ABSTRACT
Greece introduced a 13-valent pneumococcal conjugate vaccine (PCV13) into the infant national immunization program in 2010 (3 + 1 schedule until June 2019). Since 2015, PCV13 has been recommended for adults aged 19–64 years with comorbidities and adults ≥65 years sequentially with 23-valent pneumococcal polysaccharide vaccine (PPSV23). We examined pneumococcal serotype distribution among Greek adults aged ≥19 years hospitalized with community-acquired pneumonia (CAP) during November 2017-April 2019. This was an interim analysis of EGNATIA, a prospective study of adult hospitalized CAP in the cities of Ioannina and Kavala. Pneumococcus was identified using cultures, BinaxNow®, serotype-specific urinary antigen detection assays (UAD-1/2). Our analysis included overall 482 hospitalized CAP patients (mean age: 70.5 years; 56.4% male). 53.53% of patients belonged to the highest pneumonia severity index (PSI) classes (IV-V). Pneumococcus was detected in 65 (13.5%) patients, with more than half (57%) of cases detected only by UAD. Approximately two-thirds of pneumococcal CAP occurred in those aged ≥65 years (n = 40, 8.3% of CAP). More than half of pneumococcal CAP (n = 35, 53.8%) was caused by PCV13 serotypes. Most frequently detected PCV13 serotypes were 3, 19A, 23F, collectively accounting for 83% of PCV13 vaccine-type (VT) CAP and 6% of all-cause CAP. Overall, 82.9% of PCV13 VT CAP occurred among persons with an indication (age/risk-based) for PCV13 vaccination. Even with a mature PCV13 childhood immunization program, a persistent burden of PCV13 VT CAP exists in Greek adults. Strategies to increase PCV13 (and higher-valency PCVs, when licensed) coverage in adults should be implemented to reduce the disease burden.
HIGHLIGHTS
An interim analysis of a prospective study in adults hospitalized with CAP in Greece.
Serotype-specific urinary antigen detection assays were used to detect pneumococcus.
A persistent burden of PCV13 vaccine-type CAP was observed in Greek adults.
Improved PCV13 uptake and higher-valency PCVs may reduce the pneumococcal disease burden.
Introduction
Community-acquired pneumonia (CAP) is one of the leading causes of morbidity and mortality in adults, especially in the elderly and in those with comorbidities, including chronic respiratory disease, chronic heart disease, diabetes mellitus, and immunocompromising conditions.Citation1,Citation2 Streptococcus pneumoniae (pneumococcus) is the most frequent cause of bacterial CAP.Citation3 However, due to the lack of highly sensitive diagnostic methods, the prevalence of pneumococcal CAP, especially non-bacteremic disease, is underestimated.Citation4,Citation5
The epidemiology of pneumococcal disease has largely shifted following the introduction of pneumococcal conjugate vaccines (PCVs) into pediatric populations. Remarkable reductions in invasive pneumococcal disease (IPD) due to vaccine serotypes have been observed not only in children but also in the unvaccinated population through the indirect (herd) effect.Citation6–8 However, serotype replacement in pneumococcal carriage and disease remains a concern.Citation9,Citation10 Furthermore, the reductions in non-bacteremic pneumococcal CAP are limited in adults. Even with longstanding pediatric PCV programs, evidence from several countries suggests that there is persistent vaccine-serotype pneumococcal CAP.Citation4,Citation5,Citation11,Citation12 Considering non-bacteremic pneumococcal CAP is the most common presentation of pneumococcal disease among adults,Citation13 decreases of adult IPD rates do not reflect the true disease burden. One potential approach to addressing residual pneumococcal disease is direct immunization of adults with the 13-valent PCV (PCV13), which has demonstrated efficacy against both IPD and non-bacteremic pneumococcal CAP in the adult population.Citation14
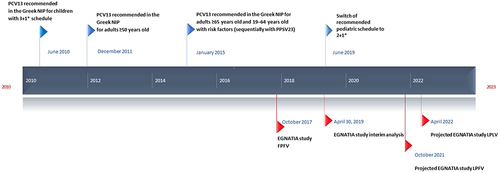
In Greece, PCVs have been available since 2004.Citation15 PCV13 (including serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V9V, 14, 18C, 19F, 19A, 23F) was introduced into the infant national immunization program (NIP) in 2010 (3 + 1 schedule until June 2019 and 2 + 1 thereafter), and in the adult NIP for all adults ≥50 years of age in December 2011 ().Citation15,Citation16 Before that, only the 23-valent pneumococcal polysaccharide vaccine (PPSV23) was recommended to persons with increased risk of pneumococcal infections. In January 2015, the Greek NIP was revised and PCV13 is now recommended together with PPSV23 for all adults ≥65 years of age and for adults 19–64 years of age at increased risk for pneumococcal infections.Citation17 For non-vaccinated adults, it is recommended that they receive PCV13 first, followed by PPSV23. No formal adult vaccination registries exist in Greece, but according to market sales data, as of 2019, PCV13 uptake was estimated to be > 30% in adults >65 years and <10% in adults ≤65 years of age countrywide.
Figure 1. Timeline of Greek PCV13 recommendations and study recruitment period.
Since the launch of PCV13 into the Greek market several years ago, limited data on the epidemiology of CAP are available.Citation18 To gain further insights on CAP epidemiology, this study was designed to determine the remaining burden of pneumococcal CAP and serotype distribution among hospitalized Greek adults. In addition to the standard-of-care diagnostic methods, serotype-specific urinary antigen detection (UAD) and BinaxNow® assays were conducted on research specimens for the identification of pneumococcal CAP cases.
Here, we present the results of the interim analysis covering the first 18 months of the ongoing study. In light of the recent development of higher-valency PCVs, these data may better inform the discussions on pneumococcal epidemiology and the potential vaccination impact.
Patients and methods
Study design
This is an ongoing, prospective, epidemiological, multicenter study in adults ≥19 years of age hospitalized with clinically and radiographically confirmed CAP. This interim analysis included patients enrolled from the first 18 months of the study, i.e., from November 2017 to April 2019, before the outbreak of the coronavirus disease 2019 (COVID-19) pandemic ().
Two cities in Greece—Ioannina and Kavala—were selected as catchment areas to ensure most CAP admissions were included in our study. All hospitals of Ioannina and Kavala, including all relevant departments with routine CAP admissions, participated in the study. The study was approved by the ethics committees at all hospitals and patients signed written informed consent prior to enrollment.
Patients
All adults who presented to the participating hospitals or emergency rooms with signs, symptoms, and radiographic evidence of pneumonia were prospectively identified and screened for inclusion. To be included, patients had to: (1) be aged ≥19 years; (2) present to a participating healthcare facility with suspected pneumonia based on the presence of two or more of the following signs or symptoms: fever or hypothermia within 24 h of enrollment, chills or rigors, pleuritic chest pain, cough, sputum production, dyspnea, tachypnea, malaise, or abnormal auscultatory findings suggestive of pneumonia; (3) have radiographic findings consistent with pneumonia and obtained no more than 72 hours prior to study enrollment; and (4) successfully provide a urine sample.
Patients were excluded if any of the following criteria applied: (1) they were not residents of Ioannina or Kavala; (2) they were transferred to a participating healthcare facility after already having been hospitalized for ≥48 hours at another inpatient facility; (3) they had hospital-acquired pneumonia (defined as pneumonia that developed ≥48 hours after hospital admission); (4) they had been previously enrolled in this study within the past 30 days.
Data on socio-demographic information, medical and vaccination (influenza and pneumococcal vaccines) history, medical treatment, and clinical outcomes were recorded for every patient. For influenza, vaccination history was primarily based on patient-reported vaccine history. For PCV13 and PPSV23, a nationwide e-prescribing system was accessed to ascertain vaccination status, as e-prescribing was expected to be used based on the vaccines’ costs. Pneumonia severity at admission was estimated using the Pneumonia Severity Index and the CURB-65 score. Patients were followed for 10 days or until hospital discharge and subsequently vital status was assessed at 1 month and 6 months post-enrollment.
Risk profile definitions
Patients were classified into risk groups (high-risk, at-risk, and low-risk) based on whether they had certain medical conditions. High-risk conditions included asplenia, cerebrospinal fluid leak, cochlear implant, hemoglobinopathy, immunosuppression therapy, nephrotic syndrome, chronic renal failure, immunodeficiency, human immunodeficiency virus (HIV) infection, acquired immunodeficiency syndrome (AIDS), cancer (including solid tumor, multiple myeloma, and other hematologic cancer treated currently or within the past 5 years), and organ/bone marrow transplantation. At-risk conditions included chronic obstructive pulmonary disease (COPD), asthma, congestive heart failure (CHF), coronary artery disease (CAD), chronic kidney disease [excluding end-stage renal disease (ESRD)], diabetes mellitus, chronic liver disease, autoimmune disorders, cystic fibrosis, tuberculosis in the past, bronchiectasis, pulmonary fibrosis, other chronic pulmonary diseases, cerebrovascular disease, obesity, smoking, and alcohol abuse. The aforementioned conditions are included in the Greek adult NIP recommendations for pneumococcal vaccination, with the exception of cerebrovascular disease and obesity, which have been associated with an increased risk for CAP as reported in other studies.Citation19–21 Patients were classified as low-risk if they were not considered high-risk or at-risk.
Laboratory evaluation
Microbiology tests were performed as part of standard of care. All standard-of-care samples (e.g., blood, respiratory tract, and pleural fluid) were collected and underwent bacterial culture at the local laboratory. All S. pneumoniae isolates were sent to a central laboratory (Infectious Diseases Laboratory of Choremion Research Center, University of Athens) for the confirmation of S. pneumoniae and serotype identification. Pneumococcal serotyping was performed by latex agglutination and the Neufeld-Quellung reaction using antisera from the Statens Serum Institute (Copenhagen, Denmark).
In addition to culture, urine samples from all patients were tested for S. pneumoniae using the BinaxNow assay and the UAD assays. For each urine collection, a specimen was processed at the local laboratory and subsequently shipped to Pfizer’s Vaccines Research and Development Laboratory (Pearl River, NY), where both BinaxNow and the UAD assays were performed.
The UAD assays—termed UAD-1 and UAD-2—are Luminex technology-based multiplex assays that can simultaneously detect multiple S. pneumoniae serotypes in a single human urine sample.Citation22 The original UAD-1 assay detects all 13 serotypes contained in PCV13, while the recently developed UAD-2 assay detects an additional seven serotypes (serotypes 8, 10A, 11A, 12F, 15B, 22F, 33F) in the experimental 20-valent PCV (PCV20) plus serotypes 2, 9N, 17F, and 20. UAD-1/2 assays have been clinically validated and demonstrated high sensitivity and specificity when compared against a gold standard of bacteremic pneumonia.Citation22,Citation23 The UAD assays are not commercially available and have not undergone the FDA approval process. They were designed as a tool for vaccine effectiveness studies and epidemiologic investigations, rather than clinical management. In every study, 400 controls are collected to ensure that the appropriate cutoff points are used for the specific study population. Urine samples from volunteers aged 19 years and older, willing to provide written consent and meeting the exclusion criteria listed in Table S1, comprised the controls in the present study. Control values are set to restrict total control positivity to be less than 2% with 99.73% confidence. Both UAD assays were performed in this study to collectively detect 24 S. pneumoniae serotypes.
Statistical analysis
Statistical analysis was performed based on the number of eligible patients with available data. Descriptive statistics were used to summarize all study data. Continuous variables were summarized with descriptive statistical measures (mean value, standard deviation [SD], minimum value, and maximum value). Categorical variables were displayed as frequency tables (N, %). Differences in mean values of continuous variables were examined using the two tailed t-test or the U-Mann Whitney test for independent samples. Associations between categorical variables were assessed using either χ2 (chi-square test) or Fisher’s exact test as appropriate. No imputation rules for missing data were implemented.
All statistical analyses were performed using SAS® version 9.3 (SAS Institute, Inc., Cary, NC, USA). The proportions of PCV13, 15-valent PCV (PCV15), PCV20 and PPSV23 serotypes as % of all-cause CAP were summarized for all patients, and by age group, risk group, and region. Statistical significance was declared at the <5% level for the comparisons between pneumococcal CAP and non-pneumococcal CAP. The exact p-values were reported.
Results
Patient characteristics
A total of 482 hospitalized patients met the eligibility criteria and were included in the analysis (). In the overall study population, in-hospital mortality was 7.1%. Patients who died in hospital had a mean age (SD) of 85.1 (9.0) years and a mean number (SD) of 2.0 (1.3) comorbidities, the most frequent comorbidities being congestive heart failure, diabetes mellitus, and coronary artery disease.
Table 1. Patient demographics for those with CAP, n = 482.
Of 65 pneumococcal CAP patients, the mean age (SD) was 67.1 (19.3) years and 55.4% were male; of these, 61.5% (n = 40) were at-risk and 10.8% (n = 7) were high-risk. Among all pneumococcal CAP patients, 49.23% (n = 32) fell into the highest PSI risk classes IV-V; the mean duration of hospital stay was 8.5 days and the in-hospital mortality was 10.8%.
No statistically significant differences were observed in CAP severity on admission or in the risk profile between patients with pneumococcal CAP and patients with non-pneumococcal CAP. When compared with non-pneumococcal CAP patients, a statistically significantly lower proportion of pneumococcal CAP patients had received influenza vaccine (18.5% vs. 31.2%, p = 0.036). In addition, pneumococcal CAP patients had a statistically significantly higher 30-day mortality rate (17% vs. 8.9%, p = 0.044) than non-pneumococcal CAP patients.
Pneumococcal CAP
Overall, S. pneumoniae was identified in 65/482 (13.5%) hospitalized CAP patients using UAD assays, BinaxNow, or culture. S. pneumoniae was detected by UAD alone in 37/482 (7.7%) patients (representing 37/65 [57%] of all pneumococcal cases) and by BinaxNow alone in 10/482 (2.1%) patients, while no pneumococcal cases were detected by culture alone (, Table S2). Another 17/482 (3.5%) cases were detected by UAD and BinaxNow, and 1/482 (0.2%) case by culture (this was blood culture) and BinaxNow. With any detection method, S. pneumoniae was detected in 40 (12.2%) patients aged ≥65 years and in 25 (16.2%) patients aged 19–64 years (). Considering risk profiles, S. pneumoniae was detected in 18 (18.2%) patients with low-risk conditions, 40 (12.8%) with at-risk conditions, and 7 (9.9%) with high-risk conditions, respectively. Among the patients who did not have an indication for pneumococcal vaccination (19–64 years of age and low-risk), S. pneumoniae was detected in 10 (19.6%) patients.
Figure 2. Venn diagram for S. pneumoniae detection by different diagnostic methods (serotype-specific urinary antigen detection assay 1/2, BinaxNow, and culture) among 65 patients with radiographically confirmed community-acquired pneumococcal pneumonia.
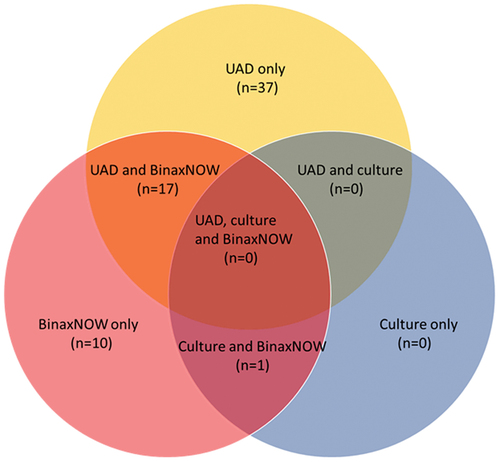
Table 2. Distribution of pneumococcus and pneumococcal serotypes as % of all-cause CAP overall, per age groups and per risk groups*, N (%).
shows S. pneumoniae status by underlying conditions and age groups. Among pneumococcal CAP patients aged 19–64 years, the most common underlying comorbidity was COPD (3/25, 12%), followed by immunocompromising/immunosuppressive conditions, diabetes mellitus, coronary artery disease, and cerebrovascular disease (1/25 each, 4%). For pneumococcal CAP patients aged ≥65 years, common underlying conditions included CAD (12/40, 30%), COPD (7/40, 17.5%), diabetes mellitus, and cerebrovascular disease (5/40 each, 12.5%).
Table 3. Smoking history and the presence of comorbidities* in the pneumococcal and non-pneumococcal CAP groups, overall and per age groups, N (%).
Regarding other pathogens, diagnostic work-up for CAP was performed as per standard of practice. 300/482 (62.2%) patients had at least 1 blood culture performed and 73/482 (15.1%) patients had at least 1 sputum culture performed. The other bacteria identified in CAP patients were Escherichia coli, Enterococcus faecium, and Enterococcus faecalis (one case each), all identified in blood cultures.
Pneumococcal serotype distribution by age and risk profiles
Among pneumococcal CAP patients, the most frequently detected S. pneumoniae serotype was serotype 3 (20 of 482 CAP cases, 4.1%), followed by 19A (6/482, 1.2%), both of which are PCV13 serotypes (). Serotypes 23F, 20, and 9N were also commonly detected (4/482 each, 0.8%). For patients aged 19–64 years, the most common serotype was 3 (11/154, 7.1%), followed by 19A/22F/9N (2/154 each, 1.3%), while among patients aged ≥65 years, common serotypes included 3 (9/328, 2.7%), 19A (4/328, 1.2%), and 23F/8/20 (3/328 each, 0.9%). In addition, of all age groups, serotype 3 was the most frequently identified serotype for those classified as low-risk (6/99, 6.1%) and at-risk (14/312, 4.5%).
Overall, PCV13, PCV15, PCV20, and PPSV23 serotypes were detected in 35 (7.3%), 39 (8.1%), 47 (9.8%), and 53 (11.0%) CAP cases, respectively (). Among PCV13-serotype-associated cases, more than half were detected in those aged ≥65 years (19/35, 54%) and almost all were detected in those classified as low-risk or at-risk (33/35, 94%). A similar trend was also observed for PCV15, PCV20, and PPSV23-serotypes-associated cases.
Overall, 82.9% of PCV13 VT CAP occurred among persons with an indication (age- or risk-based) for PCV13 vaccination.
Regional differences
presents the percentages of all-cause CAP caused by vaccine serotypes in two catchment areas: city of Ioannina and city of Kavala. Among all CAP patients, a higher proportion of cases caused by PCV13, PCV15, PCV20 and PPSV23 serotypes was found in patients from Kavala than those from Ioannina. These differences were not statistically significant.
Table 4. Patient risk profiles and proportion of CAP cases due to PCV7, PCV13, PCV15, PCV20, and PPSV23 vaccine serotypes in patients hospitalized in Greek cities of Ioannina and Kavala.
Discussion
This study is one of only a handful of studies globally reporting on results of UAD-1 and UAD-2 assays among CAP patients, allowing for ascertainment of the contribution of all pneumococcal serotypes included in higher valency vaccines under development (PCV15 and PCV20) as well as the existing PCV13 and PPSV23. It is also one of the first studies to describe the serotype distribution among hospitalized CAP due to S. pneumoniae using the serotype-specific UAD assays in Greek adults. S. pneumoniae was detected in 13.5% of adults hospitalized with radiographically confirmed CAP, more than half (7.3%) of which were caused by PCV13 serotypes. Serotypes 3, 19A, and 23F were the most prevalent PCV13 serotypes, accounting for 86% of PCV13 VT CAP and 6.2% of all-cause CAP. These results suggest that although Greece has a well-established pediatric PCV13 vaccination program since 2010, persistent PCV13 serotype disease remains in the adult population. The persistence of PCV13 serotype disease in adults has been described in both IPD and pneumococcal pneumonia in other settings also,Citation24–28 pointing out the limitations of herd immunity and underlining the importance of direct adult pneumococcal vaccination.
Serotype 3 was the most prevalent serotype among pneumococcal CAP cases in our study, consistent with other settings, where serotype 3 continues to be prevalent in both IPD and pneumococcal pneumonia.Citation24–28 PCV13 has demonstrated direct vaccine effectiveness against serotype 3 in both children and adults.Citation29,Citation30 However, surveillance data have shown minor or no change or even increases in serotype 3 incidence over time in unvaccinated adult cohorts, compared with substantial declines for other previously prevalent vaccine serotypes.Citation31,Citation32 This limited indirect protection and continued circulation of serotype 3 in the population is likely due to the reduced ability of PCV13 to impact serotype 3 carriage acquisition or density.Citation33 Different biologic characteristics of this specific serotype may also play a role.Citation34,Citation35
The proportions of S. pneumoniae and PCV13 serotypes in our cohort (13.5%, 7.3%, respectively) were comparable to those reported in a US CAP study (9.9%, 4.6%)Citation11 but substantially lower than those recently reported in a Spanish study (28.8%, 14.1%).Citation26 In addition, a lower proportion of PCV13 VT CAP and serotype 3 CAP was observed among adults aged ≥65 years in our study and the US study than the Spanish study. Another interesting finding was related to serotype 8, which has emerged as one of the most prevalent serotypes in IPD in adults in Europe and other settings.Citation25,Citation36 Serotype 8 was negligible in our study and the US study but was the second most prevalent serotype in the Spanish study. Reasons for these differences in pneumococcal epidemiology are not fully understood. However, differences in pneumococcal vaccine use may have contributed. In Greece, PCV13 has been included as part of the NIP for: 1) infants since 2010 with a 3 + 1 schedule until June 2019, when the schedule changed to 2 + 1, 2) adults aged ≥65 years and 19–64 years with comorbidities since 2015 (sequentially with PPSV23). Similarly in the US, PCV13 is used in a 3 + 1 infant schedule, and both PCV13 and PPSV23 are used for direct adult immunization.Citation37 For Spain, PCV13 has been used in a 2 + 1 infant schedule since 2015–2016 and there is almost no direct immunization with PCV13 in older adults.Citation26 It could be that the lower proportions of PCV13 VT CAP and serotype 3 CAP in Greece and the US compared to Spain are related to both better herd immunity through the 3 + 1 infant schedule (versus the 2 + 1 schedule used in Spain) and better protection through the direct vaccination of adults with PCV13. Likewise, the low prevalence of serotype 8 CAP in Greece and the US compared to Spain could be related to a lower level of serotype replacement overall in Greece and the US, which in turn may be related to the 3 + 1 schedule, the original serotype distribution at the time of pediatric PCV13 use, or other issues.
The contribution of S. pneumoniae to nonbacteremic CAP is likely underestimated due to limited diagnostic capability.Citation28 Respiratory cultures are often unavailable and of insufficient quantity and suboptimal quality to aid diagnosis. BinaxNow detects the C-polysaccharide from S. pneumoniae in urine samples from patients with CAP but does not distinguish between the different serotypes. Our study utilized culture, BinaxNow, and serotype-specific UAD-1/2 assays for the detection of S. pneumoniae. Importantly, 57% (n = 37) of pneumococcal CAP cases were detected by UAD alone, which would otherwise have been missed using traditional methods only (culture or BinaxNow). Further, compared with BinaxNow alone, combining BinaxNow and UAD considerably enhanced the diagnostic capability by 2.3-fold. An increase in the diagnostic capability of pneumococcal CAP with UAD assays has also been shown in other studies.Citation28,Citation38
Nevertheless, recent adult PCV13 vaccine efficacy or effectiveness studies have demonstrated that the proportion of CAP prevented by PCV13 is substantially higher than predicted by use of currently available diagnostic tools, including UAD-1/2 assays. For example, in the CAPiTA randomized controlled trial among Dutch adults, the rate reduction for etiologically and radiologically confirmed CAP was 25 per 100,000 person-years of observation (PYO) vs. 72 per 100,000 PYOs for clinical CAP.Citation39 Rate reductions for combined inpatient and outpatient lower respiratory tract infection were even higher at 570 per 100,000 PYOs. Similar underestimates in burden from studies based on etiologically confirmed pneumonia were derived from a US Centers for Disease Control and Prevention (CDC) study of the US Medicare population combined with a study on serotype distribution, as recently summarized.Citation40 These data emphasize that diagnostic tests such as UAD and blood culture should not be used to estimate the proportion of CAP due to pneumococcus or vaccine-type pneumococcus, except as a minimum estimate, but rather should be used to assess relative serotype distribution.
Among all CAP patients, most (79.5%) had at least one underlying condition, confirming that comorbid conditions—including diabetes mellitus, COPD, and chronic heart disease—are recognized risk factors for CAP.Citation21 Importantly, PCV13 VT CAP accounted for a larger percentage of CAP cases in those with neither an age- nor a risk-based indication for vaccination (11.8%) than those with an at-risk condition (7.1%) or a high-risk condition (2.8%). Thus, these results suggest that in addition to the targeted population with recognized risk factors for CAP (older age and certain medical conditions), direct vaccination of PCV13 in the overall adult population might be beneficial. However, in line with recent recommendations from the US Advisory Committee on Immunization Practices,Citation41 the key adult groups to target for PCV immunization remain older adults due to immunosenescence,Citation42 beginning around the age of 50 years and accelerating afterward, and younger adults with underlying risk factors for pneumococcal disease.
At the time of study conduct, adult PCV13 vaccination coverage in Kavala and Ioannina in Greece was estimated, based on market sales data, to be > 30% of those aged >65 years and <10% in those 18–65 years. Among all CAP patients in our study, 5.8% had received PCV13 prior to study enrollment. It is not clear why PCV13 uptake in our cohort was lower than the regional estimates. However, these estimates were based on the uptake of adults in the overall population, and the uptake in persons with increased risk of pneumonia was unknown. Also, it is possible that the population uptake of PCV13 was overestimated, because the estimate was based solely on sales data due to a lack of adult vaccination registries. In our study, PCV13 vaccination history data were verified through the e-prescribing system, which is required to have reimbursement for vaccination. We expect that for PCV13, which currently has a relatively high cost compared to other respiratory vaccines (i.e., 60€ in Greece), such registries would be used. Further, hospitalized patients may be the ones who were least compliant with adherence to their treatment and vaccination guidelines. Regardless of the reasons of low PCV13 uptake, given the established vaccine efficacy of PCV13 against CAP and serotype-specific CAP,Citation14,Citation43 increased immunization coverage of adults should provide direct protection for this population.
The proportion of CAP patients with PSI IV/V scores on admission and in-hospital mortality in our study were higher than those reported in other UAD CAP studies.Citation11,Citation24,Citation44 The Pneumonia Severity Index (PSI/PORT) score is a prediction tool developed to help physicians assess the severity of disease at presentation and make decisions about hospitalizations for CAP patients.Citation45 Patients in classes IV and V are defined as high-risk due to their increased mortality and are thus recommended to be managed with inpatient care.Citation46,Citation47 The higher mortality and severity of CAP events in our cohort may be explained by differences in local healthcare practice (resources for management of CAP patients in the outpatient setting, guidelines on hospital admission, resources, and bed capacity for critical care admission).
Strengths of our study include that the study was conducted independently in two geographical areas, each of which acted as an internal control for the other area. This study started in October 2017, two and a half years after the introduction of PCV13 into the NIP adult programs. Although this interim analysis only reports the first 18 months of data, the longitudinal nature of the study (from 2017 to 2021) will allow us to monitor temporal changes in pneumococcal epidemiology as the pneumococcal vaccination uptake increases among Greek adults over time.
Our study has at least the following limitations. First, the decision to recommend hospitalization or not was at the physician’s discretion and could be affected by local practice and by guidelines on the management of CAP patients. This may have led to the higher severity and mortality seen in these hospitalized CAP patients, potentially limiting the generalizability of overall results to other populations. Second, confirmation of pneumococcal etiology in our study was still limited by the available laboratory methods. The sensitivity and specificity of UAD assays for non-bacteremic pneumonia remains undefined since no gold standard for this condition exists. However, given that the UAD assays are set to maximize specificity, the reported values should be considered lower bounds on the proportion of CAP due to the UAD serotypes. BinaxNOW also has diagnostic limitations, with a meta-analysis reporting an overall sensitivity of 74% and specificity of 97.2% for adult pneumococcal pneumonia.Citation48 Third, this interim analysis was conducted a few years following the introduction of PCV13 into the adult NIP, and with low documented PCV13 uptake in the study population; therefore, the results on S. pneumoniae serotype distribution do not fully reflect the direct effects of PCV13. Finally, there were few cases of invasive/bacteremic CAP and pneumococcal CAP in our cohort, which could be related to antibiotic use prior to hospital admission. Further to this, there were two CAP cases with enterococci identified in blood culture—enterococci are rarely described as a cause of pneumonia, so this may have been due to concomitant other site infections.Citation49
In conclusion, despite a mature pediatric PCV13 vaccination program in Greece, our results highlight a persistent PCV13 serotype disease burden among this adult population hospitalized with mostly severe CAP. Increased PCV13 uptake among adults, and, when licensed, PCV15 and PCV20 vaccines under development could further reduce the pneumococcal CAP burden in adults.
Supplemental Material
Download MS Word (16.5 KB)Acknowledgments
Medical writing support was provided by Qi Yan, PhD (Pfizer Inc).
Disclosure statement
VK, JS, DM, CM, RB, EB, and BG are employees of Pfizer and may hold stock or stock options. AL, AK, VT, GB, TA, CT, MT declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. HM participated in educational, research, and consulting activities sponsored by healthcare companies, including Alexion, Amgen, Bayer, Elpen, Genesis Pharma, MSD, Pfizer, Sanofi, Servier, Viatris, Winmedica.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2079923.
Additional information
Funding
References
- Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, et al. Community-Acquired pneumonia requiring hospitalization among U.S. Adults N Engl J Med. 2015;373:415–10. doi:10.1056/NEJMoa1500245.
- Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA, Nakamatsu R, Pena S, Guinn BE, Furmanek SP, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65:1806–12. doi:10.1093/cid/cix647.
- Feldman C, Anderson R. The role of streptococcus pneumoniae in community-acquired pneumonia. Semin Respir Crit Care Med. 2016;37:806–18. doi:10.1055/s-0036-1592074.
- Bewick T, Sheppard C, Greenwood S, Slack M, Trotter C, George R, Lim WS. Serotype prevalence in adults hospitalised with pneumococcal non-invasive community-acquired pneumonia. Thorax. 2012;67:540–45. doi:10.1136/thoraxjnl-2011-201092.
- Sherwin RL, Gray S, Alexander R, McGovern PC, Graepel J, Pride MW, Purdy J, Paradiso P, Tm F Jr. Distribution of 13-valent pneumococcal conjugate vaccine Streptococcus pneumoniae serotypes in US adults aged ≥50 years with community-acquired pneumonia. J Infect Dis. 2013;208:1813–20. doi:10.1093/infdis/jit506.
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi:10.1086/648593.
- Harboe ZB, Dalby T, Weinberger DM, Benfield T, Molbak K, Slotved HC, Suppli CH, Konradsen HB, Valentiner-Branth P. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014;59:1066–73. doi:10.1093/cid/ciu524.
- Hanquet G, Krizova P, Valentiner-Branth P, Ladhani SN, Nuorti JP, Lepoutre A, Mereckiene J, Knol M, Winje BA, Ciruela P, et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax. 2019;74:473–82. doi:10.1136/thoraxjnl-2018-211767.
- Lewnard JA, Hanage WP. Making sense of differences in pneumococcal serotype replacement. Lancet Infect Dis. 2019;19:e213–e20. doi:10.1016/S1473-3099(18)30660-1.
- Du QQ, Shi W, Yu D, Yao KH. Epidemiology of non-vaccine serotypes of Streptococcus pneumoniae before and after universal administration of pneumococcal conjugate vaccines. Hum Vaccin Immunother. 2021 ;2:1–10.
- Isturiz RE, Ramirez J, Self WH, Grijalva CG, Counselman FL, Volturo G, Ostrosky-Zeichner L, Peyrani P, Wunderink RG, Sherwin R, et al. Pneumococcal epidemiology among US adults hospitalized for community-acquired pneumonia. Vaccine. 2019;37:3352–61. doi:10.1016/j.vaccine.2019.04.087.
- Payeras A, Villoslada A, Garau M, Salvador MN, Gallegos MC. Evolution of pneumococcal infections in adult patients during a four-year period after vaccination of a pediatric population with 13-valent pneumococcal conjugate vaccine. Int J Infect Dis. 2015;33:22–27. doi:10.1016/j.ijid.2014.12.035.
- Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O’-Brien KL. For the AGEDD adult pneumococcal burden study team. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8:e60273. doi:10.1371/journal.pone.0060273.
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. doi:10.1056/NEJMoa1408544.
- Grivea IN, Priftis KN, Giotas A, Kotzia D, Tsantouli AG, Douros K, Michoula AN, Syrogiannopoulos GA. Dynamics of pneumococcal carriage among day-care center attendees during the transition from the 7-valent to the higher-valent pneumococcal conjugate vaccines in Greece. Vaccine. 2014;32:6513–20. doi:10.1016/j.vaccine.2014.09.016.
- Greek Ministry of Health. National immunization program for adults. ΑΔΑ: Β4Λ5ΟΞ7Μ-ΛΝ8. 2011. [accessed 2021 Aug 20]. https://www.diavgeia.gov.gr/decision/view/%CE%924%CE%9B5%CE%9F%CE%9E7%CE%9C-%CE%9B%CE%9D8.
- Greek Ministry of Health. National immunization program for adults 2015. ΑΔΑ: Ω5Φ6Θ-46Π. [accessed 2021 Aug 20]. https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko-programma-emboliasmwn-epe-enhlikwn/6353-palaiotera-epe-enhlikwn.
- Kofteridis DP, Giourgouli G, Plataki MN, Andrianaki AM, Maraki S, Papadakis JA, Zacharioudaki ME, Samonis G. Community-acquired pneumonia in elderly adults with type 2 diabetes mellitus. J Am Geriatr Soc. 2016;64:649–51. doi:10.1111/jgs.14011.
- Greek Ministry of Health. National immunization program for adults 2020-2021. ΑΔΑ: ΨΙΡ4465ΦΥΟ-374. [accessed 2021 Aug 20]. https://www.moh.gov.gr/articles/health/dieythynsh-dhmosias-ygieinhs/emboliasmoi/ethniko-programma-emboliasmwn-epe-enhlikwn/7968-ethniko-programma-emboliasmwn-enhlikwn-2020-2021.
- Maccioni L, Weber S, Elgizouli M, Stoehlker AS, Geist I, Peter HH, Vach W, Nieters A. Obesity and risk of respiratory tract infections: results of an infection-diary based cohort study. BMC Public Health. 2018;18:271. doi:10.1186/s12889-018-5172-8.
- Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68:1057–65. doi:10.1136/thoraxjnl-2013-204282.
- Pride MW, Huijts SM, Wu K, Souza V, Passador S, Tinder C, Song E, Elfassy A, McNeil L, Menton R, et al. Validation of an immunodiagnostic assay for detection of 13 Streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Vaccines Immunol. 2012;19:1131–41. doi:10.1128/CVI.00064-12.
- Kalina WV, Souza V, Wu K, Giardina P, McKeen A, Jiang Q, Tan C, French R, Ren Y, Belanger K, et al. Qualification and clinical validation of an immunodiagnostic assay for detecting 11 additional streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Infect Dis. 2020;71:e430–e438. doi:10.1093/cid/ciaa158.
- Varghese J, Chochua S, Tran T, Walker H, Li Z, Snippes Vagnone PM, Lynfield R, McGee L, Li Y, Metcalf BJ, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26(4): 512.e1-512.e10. doi:10.1016/j.cmi.2019.09.008.
- European Centre for Disease Prevention and Control. Invasive pneumococcal disease. ECDC. Annual epidemiological report for 2018. Stockholm: ECDC; 2020 [accessed 2021 Aug 20]. https://www.ecdc.europa.eu/en/publications-data/invasive-pneumococcal-disease-annual-epidemiological-report-2018#no-link.
- Torres A, Menendez R, Espana PP, Fernandez-Villar JA, Marimon JM, Cilloniz C, Méndez R, Egurrola M, Botana-Rial M, Ercibengoa M, et al. The evolution and distribution of pneumococcal serotypes in adults hospitalized with community acquired pneumonia in Spain using serotype specific urinary antigen detection test: the CAPA study, 2011-2018. Clin Infect Dis. 2021;73(6):1075–85. doi:10.1093/cid/ciab307.
- Pick H, Daniel P, Rodrigo C, Bewick T, Ashton D, Lawrence H, Baskaran V, Edwards-Pritchard RC, Sheppard C, Eletu SD, et al. Pneumococcal serotype trends, surveillance and risk factors in UK adult pneumonia, 2013-18. Thorax. 2020;75(1):38–49. doi:10.1136/thoraxjnl-2019-213725.
- Isturiz R, Grant L, Gray S, Alexander-Parrish R, Jiang Q, Jodar L, Peyrani P, Ford KD, Pride MW, Self WH, et al. Expanded analysis of 20 pneumococcal serotypes associated with radiographically confirmed community-acquired pneumonia in hospitalized US adults. Clin Infect Dis. 2021;73(7):1216–22. doi:10.1093/cid/ciab375.
- Sings HL, De Wals P, Gessner BD, Isturiz R, Laferriere C, McLaughlin JM, Pelton S, Schmitt HJ, Suaya JA, Jodar L. Effectiveness of 13-valent pneumococcal conjugate vaccine against invasive disease caused by serotype 3 in children: a systematic review and meta-analysis of observational studies. Clin Infect Dis. 2019;68(12):2135–43. Erratum in: Clin Infect Dis. 2021;72(9):1684-1685. doi:10.1093/cid/ciy920.
- McLaughlin JM, Jiang Q, Gessner BD, Swerdlow DL, Sings HL, Isturiz RE, Jodar L. Pneumococcal conjugate vaccine against serotype 3 pneumococcal pneumonia in adults: a systematic review and pooled analysis. Vaccine. 2019;37(43):6310–16. doi:10.1016/j.vaccine.2019.08.059.
- Centers for Disease Control and Prevention. Advisory committee on immunization practices (ACIP). ACIP presentation slides: February 24-25, 2021 meeting. Pneumococcal vaccines - current epidemiology of pneumococcal disease and pneumococcal vaccine coverage in US adults. [accessed 2022 Apr 4]. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-2-24-25.html
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51. doi:10.1016/S1473-3099(18)30052-5.
- Sings HL, Gessner BD, Wasserman MD, Jodar L. Pneumococcal conjugate vaccine impact on serotype 3: a review of surveillance data. Infect Dis Ther. 2021;10(1):521–39. doi:10.1007/s40121-021-00406-w.
- Choi EH, Zhang F, Lu YJ, Malley R. Capsular polysaccharide (CPS) release by serotype 3 pneumococcal strains reduces the protective effect of anti-type 3 CPS antibodies. Clin Vaccine Immunol. 2015;23(2):162–67. doi:10.1128/CVI.00591-15.
- Groves N, Sheppard CL, Litt D, Rose S, Silva A, Njoku N, Rodrigues S, Amin-Chowdhury Z, Andrews N, Ladhani S, et al. Evolution of Streptococcus pneumoniae Serotype 3 in England and Wales: a major vaccine evader. Genes (Basel). 2019;10(11):845. doi:10.3390/genes10110845.
- Cui YA, Patel H, O’-Neil WM, Li S, Saddier P. Pneumococcal serotype distribution: a snapshot of recent data in pediatric and adult populations around the world. Hum Vaccines Immunother. 2017;13(6):1–13. doi:10.1080/21645515.2016.1277300.
- Suaya JA, Mendes RE, Sings HL, Arguedas A, Reinert RR, Jodar L, Isturiz RE, Gessner BD. Streptococcus pneumoniae serotype distribution and antimicrobial nonsusceptibility trends among adults with pneumonia in the United States, 2009-2017. J Infect. 2020;81:557–66. doi:10.1016/j.jinf.2020.07.035.
- Wunderink RG, Self WH, Anderson EJ, Balk R, Fakhran S, Courtney DM, Qi C, Williams DJ, Zhu Y, Whitney CG, et al. Pneumococcal community-acquired pneumonia detected by serotype-specific urinary antigen detection assays. Clin Infect Dis. 2018;66:1504–10. doi:10.1093/cid/cix1066.
- Gessner BD, Jiang Q, Van Werkhoven CH, Sings HL, Webber C, Scott D, Neuzil KM, O’-Brien KL, Wunderink RG, Grobbee DE, et al. A public health evaluation of 13-valent pneumococcal conjugate vaccine impact on adult disease outcomes from a randomized clinical trial in the Netherlands. Vaccine. 2019;37:5777–87. doi:10.1016/j.vaccine.2018.05.097.
- Gessner BD, Isturiz R, Snow V, Grant LR, Theilacker C, Jodar L. The rationale for use of clinically defined outcomes in assessing the impact of pneumococcal conjugate vaccines against pneumonia. Expert Rev Vaccines. 2021;20:269–80. doi:10.1080/14760584.2021.1889376.
- Kobayashi M, Farrar JL, Gierke R, Britton A, Childs L, Leidner AJ, Campos-Outcalt D, Morgan RL, Long SS, Talbot HK, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the advisory committee on immunization practices - United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):109–17. doi:10.15585/mmwr.mm7104a1.
- Grant LR, Slack MPE, Yan Q, Trzciński K, Barratt J, Sobczyk E, Appleby J, Cané A, Jodar L, Isturiz RE, et al. The epidemiologic and biologic basis for classifying older age as a high-risk, immunocompromising condition for pneumococcal vaccine policy. Expert Rev Vaccines. 2021;20(6):691–705. doi:10.1080/14760584.2021.1921579.
- Gessner BD, Jiang Q, Van Werkhoven CH, Sings HL, Webber C, Scott D, Gruber WC, Grobbee DE, Bonten MJM, Jodar L. A post-hoc analysis of serotype-specific vaccine efficacy of 13-valent pneumococcal conjugate vaccine against clinical community acquired pneumonia from a randomized clinical trial in the Netherlands. Vaccine. 2019;37:4147–54. doi:10.1016/j.vaccine.2019.05.065.
- Theilacker C, Hansen KB, Rünow E, Beavon R, Palmborg A, Reinert RR, Jiang Q, Gessner BD, Riesbeck K, Ahl J Pneumococcal Serotype Distribution in Adults Hospitalized with Radiologically-Confirmed Community-Acquired Pneumonia in Malmö, Sweden. Abstract ID 904. ISPPD 12-2020.
- Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, Coley CM, Marrie TJ, Kapoor WN. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50. doi:10.1056/NEJM199701233360402.
- Bartlett JG, Dowell SF, Mandell LA, File TM Jr., Musher DM, Fine MJ. Practice guidelines for the management of community-acquired pneumonia in adults. infectious diseases society of America. Clin Infect Dis. 2000;31:347–82. doi:10.1086/313954.
- Renaud B, Coma E, Labarere J, Hayon J, Roy PM, Boureaux H, Moritz F, Cibien JF, Guérin T, Carré E, et al. Routine use of the Pneumonia Severity Index for guiding the site-of-treatment decision of patients with pneumonia in the emergency department: a multicenter, prospective, observational, controlled cohort study. Clin Infect Dis. 2007;44:41–49. doi:10.1086/509331.
- Sinclair A, Xie X, Teltscher M, Dendukuri N. Systematic review and meta-analysis of a urine-based pneumococcal antigen test for diagnosis of community-acquired pneumonia caused by Streptococcus pneumoniae. J Clin Microbiol. 2013;51(7):2303–10. doi:10.1128/JCM.00137-13.
- Li F, Wang Y, Sun L, Wang X. Vancomycin-Resistant Enterococcus faecium pneumonia in a uremic patient on hemodialysis: a case report and review of the literature. BMC Infect Dis. 2020;20(1):167. doi:10.1186/s12879-020-4892-4.
