ABSTRACT
Strategies that improve influenza vaccine immunogenicity are critical for the development of vaccines for pandemic preparedness. Hemagglutinin (HA)-specific CD4+ T cell epitopes support protective B cell responses against seasonal influenza. However, in the case of avian H7N9, which poses a pandemic threat, HA elicits only weak neutralizing antibody responses in infection and vaccination without adjuvant. We hypothesized that an immune-engineered H7N9 HA incorporating a broadly reactive H3N2 HA-specific memory CD4+ T cell epitope that replaces a regulatory T cell-inducing epitope at the corresponding position in H7N9 HA could harness preexisting influenza T cell immunity to increase CD4+ T cells that are needed for protective antibody development. We designed and produced a virus-like particle (VLP) vaccine that carries the epitope augmented H7N9 HA (OPT1) and immunized HLA-DR3 transgenic mice with established H3N2 immunity. OPT1-VLPs stimulated higher stem cell, central, and effector memory CD4+ T cell levels over wild type VLP immunization. In addition, activated, IL-21-producing follicular helper T cell frequencies were enhanced. This novel immunogen design strategy illustrates that site-specific modifications aimed to augment T cell epitope content enhance CD4+ T cell responses among critical subpopulations capable of aiding protective immune responses upon antigen re-encounter and that mobilization of immune memory can be used to overcome the poor immunogenicity of avian influenza viruses.
Introduction
Despite intensive efforts to predict and control influenza virus spread, influenza virus-induced disease remains a major global health concern. Annual epidemics occur with a seasonal pattern and sporadic zoonotic outbreaks of novel influenza A viruses can lead to pandemics. Avian-origin H7N9 influenza viruses first infected humans in China in 2013Citation1 and resulted in >1,550 confirmed cases with >600 deaths since.Citation2 Although there have been no reported human cases in the last few years, several epidemiological findings in the 2016–2017 season suggest that H7N9 still poses a significant threat to human health. For example, limited human-to-human transmission was observed as in previous seasons.Citation3 There were increased numbers of human cases of infection with geographic spread to western provinces of China.Citation4 HA sequence diversity increased with reduced antibody cross-reactivity with early isolates.Citation5,Citation6 Additonally, highly pathogenic virus outbreaks in chickens and infections in humans were seen.Citation7 When combined together with a mortality risk that is just under 40%, these findings raise concern that an H7N9 pandemic may occur should the virus adapt to increase human-to-human transmissibility. As a result, the Center for Disease Control and Prevention Influenza Risk Assessment Tool classifies low pathogenicity H7N9 a moderate-to-high pandemic risk with high risk for potential emergence and impact.Citation8
Vaccination is the most effective strategy to control seasonal influenza and is a critical part of pandemic influenza preparedeness. Because humans are immunologically naïve to avian influenza, vaccination using the native antigen sequence may not recruit adequate levels of cross-reactive memory B and T cells generated in seasonal influenza virus vaccination or infection to induce protective antibody responses due to sequence divergence with seasonal influenza.Citation9 In H7N9 influenza virus infection, the neutralizing antibodies are not detected in the acute phase.Citation10 Anti-H7 antibodies are significantly delayed and exhibit low avidity, in comparison with antibodies generated following seasonal influenza virus vaccination and infection. Furthermore, H7N9 inactivated virus and subunit vaccines that are not adjuvanted elicit weak hemagglutinin-inhibition (HAI) antibody titers in clinical trials.Citation11,Citation12 Poor immunogenicity may be explained, in part, by low T cell epitope content in H7N9 HA in comparison to seasonal influenza HA proteins, as well as Treg induction that inhibits protective antibody development (De Groot et al.Citation13,Citation14). We showed a T cell epitope in H7N9 HA activates human CD4+CD25highCD39+FoxP3+ Tregs and suppresses H7N9-specific effector (IFNγ+) T cell responses.Citation15
We postulated that removal of the Treg epitope in H7-HA would improve the antibody response to HA, consistent with current understanding of the impact of follicular Tregs on antibody maturation in germinal center reactions.Citation16 To generate an antigenically improved H7-HA, we engineered out this Treg-inducing epitope with three amino acid substitutions that simultaneously introduced a broadly reactive and conserved H3-HA CD4+ T cell epitope (H3-HA306-318) capable of mobilizing seasonal influenza CD4+ T cell memory. This novel immunogen, named OPT1, demonstrated improved immunogenicity and efficacy over wild type (WT) H7N9 HA. First, we showed that immunizations in NOD/SCID/JAK3(null) immune-deficient mice reconstituted with human PBMCs with OPT1, stimulated an average 5-fold greater anti-H7-HA IgG titer and 20-fold greater anti-H7-HA B cell frequency over mice immunized with WT protein.Citation17 Then, in H3N2 pre-immune HLA-DR3 transgenic mice, we showed that OPT1 H7-HA vaccination elicited higher H7-HA-specific IgG titers that resulted in lower mortality, weight loss, and lung viral titer following lethal challenge with the H7N9 A/Anhui/1/2013 influenza virus compared to WT-vaccinated mice.Citation18 In this study, we assessed CD4+ T cell responses to OPT1 immunization in H3N2 pre-immune HLA-DR3 mice to evaluate the relationship between the T cell epitope modifications designed to increase CD4+ T cell responses and enhanced OPT1 immunogenicity and efficacy.
Methods
Mice
HLA-DR3 transgenic mice were obtained from Dr. Chella David (Mayo Clinic) under commercial license. The mice express the HLA-DRA and DRB1*03:01 genes on a B.10-Ab0 mouse class II-negative background. All mice were housed in microisolator units, allowed free access to food and water, and were cared for under USDA guidelines for laboratory animals. Animal research protocols for mouse studies were reviewed and approved by the Absorption Systems Inc. (IACUC #P07-10R31) and University of Georgia Institutional Animal Care and Use Committees (IACUC #A2017 11-021-Y3-A11).
VLP production and characterization
H7-HA sequences were expressed on the surface of virus-like particles (VLPs), as previously described.Citation19 Briefly, full-length H7-HA amino acid sequences were subjected to codon optimization for expression in a human cell line (Genewiz) and inserted into the pTR600 expression vector. OPT1 H7-HA is a three amino acid variant of A/Anhui/1/2013 H7-HA (R320N/S321T/L323K) that was designed using the iVAX T cell epitope discovery and vaccine design immunoinformatic platform, as previously described.Citation19 HEK293T cells were transiently co-transfected (Lipofectamine™ 3000, Thermo Fisher Scientific) with plasmids expressing H7 HAs, HIV-1 Gag (optimized for expression in mammalian cells; Genewiz), and NA (A/Thailand/1(KAN-1)/2004 H5N1) (optimized for expression in mammalian cells; Genewiz). The cells were incubated for 72 h at 37°C. Supernatants were centrifuged at low speed and filtered through a 0.22-μm sterile filter. Filtered supernatant was purified via ultracentrifugation (100,000 g through 20% glycerol, weight per volume) for 4 h at 4°C. Pellets were subsequently resuspended in PBS (pH 7.2) and stored in single-use aliquots at 4°C until use.
The HA content of H7 VLPs was determined as previously described with minor modification.Citation20 Briefly, a high-affinity, 96-well flat bottom enzyme-linked immunosorbent assay (ELISA) plate was coated with 5–10 μg of total protein of VLPs and serial dilutions of a recombinant H7 antigen (A/Anhui/1/2013 HA generated in house as previously describedCitation18 in ELISA carbonate buffer (50 mM carbonate buffer, pH 9.5), and the plate was incubated overnight at 4°C. The next morning, plates were washed in PBS with 0.05% Tween 20 (PBST), and then nonspecific binding was blocked with 1% bovine serum albumin (BSA) in PBST solution for 1 h at room temperature (RT). Buffer was removed, and then stalk-specific group 2 antibodyCitation21 was added to the plate and incubated for two hours at 37°C. Plates were washed and probed with goat anti-human IgG horseradish peroxidase-conjugated secondary antibody at a 1:3,000 dilution and incubated for 2 h at 37°C. The plates were washed 7 times with the wash buffer prior to development with 100 μL of 0.1% 2,2’-azino-bis(3-ethylbenzothiaozoline-6–sulfonic acid; ABTS) solution with 0.05% H2O2 for 40 min at 37°C. The reaction was terminated with 1% (w/v) sodium dodecyl sulfate (SDS). Colorimetric absorbance at 414 nm was measured using a PowerWaveXS plate reader (Biotek). The background was subtracted from negative wells. A linear regression standard curve analysis was performed using the known concentrations of recombinant standard antigen to estimate the HA content in VLP lots.
Vaccinations
Vaccine- and sham-treated HLA-DR3 mice were female and 6–8 weeks old at the start of studies. All mice were bled to confirm they were seronegative to influenza A/Anhui/1/2013 (H7N9) and A/Hong Kong/4108/2014 (H3N2) viruses. To generate influenza pre-immunity, mice were exposed to H3N2 virus by intranasal inoculation (1×106 PFU in 0.05 ml/mouse) with A/Hong Kong/4108/2014. Eight weeks later, mice were vaccinated by intramuscular injection with WT or OPT1 VLPs (1.2 µg HA content) without adjuvant and similarly boosted four weeks later. A control group received PBS. Mice were sacrificed either one week (N = 4/group) or three weeks (N = 4/group) after the boost immunization. Blood was sampled at baseline, pre-prime, pre-boost, and at termination. Blood was collected via submandibular bleeding using a lancet and transferred to a microfuge tube. Tubes were incubated at room temperature for at least 30 min prior to centrifugation, sera were collected and frozen at − 20°C ±5°C Spleens and draining lymph nodes were harvested and single-cell suspensions were prepared for T cell monitoring.
Infections
Three weeks following boost immunization, mice (N = 10/group) were transferred to a biosafety level 3 (BSL-3) facility for viral challenge. On the following week, mice were briefly anesthetized and infected with a 10×LD50 dose of A/Anhui/1/2013(H7N9) via the intranasal route (1×104 PFU in 0.05 mL). At 4 days post-challenge, four mice in each group were randomly selected and sacrificed to harvest lung tissue. The remaining mice were monitored for clinical symptoms and euthanized at 1 day post-challenge. All procedures were in accordance with the NRC Guide for Care and Use of Laboratory Animals, the Animal Welfare Act, and the CDC/NIH Biosafety and Microbiological and Biomedical Laboratories.
Hemagglutination-inhibition (HAI) assay
A hemagglutination-inhibition assay (HAI) assay was used to assess receptor-blocking antibodies to the HA protein to inhibit agglutination of turkey red blood cells (TRBCs). The protocol is taken from the CDC laboratory influenza surveillance manual. To inactivate nonspecific inhibitors, mouse sera was treated with receptor destroying enzyme (RDE, Denka Seiken, Co.) prior to being tested. Three parts of RDE was added to one-part sera and incubated overnight at 37°C. The RDE was inactivated at 56°C for 30 min; when cooled, 6 parts of sterile PBS was added to the sera and was kept at 4°C until use. RDE treated sera was two-fold serially diluted in v-bottom microtiter plates. Twenty-five µl of virus at 8 HAU/50 µL was added to each well (4 HAU/25 µL). Plates were covered and incubated with virus for 20 min at room temperature before adding 0.8% TRBCs in PBS. The plates were mixed by agitation and covered; the RBCs were then allowed to settle for 1 h at room temperature. HAI titer was determined by the reciprocal dilution of the last well that contained non-agglutinated RBC. Negative and positive serum controls were included for each plate. All mice were negative (HAI <1:10) for preexisting antibodies to currently circulating human influenza viruses prior to study onset.
Flow cytometry
Splenocytes were isolated and washed with 1X PBS +5% fetal bovine serum (FBS) and stained with Live/Dead Violet fixable stain (Life Technologies) in 1X PBS (Gibco) at 4°C, washed and stained with surface receptor antibodies in 1X PBS + 5% FBS in the dark at 4°C. Cells were fixed with Fixation/Permeabilization Buffer (eBioscience) in the dark at room temperature for 30 min and stained for intracellular markers in Permeabilization Buffer (eBioscience) at 4°C. Stained cells were acquired and data collected on an Attune NxT Cytometer with three laser capacity (violet-405 nm, blue-488 nm, and red-637 nm) (Life Technologies) and analyzed using FlowJo software (Becton, Dickinson and Company). For gating, after exclusion of doublets, lymphocytes were first identified by a low forward scatter (FSC) and low side scatter (SSC) gate. Dead cells were excluded by Live-Dead staining. The following antibody panels were used in this assay. T effector cells: CXCR3 (APC), CD62 L (AF700), CD3 (APC-Cy7), CD4 (BV510), CD44 (BV650), and IFNγ (PE). T follicular helper cells: IL-21 (PE-Cy7), PD-1 (FITC), ICOS (PE), Bcl6 (PerCP-Cy5.5), CXCR5 (APC), CD62L (AF700), CD3 (APC-Cy7), CD4 (BV510), and CD44 (BV650).
Statistical analysis
Differences between vaccination groups were determined by Student’s t test. One-way ANOVA was used for multiple group comparisons (GraphPad Software v8.4.0). Correlation analyses were determined by linear regression, body weight significance determined by ANOVA, and survival curves were analyzed by log-rank test. p values <0.05 were considered significant, <0.01 very significant, and <0.001 highly significant.
Results
OPT1-VLP vaccination yields enhanced helper T cell responses over vaccination with WT-HA
We immunized H3N2 pre-immune HLA-DR3 transgenic mice with VLPs carrying H7N9 HA modified to circumvent Treg induction and harness effector CD4+ T cell memory (OPT1) in order to evaluate the ability of the engineered antigen to improve CD4+ T cell and antibody responses (). CD4+ T cell immunity is fully restricted by HLA-DR3 in this transgenic mouse model as mouse MHC II is knocked out, allowing for presentation of H3-HA306-318 in the context of human MHC and evaluation of its contribution to OPT1 immunity in comparison with WT HA. Additionaly, seasonal influenza immunity was established in the mice prior to immunization with the experimental H7 VLP vaccine. This would not be required in the clinical setting, as humans generally develop influenza immunity in the first year of life by natural infection or vaccination.
Figure 1. Study design. H3N2 pre-immune HLA-DR3 transgenic mice were used to evaluate the ability of the epitope enhancements to improve immunogenicity and protective efficacy against lethal challenge. Pre-immunity to H3N2 (1×106 PFU, Hong Kong/2014) was established by a single intranasal exposure prior to vaccination. Mice (N = 18/group) were immunized by the intramuscular route with wild-type H7N9 VLPs (A/Anhui/1/2013) or VLPs carrying OPT1 engineered H7N9 HA without adjuvant eight weeks post-exposure and boosted four weeks later. A group of H3N2 pre-immune mice was not vaccinated (control). Mice were sacrificed one week (N = 4/group) or four weeks (N = 4/group) after the boosting (N = 10/group) for immunogenicity analysis or mice (N = 10/group) were challenged intranasally with H7N9 virus (1×104 PFU, Anhui/2013) two weeks after boosting and monitored for weight loss and survival.
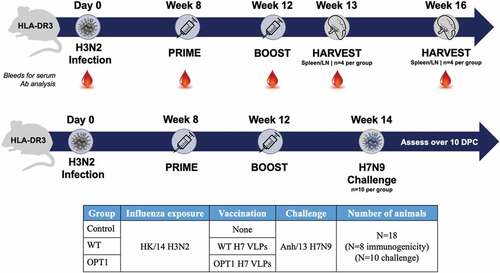
Immunity to H3N2 (1×106 PFU, Hong Kong/2014) was established by a single intranasal exposure prior to vaccination, as evidenced by seroconversion to H3N2, but not H7N9 (data not shown). Mice (N = 8/group) were immunized by the intramuscular route with wild-type H7-HA VLPs (A/Anhui/1/2013) or VLPs carrying OPT1-engineered H7N9 HA without adjuvant eight weeks post-exposure and boosted four weeks later. A group of H3N2 pre-immune mice did not receive VLP vaccine (control). CD4+ T cells were assessed for type-1 skewed helper (Th1) and T follicular helper (Tfh) cell phenotypes via flow cytometry one week (peak T cell response; N = 4/group) and four weeks following the boost immunization (memory response; N = 4/group). CD4+ T cell differentiation status was determined using CD62L, CD44, and Sca-1 surface markers to characterize proliferation and effector capacity of vaccine-induced Th/Th1 responses. For less differentiated stem cell memory T cells (TSCM, CD62L+CD44−Sca1+) and central memory T cells (TCM, CD62L+CD44+) that have higher proliferative potential, cell frequency was used as a surrogate for proliferation; for more differentiated effector memory T cells (TEM, CD62L−CD44+) and terminally differentiated T cells (TTE, CD62L−CD44−), effector function was used to monitor activation. OPT1-VLP immunization enhanced Th responses in multiple CD4+ T cell compartments. Significantly elevated frequencies of splenic CD3+/CD4+ T cells were observed in TSCM and TCM subpopulations one week post-boost, indicating enhanced antigen-driven proliferation over mock immunized mice and trended higher than mice that received WT-VLPs (). By four weeks after the boost immunization, these populations contracted to levels comparable across groups. Cells within the TEM and TTE subpopulations show no significant vaccine-induced expansion (data not shown). However, OPT1-VLP stimulated a significantly higher frequency of antigen experienced TEM cells than WT-VLP one week post-boost () and enhanced effector function (IFNγ production) one week post-boost, which persisted for three more weeks ().
Figure 2. OPT1-VLP vaccination yields enhanced helper T cell responses over vaccination with WT-HA. (a) CD3+/CD4+ splenocytes within TSCM and TCM subpopulations display significantly elevated frequencies one week post-boost with OPT1-VLP, indicating enhanced antigen-driven proliferation in these compartments. Cells within the TEM subpopulation show no significant vaccine-induced expansion (data not shown). (b) However, OPT1-VLP vaccinees exhibit a higher frequency of antigen-experienced TEM cells than those with WT-VLP one week post-boost, while also exhibiting enhanced effector function (IFNγ production) at both one and four week post-boost timepoints (C). *=p < .05, **= p < .01, ***=p < .001.
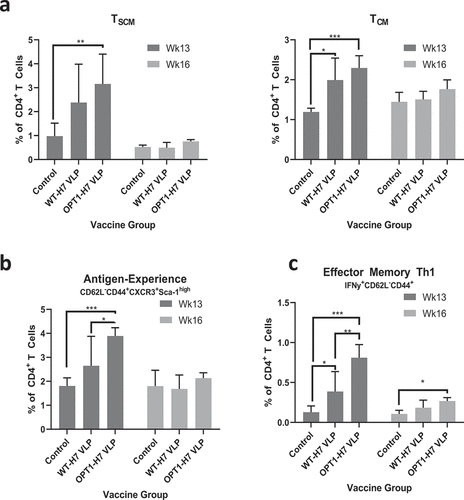
OPT1-VLP vaccination yields sustained Tfh responses within splenic reservoirs
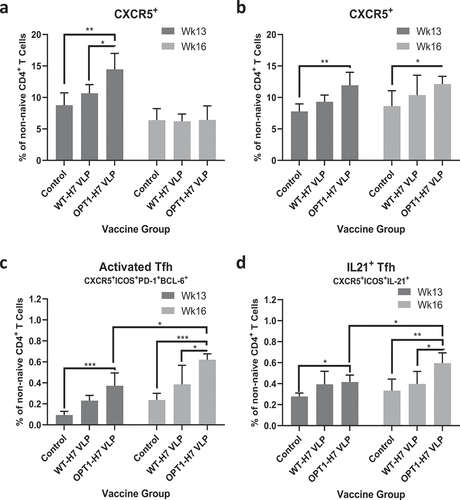
For Tfh characterization, upregulation of Tfh-associated markers was monitored over TCM/TEM/TTE populations. The frequency of CXCR5+ T cells in OPT1-VLP mice was significantly increased over both non-immunized pre-immune control mice and WT-VLP immunized mice one week following the boost immunization in lymph nodes () and increased over control mice in spleen (). While WT-VLP immunization stimulated a slight increase in this population at both sites, neither were significantly elevated over controls at one week post-boost. Four weeks after boost immunization, CXCR5+ T cells remained significantly elevated in the spleen of OPT1-VLP mice. In spleen, OPT1-VLP vaccination induced differentiation of Bcl6+/PD-1+/ICOS+ Tfh that are needed to induce germinal centers by one week following boost immunization (). Differentiated Tfh persisted in OPT-VLP mice three weeks later and were significantly increased over one week post-boost. Differentiated Tfh in WT-VLP mice trend higher at both timepoints but were significantly lower than OPT1-VLP at four weeks post-boost. Similar results were observed for IL-21-producing Tfh ().
Figure 3. OPT1-VLP vaccination yields sustained Tfh responses within splenic reservoirs. CD4+ T cells within TCM/TEM/TTE subpopulations from the lymph nodes (a) and spleen (b) were assessed ex vivo for CXCR5 expression. Splenic Tfh activation and function in OPT1-VLP vaccinated mice were measured by the upregulation of effector Tfh markers (c) and IL-21 production (D). *=p < .05, **= p < .01, ***=p < .001.
OPT1-VLP vaccination optimizes early antibody responses that correlate with spleen-derived Tfh
The humoral response to H7 VLP vaccination was characterized by HAI antibody measurement. Both OPT1-VLP and WT-VLP vaccines induced HAI antibodies after the prime immunization, however no mice seroconverted even after boosting (). HAI titers were significantly greater in OPT1-VLP mice over WT-VLP mice four weeks post-prime and one week post-boost but no different by four weeks post-boost. No significant correlation of HAI titers and lymph node Tfh was found at either timepoint after boosting (); however, a positive correlation was observed in spleen one week after boosting (). A similar correlation was found for activated Tfh ().
Figure 4. OPT1-VLP vaccination optimizes early Ab responses that correlate with spleen-derived Tfh. HAI titers were assessed in each animal enrolled in the study at four time points: pre-H3N2 exposure, pre-prime, pre-boost, and at termination (a). H7-specific HAI titers were measured starting at Wk12, four weeks post-prime. At both Wk12 and Wk13, OPT VLP vaccinated mice display significantly higher HAI than with WT-VLP. However, by wk16, HAI is comparable between the two groups. Furthermore, we find that HAI titer from terminal bleeds do not correlate with LN-derived Tfh at either timepoint (b). However, both total Tfh (c) and activated Tfh (d) frequencies in the spleen correlate with HAI titers at one-week post boost. *=p < .05, **= p < .01, ***=p < .001. Correlation determined by linear regression.
H7-VLP vaccines demonstrate similar efficacy against lethal challenge
We challenged mice immunized with OPT1-VLPs to evaluate the ability of the epitope enhancements to improve protective efficacy against lethal H7N9 infection (). Mice were challenged intranasally with H7N9 virus (1×104 PFU, Anhui/2013) two weeks after the boost immunization and monitored for weight loss and survival. Over nine days following challenge, the OPT1-VLP and WT-VLP groups demonstrated similar weight change overall with lower weight loss in WT-VLP mice at days 3 and 4 post-challenge but no difference in the critical phase of illness at day 5 through 8 (). No significant difference in survival between vaccinated mice was observed, although both were better protected than non-immunized H3N2 pre-immune ().
Figure 5. H7-VLP vaccines demonstrate similar efficacy against lethal challenge. (a) Mice challenged with H7N9 were monitored over a 9-day period post infection. We find a benefit in weight maintenance in animals vaccinated with WT-VLP over OPT1-VLP (blue) only at 3 days post-challenge, and over both H3N2 pre-immune, not immunized control (black) and OPT1 (blue) at 4 days post-challenge. However, during the more critical phase of illness, days 5–8, the two VLP vaccines were statistically similar. (b) Survival curves for both H7-VLP vaccines were also similar providing an additional 40–50% protection over that seen in pre-immune control animals. Body weight significance determined by ANOVA. Survival curves analyzed by log-rank test. *=p <.05, **=p <.01.
Discussion
Routine influenza virus exposure and vaccination generate inadequate cross-protective antibody immunity against novel avian influenza viruses of different subytpes. Avian subtype-specific or universal influenza vaccines are needed for pandemic preparedness. Although avian influenza viruses share a number of T cell epitopes with seasonal viruses and vaccines, cross-reactive CD4+ memory T cells induced by unadjuvanted H5N1 and H7N9 vaccines do not sufficiently support protective antibody responses.Citation11,Citation12,Citation22,Citation23 Strategies that can overcome poor vaccine immunogenicity, such as higher antigen doses and adjuvant formulation, increase immune response, but are not without risk. High antigen dose can limit vaccine availability when mass immunization is urgent and manufacturing facilities are limiting. Adjuvants enable dose-sparing, but adverse events have been associated with some adjuvanted influenza virus vaccinations.Citation24,Citation25
We are developing an alternative approach to redress the low immunogenicity of H7N9 influenza that is linked to fewer CD4+ T cell epitopes in HA when compared with seasonal H1-HA and H3-HA,Citation13 and the presence of an epitope that induces functional regulatory T cells that may inhibit helper T cells needed to support a protective antibody response.Citation15 Our approach introduces seasonal HA-specific CD4+ T cell epitopes into H7N9 HA to produce a novel immunogen capable of priming protective responses by inducing CD4+ T cell memory. The premise for this vaccine concept rests on the significance of CD4+ T cell memory to influenza immunity,Citation26,Citation27 the essential role CD4+ T cells play in development of neutralizing antibodies,Citation28 linked specificity of HA-derived CD4+ T cell epitopes to antibody responses,Citation29–31 and the structural plasticity of HA.Citation32,Citation33 Indeed, immune memory is the basis for giving (non-adjuvanted) annual seasonal influenza vaccines as a single dose to boost humoral memory responses,Citation34 and when a novel seasonal HA emerges like pH1N1 in 2009, CD4+ T cell memory can support naïve B cell responses with a single vaccine dose to generate neutralizing antibodies.Citation30,Citation35,Citation36 Our ultimate goal is to design novel HA immunogens to produce vaccines capable of priming protective antibody responses by harnessing CD4+ T cell memory generated by influenza infection and vaccination. This approach can be applied to development of seasonal, avian, and universal influenza vaccines. CD4+ T cell epitope-augmented seasonal and universal vaccine antigens may extend duration of protective antibody levels or increase the magnitude of protective antibodies in a single season and reduce morbidity, mortality, and spread. The availability of augmented avian and universal antigens could enable prepandemic immunization and shorten timelines between outbreak onset and mass vaccination. Moreover, the approach is platform agnostic. Novel designs can be delivered in inactivated and live attenuated virus and recombinant protein formats of currently licensed influenza vaccines, as well as next generation formats, such as mRNA.
Previously, we designed a novel H7N9 HA immunogen (OPT1) that simultaneously removes a native Treg-inducing epitope and introduces the homologous sequence H3-HA306-318 into the corresponding site. H3-HA306-318 is a highly conserved and broadly reactive CD4+ T cell epitope. It is likely there have been large numbers of exposures to this epitope over many influenza seasons and >95% of infected humans may have developed H3-HA306-318-specific CD4+ T cell memory as the epitope binds eight class II HLA supertype alleles.Citation37,Citation38 Indeed, this epitope is commonly used as a positive control for influenza immunoreactivity in cellular assays. Thus, a novel H7N9 HA vaccine bearing H3-HA306-318 could preferentially recruit memory CD4+ T cells to increase antibody responses to an otherwise poorly immunogenic HA. In this study, we showed OPT1 immunization in HLA transgenic mice with H3N2 experience stimulates increases in CD4+ TCM cells, a self-renewing population with long term homeostatic maintenance that can rapidly proliferate and differentiate into effector T cells, as well as TEM cells that serve as a first line of defense upon re-encoutnering antigen with rapid production of effector functions. In addition, OPT1 stimulated greater TSCM over the native H7-HA sequence, which elevate the capacity to repopulate memory and effector CD4+ T cell compartments upon antigen re-exposure. Last, we found OPT1 immunization increased frequencies of activated and functional Tfh that are critical to providing help to B cells to support class switch recombination, affinity maturation and B cell differentiation into long-lived plasma cells and memory cells in germinal centers in secondary lymphoid tissue. These results corroborate a similar study using chimeric HA composed of a H7 head and H3 stalk that elicited H3-specific IL-2-producing CD4+ T cell recall in H3N2 pre-immune wild type mice.Citation39
Although enhanced T cell immunity was observed in this study, it did not translate into increased HAI. In a prior study, we also found OPT1, delivered then in recombinant protein format, did not increase HAI over WT, however, HA-specific IgG was elevated and detected sooner in OPT1-immunized HLA-DR3 transgenic mice, and these mice demonstrated greater survival and lower morbidity than WT-immunized mice.Citation18 This enhanced antibody response associated with greater protection may be related to increased stem-targeting neutralizing antibodies and Fc-mediated effector functions of antibodies that can contribute to protection. Additional studies bridging these antibody functions to T cell epitope modifications to HA are needed to support the hypothesis that increased CD4+ T cell immunity enhances protective antibody responses.
Low HAI titers were observed in both OPT1-VLP and WT-VLP immunized mice. Nonetheless, both the WT and OPT1 VLP vaccines protected against lethal H7N9 virus challenge with most mice surviving and undergoing only moderate weight loss. Other types of antibodies (stem-targeting neutralizing antibodies and antibodies that engage innate immune mechanisms) and/or cellular immunity may explain the observed protection and could be considered in future work.
Additionally, the dynamic range for detecting enhanced OPT1 survival over WT was insufficient as WT survival was 80–90%. Future studies could use lower vaccine doses or a stronger challenge dose to widen the window for discerning enhanced protection. Vaccine challenge timing can also be modified to align with T cell immunity differences between OPT1 and WT to better detect efficacy differences that are attributable to immunogenicity-augmenting epitope modifications. Significant differences between OPT1 and WT immunized mice in the Tfh compartment were seen only four weeks after boost immunization and mice were challenged two weeks after boosting. Challenge at four weeks following boost immunization or later would better model real-world conditions and could discern enhancement of an engineered vaccine design over the native antigen. Indeed, we previously showed OPT1 immunization outperforms the natural sequence in anti-H7 IgG response and efficacy in H3N2 pre-immune HLA-DR3 mice when formulated as a non-adjuvanted recombinant protein vaccine.Citation18
In summary, we showed a novel immunogen design strategy that uses site-specific modifications to augment T cell epitope content enhances CD4+ T cell responses among critical subpopulations needed for protective immunity upon antigen re-encounter. This strategy, relying on mobilization of immune memory, can be used to overcome the poor immunogenicity of avian influenza viruses. A similar approach may also be used to sustain protective antibody titers against seasonal influenza longer than current vaccines are capable.
Disclosure statement
A.S. De Groot is a senior officer and shareholder, and L.M. Meyers, C.M. Boyle, M. Grizotte-Lake, B.G. McGonnigal, and L. Moise are employees of EpiVax, Inc., a privately owned biotechnology company located in Providence, RI. These authors acknowledge that there is a potential conflict of interest related to their relationship with EpiVax and attest that the work contained in this research report is free of any bias that might be associated with the commercial goals of the company. The other authors declare no competing interests exist.
Additional information
Funding
References
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, et al. Human infection with a novel avian-origin influenza a (H7N9) virus. N Engl J Med [Internet]. 2013 [accessed 2021 Dec 28];368(20):1–10. doi:10.1056/NEJMOA1304459. Cited: in: PMID: 23577628.
- Qi W, Jia W, Liu D, Li J, Bi Y, Xie S, Li B, Hu T, Du Y, Xing L, et al. Emergence and adaptation of a novel highly pathogenic H7N9 influenza virus in birds and humans from a 2013 human-infecting low-pathogenic ancestor. J Virol [Internet]. 2018 [accessed 2021 Dec 28];92(2). doi:10.1128/JVI.00921-17. Cited: in: PMID: 29070694.
- Wang X, Wu P, Pei Y, Tsang TK, Gu D, Wang W, Zhang J, Horby PW, Uyeki TM, Cowling BJ, et al. Assessment of human-to-human transmissibility of avian influenza A(H7N9) virus across 5 waves by analyzing clusters of case patients in mainland China, 2013–2017. Clin Infect Dis [Internet]. 2019 [accessed 2021 Dec 28];68(4):623–31. doi:10.1093/CID/CIY541. Cited: in: PMID: 29961834.
- Wang X, Jiang H, Wu P, Uyeki TM, Feng L, Lai S, Wang L, Huo X, Xu K, Chen E, et al. Epidemiology of avian influenza a H7N9 virus in human beings across five epidemics in mainland China, 2013–17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis [Internet]. 2017 [accessed 2021 Dec 28];17(8):822–32. doi:10.1016/S1473-3099(17)30323-7. Cited: in: PMID: 28583578.
- WHO. WHO zoonotic influenza viruses: antigenic and genetic characteristics and development of candidate vaccine viruses for pandemic preparedness. Wkly Epidemiol Rec. 2017;92(12):129–44.
- Kile JC, Ren R, Liu L, Greene CM, Roguski K, Iuliano AD, Jang Y, Jones J, Thor S, Song Y, et al. Update: increase in human infections with novel Asian lineage avian influenza A(H7N9) viruses during the fifth epidemic — China, october 1, 2016–august 7, 2017. MMWR Morb Mortal Wkly Rep [Internet]. 2017 [accessed 2021 Dec 28];66(35):928–32. doi:10.15585/MMWR.MM6635A2. Cited: in: PMID: 28880856.
- Su S, Gu M, Liu D, Cui J, Gao GF, Zhou J, Liu XE. Evolution, and pathogenesis of H7N9 influenza viruses in five epidemic waves since 2013 in China. Trends Microbiol [Internet]. 2017 [accessed 2021 Dec 28];25(9):713–28. doi:10.1016/J.TIM.2017.06.008. Cited: in: PMID: 28734617.
- Burke SA, Trock SC. Use of influenza risk assessment tool for prepandemic preparedness. Emerg Infect Dis [Internet]. 2018 [accessed 2021 Dec 28];24(3):471–77. doi:10.3201/EID2403.171852. Cited: in: PMID: 29460739.
- Zhou J, Wang D, Gao R, Zhao B, Song J, Qi X, Zhang Y, Shi Y, Yang L, Zhu W, et al. Biological features of novel avian influenza a (H7N9) virus. Nature [Internet]. 2013 [accessed 2021 Dec 28];499(7459):500–03. doi:10.1038/NATURE12379. Cited: in: PMID: 23823727.
- Guo L, Zhang X, Ren L, Yu X, Chen L, Zhou H, Gao X, Teng Z, Li J, Hu J, et al. Human antibody responses to avian influenza A(H7N9) virus, 2013. Emerg Infect Dis [Internet]. 2014 [accessed 2021 Dec 28];20(2):192–200. doi:10.3201/EID2002.131094. Cited: in: PMID: 24447423.
- Mulligan MJ, Bernstein DI, Winokur P, Rupp R, Anderson E, Rouphael N, Dickey M, Stapleton JT, Edupuganti S, Spearman P, et al. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. Jama [Internet]. 2014 [accessed 2021 Dec 28];312(14):1409–19. doi:10.1001/JAMA.2014.12854. Cited: in: PMID: 25291577.
- Fries LF, Smith GE, Glenn GM. A recombinant viruslike particle influenza a (H7N9) vaccine. N Engl J Med [Internet]. 2013 [accessed 2021 Dec 28];369(26):2564–66. doi:10.1056/NEJMC1313186. Cited: in: PMID: 24224560.
- de Groot AS, Ardito M, Terry F, Levitz L, Ross T, Moise L, Martin W. Low immunogenicity predicted for emerging avian-origin H7N9: implication for influenza vaccine design. Hum Vaccin Immunother [Internet]. 2013 [accessed 2021 Dec 28];9(5):950–56. doi:10.4161/HV.24939. Cited: in: PMID: 23807079.
- de Groot AS, Moise L, Liu R, Gutierrez AH, Terry F, Koita OA, Ross TM, Martin W. Cross-Conservation of T-cell epitopes: now even more relevant to (H7N9) influenza vaccine design. Hum Vaccin Immunother [Internet]. 2014 [accessed 2021 Dec 28];10(2):256–62. doi:10.4161/HV.28135. Cited: in: PMID: 24525618.
- Liu R, Moise L, Tassone R, Gutierrez AH, Terry FE, Sangare K, Ardito MT, Martin WD, de Groot AS. H7N9 T-cell epitopes that mimic human sequences are less immunogenic and may induce Treg-mediated tolerance. Hum Vaccin Immunother [Internet]. 2015 [accessed 2021 Dec 28];11(9):2241–52. doi:10.1080/21645515.2015.1052197. Cited: in: PMID: 26090577.
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, et al. Follicular regulatory T cells expressing foxp3 and bcl-6 suppress germinal center reactions. Nat Med [Internet]. 2011 [accessed 2021 Dec 29];17(8):983–88. doi:10.1038/NM.2426. Cited: in: PMID: 21785430.
- Wada Y, Nithichanon A, Nobusawa E, Moise L, Martin WD, Yamamoto N, Terahara K, Hagiwara H, Odagiri T, Tashiro M, et al. A humanized mouse model identifies key amino acids for low immunogenicity of H7N9 vaccines. Sci Rep [Internet]. 2017 [accessed 2021 Dec 28];7(1). doi:10.1038/S41598-017-01372-5. Cited: in: PMID: 28455520.
- Jang H, Meyers LM, Boyle C, de Groot AS, Moise L, Ross TM. Immune-Engineered H7N9 influenza hemagglutinin improves protection against viral influenza virus challenge. Hum Vaccin Immunother [Internet]. 2020 [accessed 2021 Dec 23];16(9):2042–50. doi:10.1080/21645515.2020.1793711. Cited: in: PMID: 32783766.
- Carter DM, Darby CA, Lefoley BC, Crevar CJ, Alefantis T, Oomen R, Anderson SF, Strugnell T, Cortés-Garcia G, Vogel TU, et al. Design and characterization of a computationally optimized broadly reactive hemagglutinin vaccine for H1N1 influenza viruses. J Virol [Internet]. 2016 [accessed 2021 Dec 23];90(9):4720–34. doi:10.1128/JVI.03152-15. Cited: in: PMID: 26912624.
- Huang Y, Owino SO, Crevar CJ, Carter DM, Ross TM. N-Linked glycans and K147 residue on hemagglutinin synergize to elicit broadly reactive H1N1 influenza virus antibodies. 2020. doi:10.1128/JVI.01432-19.
- Tan GS, Lee PS, Hoffman RMB, Mazel-Sanchez B, Krammer F, Leon PE, Ward AB, Wilson IA, Palese P. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza a virus hemagglutinin. J Virol [Internet]. 2014 [accessed 2021 Dec 23];88(23):13580–92. doi:10.1128/JVI.02289-14. Cited: in: PMID: 25210195.
- Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HKB, Graham IL, Noah DL, He F, Hill H. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis [Internet]. 2008 [accessed 2021 Dec 28];197(5):667–75. doi:10.1086/527489. Cited: in: PMID: 18260764.
- Keitel WA, Campbell JD, Treanor JJ, Walter EB, Patel SM, He F, Noah DL, Hill H. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I–II randomized clinical trial. J Infect Dis [Internet]. 2008 [accessed 2021 Dec 28];198(9):1309–16. doi:10.1086/592172. Cited: in: PMID: 18808338.
- Eurosurveillance editorial team. Swedish medical products agency publishes report from a case inventory study on pandemrix vaccination and development of narcolepsy with cataplexy. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin [Internet].2011 [accessed 2021 Dec 28];16. doi:10.2807/ESE.16.26.19904-EN. Cited: in: PMID: 21745441.
- Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, Hublin C, Linna M, Olsén P, Nokelainen P, Alén R, Wallden T, Espo M, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One [Internet]. 2012 [accessed 2021 Dec 28];7(3):e33723. doi:10.1371/JOURNAL.PONE.0033723. Cited: in: PMID: 22470463.
- Jansen JM, Gerlach T, Elbahesh H, Rimmelzwaan GF, Saletti G. Influenza virus-specific CD4+ and CD8+ T cell-mediated immunity induced by infection and vaccination. J Clin Virol [Internet]. 2019 [accessed 2021 Dec 28];119:44–52. doi:10.1016/J.JCV.2019.08.009. Cited: in: PMID: 31491709.
- Spensieri F, Borgogni E, Zedda L, Bardelli M, Buricchi F, Volpini G, Fragapane E, Tavarini S, Finco O, Rappuoli R, et al. Human circulating influenza-CD4+ICOS1+IL-21+T cells expand after vaccination, exert helper function, and predict antibody responses. Proc Natl Acad Sci USA [Internet]. 2013 [accessed 2021 Dec 28];110(35):14330–35. doi:10.1073/PNAS.1311998110. Cited: in: PMID: 23940329.
- Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol [Internet]. 2011 [accessed 2021 Dec 28];29(1):621–63. doi:10.1146/ANNUREV-IMMUNOL-031210-101400. Cited: in: PMID: 21314428.
- Alam S, Knowlden ZAG, Sangster MY, Sant AJ. CD4 T cell help is limiting and selective during the primary B cell response to influenza virus infection. J Virol [Internet]. 2014 [accessed 2021 Dec 28];88(1):314–24. doi:10.1128/JVI.02077-13. Cited: in: PMID: 24155379.
- Nayak JL, Fitzgerald TF, Richards KA, Yang H, Treanor JJ, Sant AJ. CD4+ T-cell expansion predicts neutralizing antibody responses to monovalent, inactivated 2009 pandemic influenza A(H1N1) virus subtype H1N1 vaccine. J Infect Dis [Internet]. 2013 [accessed 2021 Dec 28];207(2):297–305. doi:10.1093/INFDIS/JIS684. Cited: in: PMID: 23148285.
- Nayak JL, Richards KA, Yang H, Treanor JJ, Sant AJ. Effect of influenza A(H5N1) vaccine prepandemic priming on CD4+ T-cell responses. J Infect Dis [Internet]. 2015 [accessed 2021 Dec 28];211(9):1408–17. doi:10.1093/INFDIS/JIU616. Cited: in: PMID: 25378637.
- Das SR, Hensley SE, Ince WL, Brooke CB, Subba A, Delboy MG, Russ G, Gibbs JS, Bennink JR, Yewdell JW. Defining influenza a virus hemagglutinin antigenic drift by sequential monoclonal antibody selection. Cell Host Microbe [Internet]. 2013 [accessed 2021 Dec 28];13(3):314–23. doi:10.1016/J.CHOM.2013.02.008. Cited: in: PMID: 23498956.
- Thyagarajan B, Bloom JD. The inherent mutational tolerance and antigenic evolvability of influenza hemagglutinin. Elife [Internet]. 2014 [accessed 2021 Dec 28];3. doi:10.7554/ELIFE.03300. Cited: in: PMID: 25006036.
- Fiore AE, Bridges CB, Cox NJ. Seasonal influenza vaccines. Curr Top Microbiol Immunol [Internet]. 2009 [accessed 2021 Dec 28];333:43–82. doi:10.1007/978-3-540-92165-3_3. Cited: in: PMID: 19768400.
- Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, et al. Response to a monovalent 2009 influenza a (H1N1) vaccine. N Engl J Med [Internet]. 2009 [accessed 2021 Dec 28];361(25):2405–13. doi:10.1056/NEJMOA0907413. Cited: in: PMID: 19745216.
- Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza a (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med [Internet]. 2009 [accessed 2020 Dec 23];361(25):2424–35. doi:10.1056/nejmoa0907650.
- Weber CA, Mehta PJ, Ardito M, Moise L, Martin B, de Groot AS. T cell epitope: friend or foe? Immunogenicity of biologics in context. Adv Drug Deliv Rev [Internet]. 2009 [accessed 2021 Dec 28];61(11):965–76. doi:10.1016/J.ADDR.2009.07.001. Cited: in: PMID: 19619593.
- Posch PE, Hastings AE, Rosen-Bronson S, Richert JR, Hurley CK. The relative importance of individual DR binding motif positions as defined by peptide anchor analysis of influenza hemagglutinin peptide 306-318 and human myelin basic protein peptide 152-165 binding to several DR molecules: definition of a common extended DR binding motif. Eur J Immunol [Internet]. 1996 [accessed 2021 Dec 28];26:1884–91. doi:10.1002/EJI.1830260832. Cited: in: PMID: 8765035.
- DiPiazza AT, Fan S, Rattan A, DeDiego ML, Chaves F, Neumann G, Kawaoka Y, Sant AJ. A novel vaccine strategy to overcome poor immunogenicity of avian influenza vaccines through mobilization of memory CD4 T cells established by seasonal influenza. J Immunol [Internet]. 2019 [accessed 2021 Dec 28];203(6):1502–08. doi:10.4049/JIMMUNOL.1900819. Cited: in: PMID: 31399519.
