42,Citation104,Citation142,Citation175
Effective vaccines for RSV are not yet commercially available.Citation26,Citation52,Citation69,Citation72,Citation122 Available data suggest its use in children will be limited by the age of use, timing its use on above 4/6 months of age. On the other hand, vaccines can be used on pregnant women to protect the new born but while maternal immunization approaches are of success in preventing other respiratory diseases short half-life transplacental antibodies might impair the reliability of maternal immunization,Citation30,Citation64,Citation65,Citation78 Further studies on these vaccines are needed to understand efficacy, duration, and effectiveness of this intervention on the new born. Furthermore, it would be then important to evaluate correct health system strategies to address all pregnant women giving birth during a season in order to protect all newborns. From this point of view, it is important to stress that even for vaccines with a consolidated preventive value for both mothers and infants, such as seasonal influenza vaccine, are still struggling to achieve substantial coverage rates.Citation176,Citation177
The only currently available pharmacological prophylaxis is a short-life mAb that requires monthly injections and is restricted to the most vulnerable infants like preterms, less than 7% of all newborns.Citation10–27–Citation178–180
The use of monoclonal antibodies with extended half-life is an appealing prospect, which could address many of the problems associated with other approaches in order to prevent the disease in all newborns, from birth, during a season.Citation1,Citation28,Citation30
Immunization of neonates and infants through extended half-life mAbs seem to provides rapid and consistent protection against RSV from birth and for at least 5 months, which covers the entire duration of a typical RSV season,Citation121,Citation157 and support an increased flexibility in the timing of administration that could address the need in infants born both within and outside of the season by immunizing them at the start of the season itself.Citation1,Citation121
The WHO recommendations for preferred product characteristics for mAbs seem to be covered in the recent clinical trials phase 3 publication of an almost ready to market extended half-life,Citation1,Citation121,Citation162 and we wait to see final product registration and characteristics. Meanwhile, early phase II studies on maternal immunization strategies are ongoing,Citation124,Citation126,Citation127,Citation130 and although too early to understand if the results will cover WHO recommended PPC,Citation123 they might be a promising alternative solution to evaluate in the future.
The burden of RSV disease in infants throughout their first year of life, irrespective of gestational age at birth, preexisting conditions, or age at entry into the season, suggests that there is a need to evaluate new strategies to help protect all infants against RSV and mitigate this burden for infants, their caregivers, and the health-care systems.
Disclosure statement
In accordance with Taylor & Francis policy and their ethical obligation as researchers we declare that all external authors have been participants in the past at advisory boards and/or have been speakers at symposia and/or lecture sponsored by Sanofi. Dr. CHECCUCCI LISI, Dr. ROBERTS, Dr. HEINRICHS, and Dr. VASSILOUTHIS, are reporting that they are employed by SANOFI and may hold shares and/or stock options in the company, a company that may be affected by the research reported in the enclosed paper. Authors have disclosed those interests fully to Taylor & Francis, and they have in place an approved plan for managing any potential conflicts arising from having been involved in the writing of this review.
Additional information
Funding
References
- Azzari C, Baraldi E, Bonanni P, Bozzola E, Coscia A, Lanari M, Manzoni P, Mazzone T, Sandri F, Checcucci Lisi G, et al. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital J Pediatr. 2021;47(1):1. doi:10.1186/s13052-021-01148-8.
- Pellegrinelli L, Galli C, Bubba L, Cereda D, Anselmi G, Binda S, Gramegna M, Pariani E. Respiratory syncytial virus in influenza-like illness cases: epidemiology and molecular analyses of four consecutive winter seasons (2014-2015/2017-2018) in Lombardy (Northern Italy). J Med Virol. 2020;92(12):2999–14. doi:10.1002/jmv.25917.
- Shi T, Denouel A, Tietjen AK, Campbell I, Moran E, Li X, Campbell H, Demont C, Nyawanda BO, Chu HY, et al. Global disease burden estimates of respiratory syncytial virus–associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2021;222(Supplement_7):S577–83. doi:10.1093/infdis/jiz059.
- Openshaw PJM, Chiu C, Culley FJ, Johansson C. Protective and harmful immunity to RSV infection. Annu Rev Immunol. [Internet]. 2017;35(1):501–32. doi:10.1146/annurev-immunol-.
- Andeweg SP, Schepp RM, van de Kassteele J, Mollema L, Berbers GAM, van Boven M. Population-Based serology reveals risk factors for RSV infection in children younger than 5 years. Sci Rep. 2021;11(1):8953. doi:10.1038/s41598-021-88524-w.
- Mazur NI, Martinón-Torres F, Baraldi E, Fauroux B, Greenough A, Heikkinen T, Manzoni P, Mejias A, Nair H, Papadopoulos NG, et al. Lower respiratory tract infection caused by respiratory syncytial virus: current management and new therapeutics. Lancet Respir Med. 2015;3(11):888–900. doi:10.1016/S2213-2600(15)00255-6.
- Nair H, Theodoratou E, Rudan I, Nokes DJ, Ngama HM, Munywoki PK, Dherani M, Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. [Internet]. 2010;375(9725):1545–55. www.thelancet.com.
- Demont C, Petrica N, Bardoulat I, Duret S, Watier L, Chosidow A, Lorrot M, Kieffer A, Lemaitre M. Economic and disease burden of RSV-associated hospitalizations in young children in France, from 2010 through 2018. BMC Infect Dis. 2021;21(1):730. doi:10.1186/s12879-021-06399-8.
- Kramer R, Duclos A, Lina B, Casalegno JS. Cost and burden of RSV related hospitalisation from 2012 to 2017 in the first year of life in Lyon, France. Vaccine. 2018;36(45):6591–93. doi:10.1016/j.vaccine.2018.09.029.
- Brady MT, Byington CL, Davies HD, Edwards KM, Jackson MA, Maldonado YA, Murray DL, Orenstein WA, Rathore MH, Sawyer MH. American Academy of Pediatrics, Committee on Infectious Diseases and Bronchiolitis Guidelines Committee. Updated guidance for Palivizumab Prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134(2):e620–38. doi:10.1542/peds.2014-1666.
- Meissner HC, Ingelfinger JR. Viral bronchiolitis in children. N Engl J Med. 2016;374(1):62–72. doi:10.1056/NEJMra1413456.
- Greenough A, Cox S, Alexander J, Lenney W, Turnbull F, Burgess S, Chetcuti AJ, Shaw NJ, Woods A, Boorman J, et al. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child. 2001;85(6):463–68. doi:10.1136/adc.85.6.463.
- Parrott RH, Kim HW, Arrobio JO, Hodes DS, Murphy BR, Brandt CD, Camargo E, Chanock RM. Epidemiology of respiratory syncytial virus infection in Washington, D.C. Am J Epidemiol. 1973;98(4):289–300. doi:10.1093/oxfordjournals.aje.a121558.
- Meissner HC, Long SS. Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of Palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112(6):1447–52. doi:10.1542/peds.112.6.1447.
- Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JLL, Bont L. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med. 2013;368(19):1791–99. doi:10.1056/NEJMoa1211917.
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med [Internet]. 2001;344(25):1917–28. www.nejm.org.
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588–98. doi:10.1056/NEJMoa0804877.
- Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–41. doi:10.1164/rccm.200406-730OC.
- Baraldi E, Bonadies L, Manzoni P . Evidence on the link between respiratory syncytial virus infection in early life and chronic obstructive lung diseases. Am J Perinatol. 2020;37(S 02):S26–30.
- Turnham H, Agbeko RS, Furness J, Pappachan J, Sutcliffe AG, Ramnarayan P. Non-Invasive respiratory support for infants with bronchiolitis: a national survey of practice. BMC Pediatr. 2017;17(1):20. doi:10.1186/s12887-017-0785-0.
- Baraldi E, Lanari M, Manzoni P, Rossi GA, Vandini S, Rimini A, Romagnoli C, Colonna P, Biondi A, Biban P, et al. Inter-Society consensus document on treatment and prevention of bronchiolitis in newborns and infants. Ital J Pediatr. 2014;40(1):65. doi:10.1186/1824-7288-40-65.
- National Institute for Health and Care Excellence (NICE). Bronchiolitis in children Funding National Institute for Health and Care Excellence Bronchiolitis in children contents [Internet]. London; 2021 [accessed 2021 Dec 20]. www.nice.org.uk/guidance/ng9
- Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, Johnson DW, Light MJ, Maraqa NF, Mendonca EA, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–502. doi:10.1542/peds.2014-2742.
- Florin TA, Plint AC, Zorc JJ. Viral bronchiolitis. The Lancet. 2017;389(10065):211–24. doi:10.1016/S0140-6736(16)30951-5.
- Giersing BK, Karron RA, Vekemans J, Kaslow DC, Moorthy VS. Meeting report: WHO consultation on Respiratory Syncytial Virus (RSV) vaccine development, Geneva, 25–26 April 2016. Vaccine. 2019;37(50):7355–62. doi:10.1016/j.vaccine.2017.02.068.
- Ruckwardt TJ, Morabito KM, Graham BS. Immunological lessons from respiratory syncytial virus vaccine development. Immunity. 2019;51(3):429–42. doi:10.1016/j.immuni.2019.08.007.
- Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev. 2013 Apr;(4): CD006602. doi:10.1002/14651858.cd006602.pub4.
- Barbati F, Moriondo M, Pisano L, Calistri E, Lodi L, Ricci S, Giovannini M, Canessa C, Indolfi G, Azzari C. Epidemiology of respiratory syncytial virus-related hospitalization over a 5-year period in Italy: evaluation of seasonality and age distribution before vaccine introduction. Vaccines (Basel). 2020;8(1):15. doi:10.3390/vaccines8010015.
- Janet S, Broad J, Snape MD. Respiratory syncytial virus seasonality and its implications on prevention strategies. Hum Vaccin Immunother. 2018;14(1):234–44. doi:10.1080/21645515.2017.1403707.
- Pouwels KB, Bozdemir SE, Yegenoglu S, Celebi S, McIntosh ED, Unal S, Postma MJ, Hacimustafaoglu M, Cormier SA. Potential cost-effectiveness of RSV vaccination of infants and pregnant women in Turkey: an illustration based on bursa data. PLoS ONE. 2016;11(9):e0163567. doi:10.1371/journal.pone.0163567.
- Zhang Y, Yuan L, Zhang Y, Zhang X, Zheng M, Kyaw MH. Burden of respiratory syncytial virus infections in China: systematic review and meta–analysis. J Glob Health. 2015;5(2). doi:10.7189/jogh.05.020417.
- Pebody R, Moyes J, Hirve S, Campbell H, Jackson S, Moen A, Nair H, Simões EAF, Smith PG, Wairagkar N, et al. Approaches to use the WHO respiratory syncytial virus surveillance platform to estimate disease burden. Influenza Other Respir Viruses. 2020;14(6):615–21. doi:10.1111/irv.12667.
- Cromer D, Jan Van Hoek A, Newall AT, Pollard AJ, Jit M Articles burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England [Internet]. 2017. www.thelancet.com/
- Olchanski N, Hansen RN, Pope E, D’-Cruz B, Fergie J, Goldstein M, Krilov LR, McLaurin KK, Nabrit-Stephens B, Oster G, et al. Palivizumab prophylaxis for respiratory syncytial virus: examining the evidence around value. Open Forum Infect Dis. 2018;5(3):ofy031. doi:10.1093/ofid/ofy031.
- Mac S, Sumner A, Duchesne-Belanger S, Stirling R, Tunis M, Sander B. Cost-Effectiveness of Palivizumab for respiratory syncytial virus: a systematic review. Pediatrics. 2019;143(5):20184064. doi:10.1542/peds.2018-4064.
- Shi T, McAllister DA, O’-Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–58. doi:10.1016/S0140-6736(17)30938-8.
- Pitzer VE, Viboud C, Alonso WJ, Wilcox T, Metcalf CJ, Steiner CA, Haynes AK, Grenfell BT, Kaderali L. Environmental drivers of the spatiotemporal dynamics of respiratory syncytial virus in the United States. PLoS Pathog. 2015;11(1):e1004591. doi:10.1371/journal.ppat.1004591.
- Baker RE, Mahmud AS, Wagner CE, Yang W, Pitzer VE, Viboud C, Vecchi GA, Metcalf CJE, Grenfell BT. Epidemic dynamics of respiratory syncytial virus in current and future climates. Nat Commun. 2019;10(1):5512. doi:10.1038/s41467-019-13562-y.
- Weigl JAI, Puppe W, Schmitt HJ. Incidence of respiratory syncytial virus-positive hospitalizations in Germany. Eur J Clin Microbiol Infect Dis. 2001;20(7):0452–9. doi:10.1007/s100960100527.
- Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J. 2002;21(7):629–32. doi:10.1097/00006454-200207000-00005.
- Leader S, Kohlhase K, Pearlman MH, Williams JV, Engle WA. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr. 2003;143(5):S127–32. doi:10.1067/S0022-3476(03)00510-9.
- Palmer L, Hall CB, Katkin JP, Shi N, Masaquel AS, McLaurin KK, Mahadevia PJ. Healthcare costs within a year of respiratory syncytial virus among medicaid infants. Pediatr Pulmonol. 2010;45:772–81. doi:10.1002/ppul.21244.
- Mosalli R, Alqarni SA, Khayyat WW, Alsaidi ST, Almatrafi AS, Bawakid AS, Paes B. Respiratory syncytial virus nosocomial outbreak in neonatal intensive care: a review of the incidence, management, and outcomes. Am J Infect Control. [Internet]. 2021. epub ahead of print. https://linkinghub.elsevier.com/retrieve/pii/S0196655321007112
- Loubet P, Lenzi N, Valette M, Foulongne V, Krivine A, Houhou N, Lagathu G, Rogez S, Alain S, Duval X, et al. Clinical characteristics and outcome of respiratory syncytial virus infection among adults hospitalized with influenza-like illness in France. Clin Microbiol Infect. 2017;23(4):253–59. doi:10.1016/j.cmi.2016.11.014.
- McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. 2016;36(11):990–96. doi:10.1038/jp.2016.113.
- Rha B, Curns AT, Lively JY, Campbell AP, Englund JA, Boom JA, Azimi PH, Weinberg GA, Staat MA, Selvarangan R, et al. Respiratory syncytial virus-associated hospitalizations among young children: 2015-2016 [Internet]. 2020. http://publications.aap.org/pediatrics/article-pdf/146/1/e20193611/1081381/peds_20193611.pdf?casa_token=NEb8coMqaSwAAAAA:gOOjzURVbTwXJtDiOES1Dn6SX_Fpi0ONLwerBqwzm86dr4kkrutGDNTB2QJSEqsABfIP2HQ
- Arriola CS, Kim L, Langley G, Anderson EJ, Openo K, Martin AM, Lynfield R, Bye E, Como-Sabetti K, Reingold A, et al. Estimated burden of community-onset respiratory syncytial virus–associated hospitalizations among children aged <2 years in the United States. J Pediatric Infect Dis Soc. 2020;9(5):587–95. doi:10.1093/jpids/piz087.
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. [Internet] Available from. 2003;289(2):179–86.
- Bergeron HC, Tripp RA. Emerging small and large molecule therapeutics for respiratory syncytial virus. Expert Opin Investig Drugs. 2020;29(3):285–94. doi:10.1080/13543784.2020.1735349.
- Hatter L, Eathorne A, Hills T, Bruce P, Beasley R. Respiratory syncytial virus: paying the immunity debt with interest. Lancet Child Adolesc Health. 2021;5(12):e44–5. doi:10.1016/S2352-4642(21)00333-3.
- di Mattia G, Nenna R, Mancino E, Rizzo V, Pierangeli A, Villani A, Midulla F. During the COVID-19 pandemic where has respiratory syncytial virus gone? Pediatr Pulmonol. 2021;56(10):3106–09. doi:10.1002/ppul.25582.
- Mejias A, Rodríguez-Fernández R, Oliva S, Peeples ME, Ramilo O. The journey to a respiratory syncytial virus vaccine. Ann Allergy Asthma Immunol. 2020;125(1):36–46. doi:10.1016/j.anai.2020.03.017.
- Lively JY, Curns AT, Weinberg GY, Edwards KM, Staat MA, Prill MM, Gerber SI, Lengley GE. Respiratory syncytial virus–associated outpatient visits among children younger than 24 months. J Pediatric Infect Dis Soc. 2019;8(3):284. doi:10.1093/jpids/piz011.
- Rainisch G, Adhikari B, Meltzer MI, Langley G. Estimating the impact of multiple immunization products on medically-attended respiratory syncytial virus (RSV) infections in infants. Vaccine. 2020;38(2):251–57. doi:10.1016/j.vaccine.2019.10.023.
- Tramuto F, Maida CM, di Naro D, Randazzo G, Vitale F, Restivo V, Costantino C, Amodio E, Casuccio A, Graziano G, et al. Respiratory syncytial virus: new challenges for molecular epidemiology surveillance and vaccination strategy in patients with ILI/SARI. Vaccines (Basel). 2021;9(11):1334. doi:10.3390/vaccines9111334.
- Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30(1):277–319. doi:10.1128/CMR.00010-16.
- Sullender WM. Respiratory syncytial virus genetic and antigenic diversity. 2000.
- Mastrangelo P, Chin AA, Tan S, Jeon AH, Ackerley CA, Siu KK, Lee JE, Hegele RG. Identification of RSV fusion protein interaction domains on the virus receptor, nucleolin. Viruses. 2021;13(2):261. doi:10.3390/v13020261.
- Battles MB, McLellan JS. Respiratory syncytial virus entry and how to block it. Nat Rev Microbiol. 2019;17(4):233–45. doi:10.1038/s41579-019-0149-x.
- San-Juan-Vergara H, Peeples ME. Importance of virus characteristics in respiratory syncytial virus-induced disease. Immunol Allergy Clin North Am. 2019;39(3):321–34. doi:10.1016/j.iac.2019.04.001.
- Weber A, Weber M, Milligan P. Modeling epidemics caused by respiratory syncytial virus (RSV). Math Biosci [Internet]. 2001;172(2):95–113. www.elsevier.com/locate/mbsMathematicalBiosciences172
- Feng Z, Xu L, Xie Z. Receptors for respiratory syncytial virus infection and host factors regulating the life cycle of respiratory syncytial virus. Front Cell Infect Microbiol [Internet]. 2022;12. https://www.frontiersin.org/articles/10.3389/fcimb.2022.858629/full
- King T, Mejias A, Ramilo O, Peeples ME, Pietschmann T. The larger attachment glycoprotein of respiratory syncytial virus produced in primary human bronchial epithelial cultures reduces infectivity for cell lines. PLoS Pathog. 2021;17(4):e1009469. doi:10.1371/journal.ppat.1009469.
- Tripp RA, Jones LP, Haynes LM, Zheng H, Murphy PM, Anderson LJ. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nature Immunology [Internet]. 2001;2(8):732–38. http://immunol.nature.com
- Pinto RA, Arredondo SM, Bono MR, Gaggero AA, Díaz PV. T helper 1/T helper 2 cytokine imbalance in respiratory syncytial virus infection is associated with increased endogenous plasma cortisol. Pediatrics. 2006;117(5):117. doi:10.1542/peds.2005-2119.
- Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev. 2017;30(2):481–502. doi:10.1128/CMR.00090-16.
- Kombe IK, Agoti CN, Munywoki PK, Baguelin M, Nokes DJ, Medley GF. Integrating epidemiological and genetic data with different sampling intensities into a dynamic model of respiratory syncytial virus transmission. Sci Rep. 2021;11(1):11. doi:10.1038/s41598-021-81078-x.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis practice gaps. Pediatr Rev. 2014;35(12):519–30. doi:10.1542/pir.35.12.519.
- Biagi C, Dondi A, Scarpini S, Rocca A, Vandini S, Poletti G, Lanari M. Current state and challenges in developing respiratory syncytial virus vaccines. Vaccines (Basel). 2020;8(4):672. doi:10.3390/vaccines8040672.
- Tognarelli EI, Bueno SM, González PA. Immune-Modulation by the human respiratory syncytial virus: focus on dendritic cells. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.00810.
- Graham BS. Vaccine development for respiratory syncytial virus. Curr Opin Virol. 2017;23:107–12. doi:10.1016/j.coviro.2017.03.012.
- Graham BS, Modjarrad K, McLellan JS. Novel antigens for RSV vaccines. Curr Opin Immunol. 2015;35:30–38. doi:10.1016/j.coi.2015.04.005.
- Maruz NI, Horsley NM, Englund JA, Nederend M, Magaret A, Kumar A, Jacobino SR, de Haan CAM, Khatry SK, LeClerq SC, et al. Breast milk prefusion F IgG as a correlate of protection against respiratory syncytial virus acute respiratory illness. J Infect Dis. 2019;219(1):59–67. doi:10.1093/infdis/jiy477.
- Kulkarni PS, Hurwitz JL, Simões EAF, Piedra PA. Establishing correlates of protection for vaccine development: considerations for the respiratory syncytial virus vaccine field. Viral Immunol. 2018;31(2):195–203. doi:10.1089/vim.2017.0147.
- Rossey I, McLellan JS, Saelens X, Schepens B. Clinical potential of prefusion RSV F-specific antibodies. Trends Microbiol. 2018;26(3):209–19. doi:10.1016/j.tim.2017.09.009.
- Patel N, Massare MJ, Tian JH, Guebre-Xabier M, Lu H, Zhou H, Maynard E, Scott D, Ellingsworth L, Glenn G, et al. Respiratory syncytial virus prefusogenic fusion (F) protein nanoparticle vaccine: structure, antigenic profile, immunogenicity, and protection. Vaccine. 2019;37(41):6112–24. doi:10.1016/j.vaccine.2019.07.089.
- Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med. 2011;17(9):1132–35. doi:10.1038/nm.2444.
- Weiner JH. Respiratory syncytial virus infection and palivizumab: are families receiving accurate information? Am J Perinatol. 2010;27(3):219–23. doi:10.1055/s-0029-1239493.
- Kishko M, Catalan J, Swanson K, DiNapoli J, Wei CJ, Delagrave S, Chivukula S, Zhang L. Evaluation of the respiratory syncytial virus G-directed neutralizing antibody response in the human airway epithelial cell model. Virology. 2020;550:21–26. doi:10.1016/j.virol.2020.08.006.
- Fedechkin SO, George NL, Wolff JT, Kauvar LM, Dubois RM Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies [Internet]. 2018. http://immunology.sciencemag.org/
- Pica N, Bouvier NM. Environmental factors affecting the transmission of respiratory viruses. Curr Opin Virol. 2012;2(1):90–95. doi:10.1016/j.coviro.2011.12.003.
- Taleb SA, Al-Ansari K, Nasrallah GK, Elrayess MA, Al-Thani AA, Derrien-Colemyn A, Ruckwardt TJ, Graham BS, Yassine HM. Level of maternal respiratory syncytial virus (RSV) F antibodies in hospitalized children and correlates of protection. Int J Infect Dis. 2021;109:56–62. doi:10.1016/j.ijid.2021.06.015.
- Nourbakhsh S, Shoukat A, Zhang K, Poliquin G, Halperin D, Sheffield H, Halperin SA, Langley JM, Moghadas SM. Effectiveness and cost-effectiveness of RSV infant and maternal immunization programs: a case study of Nunavik, Canada. eClinicalMedicine. 2021;41:101141. doi:10.1016/j.eclinm.2021.101141.
- Buchwald AG, Graham BS, Traore A, Haidara FC, Chen M, Morabito K, Lin BC, Sow SO, Levine MM, Pasetti MF, et al. Respiratory Syncytial Virus (RSV) neutralizing antibodies at birth predict protection from RSV illness in infants in the first 3 months of life. Clin Infect Dis. 2021;73(11):e4421–7. doi:10.1093/cid/ciaa648.
- Jacoby P, Glass K, Moore HC. Characterizing the risk of respiratory syncytial virus in infants with older siblings: a population-based birth cohort study. Epidemiol Infect. 2017;145(2):266–71. doi:10.1017/S0950268816002545.
- Gabutti G, Azzari C, Bonanni P, Prato R, Tozzi AE, Zanetti A, Zuccotti G. Pertussis. Hum Vaccin Immunother [Internet]. 2015;11(1):108–17. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4514233&tool=pmcentrez&rendertype=abstract
- Meregaglia M, Ferrara L, Melegaro A, Demicheli V. Parent “cocoon” immunization to prevent pertussis-related hospitalization in infants: the case of Piemonte in Italy. Vaccine [Internet]. 2013;31(8):1135–37. doi:http://dx.doi.org/10.1016/j.vaccine.2012.12.061.
- Esposito S, Principi N. Prevention of pertussis: an unresolved problem. Human Vaccines Immunother. [Internet]. 2018;5515:1–27. https://www.tandfonline.com/doi/full/10.1080/21645515.2018.1480298
- Riccò M, Vezzosi L, Gualerzi G, Bragazzi NL, Balzarini F Pertussis immunization in healthcare workers working in pediatric settings: knowledge, attitudes and practices (KAP) of occupational physicians. Preliminary results from a web-based survey (2017). J Prev Med Hyg 2020:19–21.
- Reis J, Shaman J. Simulation of four respiratory viruses and inference of epidemiological parameters. Infect Dis Model. 2018;3:23–34. doi:10.1016/j.idm.2018.03.006.
- Reis J, Shaman J, Wilke CO. Retrospective parameter estimation and forecast of respiratory syncytial virus in the United States. PLoS Comput Biol. 2016;12(10):e1005133. doi:10.1371/journal.pcbi.1005133.
- Byington CL, Wilkes J, Korgenski K, Sheng X. Respiratory syncytial virus–associated mortality in hospitalized infants and young children. Pediatrics. 2015;135(1):e24–31. doi:10.1542/peds.2014-2151.
- Berbers G, Mollema L, van der Klis F, den Hartog G, Schepp R. Antibody responses to respiratory syncytial virus: a cross-sectional serosurveillance study in the Dutch population focusing on infants younger than 2 years. J Infect Dis. 2021;224(2):269–78. doi:10.1093/infdis/jiaa483.
- Suleiman-Martos N, Caballero-Vázquez A, Gómez-Urquiza JL, Albendín-García L, Romero-Béjar JL, Cañadas-De la Fuente GA. Prevalence and risk factors of respiratory syncytial virus in children under 5 years of age in the who European region: a systematic review and meta-analysis. J Pers Med. 2021;11(5):416. doi:10.3390/jpm11050416.
- Cusi MG, Terrosi C, Kleines M, Schildgen O. RSV and HMPV seroprevalence in Tuscany (Italy) and North-Rhine Westfalia (Germany) in the winter season 2009/2010. Influenza Other Respir Viruses. 2011;5(6):380–81. doi:10.1111/j.1750-2659.2011.00252.x.
- Haynes AK, Prill MM, Iwane MK, Gerber SI. Respiratory syncytial virus—United States, July 2012–June 2014. Morb Mortl Wkly Rep MMWR [Internet]. 2014;63:1133–36. http://www.cdc.gov/surveillance/nrevss
- Rose EB, Wheatley A, Langley G, Gerber S, Haynes A. Respiratory syncytial virus seasonality — United States, 2014–2017. Morb Mortal Wkly Rep. 2018;67(2):71–76. doi:10.15585/mmwr.mm6702a4.
- Kenmoe S, Bigna JJ, Well EA, Simo FBN, Penlap VB, Vabret A, Njouom R. Prevalence of human respiratory syncytial virus infection in people with acute respiratory tract infections in Africa: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2018;12(6):793–803. doi:10.1111/irv.12584.
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE Respiratory syncytial virus infection in elderly and high-risk adults [Internet]. 2005. doi:10.1056/NEJMoa043951.
- Shi T, Arnott A, Semogas I, Falsey AR, Openshaw P, Wedzicha JA, Campbell H, Nair H, Nair H, Campbell H. The etiological role of common respiratory viruses in acute respiratory infections in older adults: a systematic review and meta-analysis. J Infect Dis. 2020;222(Supplement_7):S563–9. doi:10.1093/infdis/jiy662.
- Choi Y, Hill-Ricciuti A, Branche AR, Sieling WD, Saiman L, Walsh EE, Phillips M, Falsey AR, Finelli L. Cost determinants among adults hospitalized with respiratory syncytial virus in the United States, 2017-2019. Influenza Other Respir Viruses. 2021;16(1):151–58. doi:10.1111/irv.12912.
- Paes BA Mitchell I Banerji A Lanctôt KL, Langley JM. A decade of respiratory syncytial virus epidemiology and prophylaxis: translating evidence into everyday clinical practice case presentation. Can Respir J. 2011;18(2):e10–e19. doi:10.1155/2011/493056.
- Auvinen R, Syrjänen R, Ollgren J, Nohynek H, Skogberg K. Clinical characteristics and population-based attack rates of respiratory syncytial virus versus influenza hospitalizations among adults-an observational study. Influenza Other Respir Viruses. Onlineahead of print. 2021;16(2):276–88. doi:10.1111/irv.12914.
- Hall CB, Long CE, Schnabel KC. Respiratory syncytial virus infections in previously healthy working adults. Clin Infect Dis [Internet]. 2001;33(6):792–96.
- Calderaro A, de Conto F, Buttrini M, Piccolo G, Montecchini S, Maccari C, Martinelli M, di Maio A, Ferraglia F, Pinardi F, et al. Human respiratory viruses, including SARS-CoV-2, circulating in the winter season 2019–2020 in Parma, Northern Italy. Int J Infect Dis. 2021;102:79–84. doi:10.1016/j.ijid.2020.09.1473.
- Sherman AC, Babiker A, Sieben AJ, Pyden A, Steinberg J, Kraft CS, Koelle K, Kanjilal S. The effect of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) mitigation strategies on seasonal respiratory viruses: a tale of 2 large metropolitan centers in the United States. Clin Infect Dis. 2021;72(5):E154–7. doi:10.1093/cid/ciaa1704.
- Kuitunen I, Artama M, Mäkelä L, Backman K, Heiskanen-Kosma T, Renko M. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. 2020;39(12):E423–7. doi:10.1097/INF.0000000000002845.
- van Brusselen D, de Troeyer K, Ter Haar E, Vander Auwera A, Poschet K, van Nuijs S, Bael A, Stobbelaar K, Verhulst S, van Herendael B, et al. Bronchiolitis in COVID-19 times: a nearly absent disease? Eur J Pediatr [Internet]. 2021;180(6):1969–73. https://www.cdc.gov/coronavirus/2019-ncov/more/
- Britton PN, Hu N, Saravanos G, Shrapnel J, Davis J, Snelling T, Dalby-Payne J, Kesson AM, Wood N, Macartney K, et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Health. 2020;4(11):e42–3. doi:10.1016/S2352-4642(20)30307-2.
- Foley DA, Phuong LK, Peplinski J, Lim SM, Lee WH, Farhat A, Minney-SmithCA, Martin AC, Mace AO, Sikazwe CT, et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch Dis Child. 2022 Mar;107(3):e7. doi:10.1136/archdischild-2021-322507.
- Foley DA, Yeoh DK, Minney-Smith CA, Martin AC, Mace AO, Sikazwe CT, Le H, Levy A, Moore HC, Blyth CC. The interseasonal resurgence of respiratory syncytial virus in Australian children following the reduction of coronavirus disease 2019–related public health measures. Clin Infect Dis. 2021;73(9):E2829–30. doi:10.1093/cid/ciaa1906.
- Varela FH, Scotta MC, Polese-Bonatto M, Sartor ITS, Ferreira CF, Fernandes IR, Zavaglia GO, de Almeida WAF, Arakaki-Sanchez D, Pinto LA, et al. Absence of detection of RSV and influenza during the COVID-19 pandemic in a Brazilian cohort: likely role of lower transmission in the community. J Glob Health. 2021;11:05007. doi:10.7189/jogh.11.05007.
- Ippolito G, la Vecchia A, Umbrello G, di Pietro G, Bono P, Scalia S, Pinzani R, Tagliabue C, Bosis S, Agostoni C, et al. Disappearance of seasonal respiratory viruses in children under two years old during COVID-19 pandemic: a monocentric retrospective study in Milan, Italy. Front Pediatr. 2021;9:721005. doi:10.3389/fped.2021.721005.
- Ujiie M, Tsuzuki S, Nakamoto T, Iwamoto N, Ujiie M. Resurgence of respiratory syncytial virus infections during COVID-19 pandemic, Tokyo, Japan. Emerg Infect Dis. 2021;27(11):2969–70. doi:10.3201/eid2711.211565.
- Lumley SF, Richens N, Lees E, Cregan J, Kalimeris E, Oakley S, Morgan M, Segal S, Dawson M, Walker AS, et al. Changes in paediatric respiratory infections at a UK teaching hospital 2016-2021; impact of the SARS-CoV-2 pandemic. J Infect [Internet]. 2021. https://linkinghub.elsevier.com/retrieve/pii/S0163445321005405
- Taylor A, Whittaker E. The changing epidemiology of respiratory viruses in children during the COVID-19 pandemic: a canary in a COVID time. Pediatr Infect Dis J. 2022;41(2):E46–8. doi:10.1097/INF.0000000000003396.
- Li Y, Wang X, Cong B, Deng S, Feikin DR, Nair H Understanding the potential drivers for respiratory syncytial virus rebound during the COVID-19 pandemic.
- Sánchez Luna M, Manzoni P, Paes B, Baraldi E, Cossey V, Kugelman A, Chawla R, Dotta A, Rodríguez Fernández R, Resch B, et al. Expert consensus on Palivizumab use for respiratory syncytial virus in developed countries. Paediatr Respir Rev. 2020;33:35–44. doi:10.1016/j.prrv.2018.12.001.
- Bloomfield A, DeVincenzo JP, Ambrose CS, Krilov LR. RSV and non-RSV illness hospitalization in RSV immunoprophylaxis recipients: a systematic literature review. J Clin Virol. 2020;129:104339. doi:10.1016/j.jcv.2020.104339.
- Rocca A, Biagi C, Scarpini S, Dondi A, Vandini S, Pierantoni L, Lanari M. Passive immunoprophylaxis against respiratory syncytial virus in children: where are we now? Int J Mol Sci. 2021;22(7):3703. doi:10.3390/ijms22073703.
- Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, Simões EAF, Esser MT, Khan AA, Dubovsky F, et al. Single-Dose Nirsevimab for prevention of RSV in preterm infants. N Engl J Med. 2020;383(5):415–25. doi:10.1056/NEJMoa1913556.
- Shan J, Britton PN, King CL, Booy R. The immunogenicity and safety of respiratory syncytial virus vaccines in development: a systematic review. Influenza Other Respir Viruses. 2021;15(4):539–51. doi:10.1111/irv.12850.
- World Health Organization (WHO). WHO preferred product characteristics for respiratory syncytial virus (RSV) vaccines [Internet]. Geneva; 2017. http://apps.who.int/bookorders
- Gunatilaka A, Giles ML. Maternal RSV vaccine development. Where to from here? Hum Vaccin Immunother. 2021;17(11):4542–48. doi:10.1080/21645515.2021.1955608.
- ClinicalTrials.gov. A trial to evaluate the efficacy and safety of RSVpreF in infants born to women vaccinated during pregnancy. 2022.
- ClinicalTrials.gov. A phase III double-blind study to assess safety and efficacy of an RSV maternal unadjuvanted vaccine, in pregnant women and infants born to vaccinated mothers (GRACE). [Internet]. 2022. https://clinicaltrials.gov/ct2/show/NCT04605159?type=Intr&cond=rsv&phase=2&draw=2
- Engmann C, Fleming JA, Khan S, Innis BL, Smith JM, Hombach J, Sobanjo-Ter Meulen A. Closer and closer? Maternal immunization: current promise, future horizons. Journal of Perinatology. 2020;40(6):844–57. doi:10.1038/s41372-020-0668-3.
- Madhi SA, Polack FP, Piedra PA, Munoz FM, Trenholme AA, Simões EAF, Swamy GK, Agrawal S, Ahmed K, August A, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383(5):426–39. doi:10.1056/NEJMoa1908380.
- Lewnard JA, Fries LF, Cho I, Chen J, Laxminarayan R. Prevention of antimicrobial prescribing among infants following maternal vaccination against respiratory syncytial virus. Proc Natl Acad Sci. 2022;119(12):e2112410119. doi:10.1073/pnas.2112410119.
- Phijffer EWEM, Bont LJ. Are we ready for maternal respiratory syncytial virus vaccination? J Infect Dis. 2021. doi:10.1093/infdis/jiab613.
- Walsh EE, Falsey AR, Scott DA, Gurtman A, Zareba AM, Jansen KU, Gruber WC, Dormitzer PR, Swanson KA, Radley D , et al. A randomized phase 1/2 study of a respiratory syncytial virus prefusion F vaccine. J Infect Dis. 2022 Apr 19;225(8):1357–1366. doi:10.1093/infdis/jiab612.
- Scheltema NM, Kavelaars XM, Thorburn K, Hennus MP, van Woensel JB, van der Ent CK, Borghans JAM, Bont LJ, Drylewicz J, van Woensel JB, van der Ent CK. Potential impact of maternal vaccination on life-threatening respiratory syncytial virus infection during infancy. Vaccine. 2018;36(31):4693–700. doi:10.1016/j.vaccine.2018.06.021.
- Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. Effectiveness of maternal pertussis vaccination in England: an observational study. The Lancet [Internet]. 2014;384(9953):1521–28. doi:10.1016/S0140-6736(14)60686-3.
- Gkentzi D, Katsakiori P, Marangos M, Hsia Y, Amirthalingam G, Heath PT, Ladhani S. Maternal vaccination against pertussis: a systematic review of the recent literature. Arch Dis Child Fetal Neonatal Ed. 2017;102(5):F456–63. doi:10.1136/archdischild-2016-312341.
- Fell DB, Bhutta ZA, Hutcheon JA, Karron RA, Knight M, Kramer MS, Monto AS, Swamy GK, Ortiz JR, Savitz DA. Report of the WHO technical consultation on the effect of maternal influenza and influenza vaccination on the developing fetus: Montreal, Canada, September 30–October 1, 2015. Vaccine. [Internet]. 2017;35(18):2279–87. doi:10.1016/j.vaccine.2017.03.056.
- Walsh EE, Wang L, Falsey AR, Qiu X, Corbett A, Holden-Wiltse J, Mariani TJ, Topham DJ, Caserta MT. Virus-Specific antibody, viral load, and disease severity in respiratory syncytial virus infection. J Infect Dis. 2018;218(2):208–17. doi:10.1093/infdis/jiy106.
- Groothuis JR, Simoes EA, Levin MJ, Hall CB, Long CE, Rodriguez WJ, Arrobio J, Meissner HC, Fulton DR, Welliver RC, et al. Prophylactic administration of respiratory syncytial virus immune globulin to high-risk infants and young children. N Eng J Med. 1993;329(21):1524–30. doi:10.1056/NEJM199311183292102.
- Glezen PW, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98(5):708–15. doi:10.1016/S0022-3476(81)80829-3.
- Jans J, Wicht O, Widjaja I, Ahout IML, de Groot R, Guichelaar T, Luytjes W, de Jonge MI, de Haan CAM, Ferwerda G. Characteristics of RSV-specific maternal antibodies in plasma of hospitalized, acute RSV patients under three months of age. PLoS ONE. 2017;12(1):e0170877. doi:10.1371/journal.pone.0170877.
- Chu HY, Newman KL, Englund JA, Cho S, Bull C, Lacombe K, Carlin K, Bulkow LR, Rudolph K, Debyle C, et al. Transplacental respiratory syncytial virus and influenza virus antibody transfer in Alaska native and Seattle Mother–Infant Pairs. J Pediatric Infect Dis Soc. 2021;10(3):230–36. doi:10.1093/jpids/piaa040.
- Chu HY, Tielsch J, Katz J, Magaret AS, Khatry S, LeClerq SC, Shrestha L, Kuypers J, Steinhoff MC, Englund JA. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. Journal of Clinical Virology. 2017;95:90–95. doi:10.1016/j.jcv.2017.08.017.
- Lloyd PC, May L, Hoffman D, Riegelman R, Simonsen L. The effect of birth month on the risk of respiratory syncytial virus hospitalization in the first year of life in the United States. Pediatr Infect Dis J. 2014;33(6):e135–40. doi:10.1097/INF.0000000000000250.
- Yildiz M, Kara M, Sutcu M, Mese S, Demircili ME, Sivrikoz TS, Torun SH, Agacfidan A, Coban A, Unuvar E, et al. Evaluation of respiratory syncytial virus IgG antibody dynamics in mother-infant pairs cohort. Eur J Clin Microbiol Infect Dis. [Internet]. 2020;39(7):1279–86. doi:10.1007/s10096-020-03841-8.
- Chu HY, Steinhoff MC, Magaret A, Zaman K, Roy E, Langdon G, Formica MA, Walsh EE, Englund JA. Respiratory syncytial virus transplacental antibody transfer and kinetics in Mother-Infant pairs in Bangladesh. J Infect Dis. 2014;210(10):1582–89. doi:10.1093/infdis/jiu316.
- Wesumperuma HL, Pharoah POD, Hart CA, Hart CA. The influence of prematurity and low birthweight on transplacental antibody transfer in Sri Lanka. Ann Trop Med Parasitol. 1999;93(2):169–77. doi:10.1080/00034983.1999.11813407.
- van den Berg JP, Westerbeek EAM, van der Klis FRM, Berbers GAM, van Elburg RM. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev. 2011;87(2):67–72. doi:10.1016/j.earlhumdev.2010.11.003.
- Okoko JB, Wesumperuma HL, Hart CA. The influence of prematurity and low birthweight on transplacental antibody transfer in a rural West African population. Trop Med Int Health. 2001;6(7):529–34. doi:10.1046/j.1365-3156.2001.00741.x.
- Schwarz TF, Johnson C, Grigat C, Apter D, Csonka P, Lindblad N, Nguyen T-A, Gao FF, Qian H, Tullio AN, et al. Three dose levels of a maternal respiratory syncytial virus vaccine candidate are well tolerated and immunogenic in a randomized trial in nonpregnant women. J Infect Dis. 2021. doi:10.1093/infdis/jiab317.
- Takashima MD, Grimwood K, Sly PD, Lambert SB, Chappell KJ, Watterson D, Young P, Kusel M, Holt B, Holt P, et al. Cord-Blood respiratory syncytial virus antibodies and respiratory health in first 5 years of life. Pediatr Pulmonol. 2021;56(12):3942–51. doi:10.1002/ppul.25688.
- Koivisto K, Nieminen T, Mejias A, Capella Gonzalez C, Ye F, Mertz S, Peeples M, Ramilo O, Saxén H . Respiratory syncytial virus (RSV)–specific antibodies in pregnant women and subsequent risk of RSV hospitalization in young infants. J Infect Dis 2022 Apr 1;225(7): 1189–1196. doi:10.1093/infdis/jiab315.
- Giles ML, Buttery J, Davey MA, Wallace E. Pregnant women’s knowledge and attitude to maternal vaccination including group B streptococcus and respiratory syncytial virus vaccines. Vaccine. 2019;37(44):6743–49. doi:10.1016/j.vaccine.2019.08.084.
- Wheeler SM, Dotters-Katz S, Heine RP, Grotegut CA, Swamy GK. Maternal effects of respiratory syncytial virus infection during pregnancy. Emerg Infect Dis. 2015;21(11):1951–55. doi:10.3201/eid2111.150497.
- Hause AM, Panagiotakopoulos L, Weintraub ES, Sy LS, Glenn SC, Tseng HF, McNeil MM. Adverse outcomes in pregnant women hospitalized with respiratory syncytial virus infection: a case series. Clin Infect Dis. 2021;72(1):138–40. doi:10.1093/cid/ciaa668.
- Regan AK, Klein NP, Langley G, Drews SJ, Buchan S, Ball S, Kwong JC, Naleway A, Thompson M, Wyant BE, et al. Respiratory syncytial virus hospitalization during pregnancy in 4 high-income countries, 2010–2016. Clin Infect Dis. 2018;67(12):1915–18. doi:10.1093/cid/ciy439.
- Madhi SA, Cutland CL, Downs S, Jones S, van Niekerk N, Simoes EAF, Nunes MC. Burden of respiratory syncytial virus infection in South African Human Immunodeficiency Virus (HIV)-infected and HIV-uninfected pregnant and postpartum women: a longitudinal cohort study. Clin Infect Dis. 2018;66(11):1658–65. doi:10.1093/cid/cix1088.
- Wilcox CR, Calvert A, Metz J, Kilich E, Macleod R, Beadon K, Heath PT, Khalil A, Finn A, Snape MD, et al. Attitudes of pregnant women and healthcare professionals toward clinical trials and routine implementation of antenatal vaccination against respiratory syncytial virus: a multicenter questionnaire study. Pediatr Infect Dis J. 2019;38(9):944–51. doi:10.1097/INF.0000000000002384.
- Bergeron HC, Tripp RA . Breakthrough therapy designation of nirsevimab for the prevention of lower respiratory tract illness caused by respiratory syncytial virus infections (RSV). Expert Opin Investig Drugs. 2022 Jan;31(1): 23–29. doi:10.1080/13543784.2022.2020248.
- Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA, Muller WJ, Zar HJ, Brooks D, Grenham A, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. New England J Med [Internet]. 2022;386(9):837–46. http://www.nejm.org/doi/10.1056/NEJMoa2110275
- Domachowske J, Madhi SA, Simões EAF, Atanasova V, Cabañas F, Furuno K, Garcia-Garcia ML, Grantina I, Nguyen KA, Brooks D, et al. Safety of Nirsevimab for RSV in infants with heart or lung disease or prematurity. New England J Med [Internet]. 2022;386(9):892–94. http://www.nejm.org/doi/10.1056/NEJMc2112186
- Li X, Bilcke J, Vázquez Fernández L, Bont L, Willem L, Wisløff T, Jit M, Beutels P, Beutels P, Bont L, et al. Cost-Effectiveness of respiratory syncytial virus disease prevention strategies: maternal vaccine versus seasonal or year-round monoclonal antibody program in Norwegian children. J Infect Dis [Internet]. 2022. https://academic.oup.com/jid/advance-article/doi/10.1093/infdis/jiac064/6549175
- Ananworanich J, Heaton PM. Bringing preventive RSV monoclonal antibodies to infants in low- and middle-income countries: challenges and opportunities. Vaccines (Basel). 2021;9(9). doi:10.3390/vaccines9090961.
- Sparrow, E, Adetifa, I, Chaiyakunapruk, N, Cherian, T, Fell, DB, Graham, BS, Innis, B, Kaslow, DC, Karron, RA, Nair, H et al . WHO preferred product characteristics for monoclonal antibodies for passive immunization against respiratory syncytial virus (RSV) disease in infants - Key considerations for global use. Vaccine. Geneva;2022 Feb 17:S0264-410X(22)00186-4. doi:10.1016/j.vaccine.2022.02.040
- Lorcy A, Gilca R, Dubé E, Rochette M, de Serres G. Feasibility and ethical issues: experiences and concerns of healthcare workers regarding a new RSV prophylaxis programme in Nunavik, Quebec. Int J Circumpolar Health. 2020;79(1). doi:10.1080/22423982.2020.1742564.
- Frogel MP, Stewart DL, Hoopes M, Fernandes AW, Mahadevia PJ. A systematic review of compliance with Palivizumab Administration for RSV immunoprophylaxis. J Managed Care Pharm [Internet]. 2010;16(1):46–58. www.amcp.org.
- Langedijk AC, Harding ER, Konya B, Vrancken B, Lebbink RJ, Evers A, Willemsen J, Lemey P, Bont LJ. A systematic review on global RSV genetic data: identification of knowledge gaps. Rev Med Virol. 2021. doi:10.1002/rmv.2284.
- Anderson LJ, Jadhao SJ, Paden CR, Tong S. Functional features of the respiratory syncytial virus G protein. Viruses. 2021;13(7):1214. doi:10.3390/v13071214.
- Esposito S, Scarselli E, Lelii M, Scala A, Vitelli A, Capone S, Fornili M, Biganzoli E, Orenti A, Nicosia A, et al. Antibody response to respiratory syncytial virus infection in children <18 months old. Hum Vaccin Immunother. 2016;12(7):1700–06. doi:10.1080/21645515.2016.1145847.
- Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, et al. Respiratory syncytial virus–associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132(2):e341–8. doi:10.1542/peds.2013-0303.
- Domachowske JB, Anderson EJ, Goldstein M. The future of respiratory syncytial virus disease prevention and treatment. Infect Dis Ther. 2021;10(S1):47–60. doi:10.1007/s40121-020-00383-6.
- Aliprantis AO, Shaw CA, Griffin P, Farinola N, Railkar RA, Cao X, Liu W, Sachs JR, Swenson CJ, Lee H, et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum Vaccine Immunother. 2021;17(5):1248–61. doi:10.1080/21645515.2020.1829899.
- Staadegaard L, Caini S, Wangchuk S, Thapa B, de Almeida WAF, de Carvalho FC, Njouom R, Fasce RA, Bustos P, Kyncl J, et al. The global epidemiology of RSV in community and hospitalized care: findings from 15 countries. Open Forum Infect Dis. 2021;8(7):ofab159. doi:10.1093/ofid/ofab159.
- Lanari M, Anderson EJ, Sheridan-Pereira M, Carbonell-Estrany X, Paes B, Rodgers-Gray BS, Fullarton JR, Grubb E, Blanken M. Burden of respiratory syncytial virus hospitalisation among infants born at 32-35 weeks’ gestational age in the Northern Hemisphere: pooled analysis of seven studies. Epidemiol Infect. 2020;148:e170. doi:10.1017/S0950268820001661.
- Halabi KC, Saiman L, Zachariah P. The epidemiology of respiratory syncytial virus in New York City during the COVID-19 pandemic compared with previous years. J Pediatr. 2022;242:242–44. doi:10.1016/j.jpeds.2021.10.057.
- Seale H, Dyer CEF, Abdi I, Rahman KM, Sun Y, Qureshi MO, Dowell-Day A, Sward J, Islam MS. Improving the impact of non-pharmaceutical interventions during COVID-19: examining the factors that influence engagement and the impact on individuals. BMC Infect Dis. 2020;20(1):20. doi:10.1186/s12879-020-05340-9.
- Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163(4):693–98. doi:10.1093/infdis/163.4.693.
- Buchy P, Badur S, Kassianos G, Preiss S, Tam JS. Vaccinating pregnant women against influenza needs to be a priority for all countries: an expert commentary. Int J Infect Dis. 2020;92:1–12. doi:10.1016/j.ijid.2019.12.019.
- Baïssas T, Boisnard F, Cuesta Esteve I, Garcia Sánchez M, Jones CE, Rigoine de Fougerolles T, Tan L, Vitoux O, Klein C. Vaccination in pregnancy against pertussis and seasonal influenza: key learnings and components from high-performing vaccine programmes in three countries: the United Kingdom, the United States and Spain. BMC Public Health. 2021;21(1). doi:10.1186/s12889-021-12198-2.
- Zylbersztejn A, Almossawi O, Gudka N, Tompsett D, de Stavola B, Standing JF, Smyth R, Hardelid P. Access to palivizumab among children at high risk of respiratory syncytial virus complications in English hospitals. Br J Clin Pharmacol. 2022;88(3):1246–57. doi:10.1111/bcp.15069.
- Chida-Nagai A, Sato H, Sato I, Shiraishi M, Sasaki D, Izumi G, Yamazawa H, Cho K, Manabe A, Takeda A. Risk factors for hospitalisation due to respiratory syncytial virus infection in children receiving prophylactic palivizumab. Eur J Pediatr. 2022;181(2):539–47. doi:10.1007/s00431-021-04216-7.
- Viguria N, Navascués A, Juanbeltz R, Echeverría A, Ezpeleta C, Castilla J. Effectiveness of palivizumab in preventing respiratory syncytial virus infection in high-risk children. Hum Vaccin Immunother. 2021;17(6):1867–72. doi:10.1080/21645515.2020.1843336.
- Rossey I, Saelens X. Vaccines against human respiratory syncytial virus in clinical trials, where are we now? Expert Rev Vaccines. 2019;18(10):1053–67. doi:10.1080/14760584.2019.1675520.
