 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In South Korea, despite the implementation of a universal single-dose vaccination program for children aged 12–15 months in 2005, the varicella incidence rate remains significant. Prior case-control studies have reported that currently used varicella vaccines are extremely inefficacious. We estimated vaccine effectiveness (VE) by fitting a dynamic transmission model to age-specific varicella incidence data from 2007 to 2015 and available vaccine coverage data. The initial vaccine efficacy and primary failure rates were estimated to be 61.1% and 38.9%, respectively. The average duration of protection was 21.4 years. The mean VE [(1-relative risk) %] for the simulated data of 2004–2014 birth cohorts decreased from 59.8% to 50.7% over 9 years. This mathematical modeling study demonstrated that the single-dose vaccine exhibits moderate effectiveness, and a high proportion of primary failure could be a main cause of breakthrough infections. Therefore, a two-dose vaccination strategy should be considered.
Introduction
Varicella, caused by varicella-zoster virus (VZV), is a highly infectious disease, with secondary attack rates reaching up to 90% for susceptible household contacts.Citation1 On a global scale, varicella vaccines are highly effective in reducing the incidence and burden of varicella. A systematic review reported the overall single-dose varicella vaccine effectiveness (VE) to be approximately 80%, whereas the two-dose VE was approximately 84–98%.Citation2,Citation3 The World Health Organization recommends varicella vaccination be introduced into routine immunization programs.Citation4
In South Korea, varicella vaccines were introduced in 1988. A universal single-dose vaccination program for children aged 12–15 months was implemented in 2005. Vaccine coverage was assumed to be approximately 70% before the universal vaccination program and increased to 97% among 12–15-month-old children.Citation5–9 According to a population-based longitudinal study, the varicella incidence rate was moderately reduced upon the introduction of the vaccination program.Citation10 However, the number of notified varicella cases remains as high as >80,000 in 2019,Citation9,Citation11 suggesting that the effectiveness of the current vaccination program may be insufficient to interrupt VZV transmission in children. Consequently, the effectiveness of varicella vaccines used in South Korea has been critically questioned.Citation1,Citation12 In a case-control study, VE was estimated to be 13%, with immunity rapidly decreasing three years after vaccination. A recent study followed a birth cohort of children born in 2011 for 8 years retrospectively and estimated the VE against severe and any varicella to be 62.7% and 40.8%, respectively.Citation13
However, estimations of VE using conventional study designs are limited because high vaccine coverage has been maintained for >10 years. Furthermore, the indirect protection of vaccination may leave reduced opportunities for varicella among unvaccinated children, which underestimates the VE.Citation10 Although the National Immunization Program provides a single-dose vaccine, second-dose vaccination has been practiced in private clinics, with approximately 26.7% of the 2011 birth cohort having received a second dose.Citation13 This could have increased the indirect effect by reducing varicella incidence. Other factors that potentially affect the varicella incidence rate include the rapid decline in child population, reduced opportunities for immunity boosting following exposure to circulating VZV in children, and a growing incidence of herpes zoster among the elderly.Citation10,Citation14
Considering the complexity of VZV transmission dynamics and interactions between direct and indirect vaccine effects in a population, mathematical modeling can be an adjunctive method to assess the effectiveness of the vaccination program. In a prior study, we developed a dynamic compartmental model of VZV that accounts for South Korea’s changing population structure.Citation14 In this study, we applied this model to estimate the effectiveness of single-dose varicella vaccines used in South Korea.
Materials and methods
Model structure and estimation
We used the VZV transmission dynamic model developed in our previous study.Citation14 The model was stratified by disease development stage, vaccination status, and age. The stages of disease development progress from uninfected to varicella susceptible, varicella exposed, varicella infectious, varicella recovered after VZV infection, zoster susceptible, zoster infectious, and zoster recovered after VZV reactivation. Vaccinated individuals are divided into three states: temporarily protected, initial failure to mount sufficient response despite seroconversion, and failure to seroconvert (primary vaccine failure). Temporarily protected individuals become susceptible to varicella as the vaccine-induced immunity wanes. We incorporated South Korea’s population demographics in our model, and the contact patterns between and within age groups were derived from the POLYMOD survey data.Citation14,Citation15
Vaccine effectiveness is determined by initial efficacy and waning immunity. We estimated the proportion of children temporarily protected by the single-dose vaccine () at the time of vaccination, initial failure to mount sufficient response despite seroconversion (
), the proportion of primary vaccine failure (
), and the average duration of protection after vaccine-induced immunity (
) by fitting the model to age-specific incidence data (). We obtained point estimates using maximum likelihood estimation assuming a Poisson distribution and estimated 95% confidence intervals by profiling likelihood.
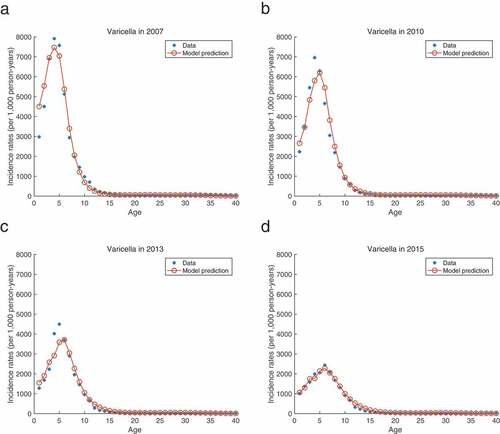
Figure 1. Age-Specific varicella incidence rates (per 1,000 person-years) and model prediction from 2007 to 2015 (a–d).
The VE of each birth cohort from 2004 to 2014 was calculated as follows using simulated data.
Data source
The age-specific varicella incidence rates per 100,000 population from 2007 to 2015 were obtained from existing literature that used the National Health Information Database (NHID). This database encompasses almost the entire Korean population. Varicella cases were defined by varicella-related ICD-10 codes (B01) in any field. Single-dose vaccine coverage data was collected from national vaccination surveys and registry reports.Citation5–7−Citation16
The two-dose vaccine efficacy () was assumed to be 92%, with the primary vaccine failure (
) being 1%.Citation3 The immunity waning (
) after two-dose vaccination was assumed to be almost lifelong (60 years). The second dose was assumed to be administered to 4-year-old children from 2011, with the coverage increasing up to 26.7% in 2015.Citation13 For sensitivity analysis, we examined the influence of lower two-dose vaccine efficacy (88%, 84%) and higher second-dose vaccine coverage (30%, 35%) on the estimates.
Results
Under the 92% two-dose vaccine efficacy, the initial single-dose vaccine efficacy was estimated at 61.1% (95% CI: 60.2–61.5), with primary vaccine failure being 38.9% (95% CI: 38.1–39.3). The average duration of vaccine-induced protection was estimated to be 21.4 years (95% CI: 20.0–23.0 years) (). As two-dose efficacy decreased, there were slight changes in the estimated parameters. However, the influence of two-dose efficacy on the single-dose VE was not significant, with second dose coverage at 26.7%. With an increase in second dose coverage, the initial single-dose vaccine efficacy and primary failure changed minimally, whereas the duration of protection significantly decreased (). The VE of each birth cohort decreased over time. When each birth cohort reached the age of 10 years, the mean VE decreased to 50.7% (range: 49.1–52.4%) in the base scenario, as shown in . In a sensitivity analysis with decreasing two-dose vaccine efficacy, the VE decreased over 9 years to a level similar to that of the base case (the mean VE: 50.7–50.9%). However, as two-dose vaccine coverage increased, a further decline in the VE was noted over time (the mean VE: 46.5–49.1%) ().
Figure 2. The effectiveness of single-dose varicella vaccinations over 9 years, calculated using simulated data by birth cohort (2004–2014) (a), sensitivity analyses with a decreasing two-dose vaccine efficacy (T2) (b,c), and an increase in two-dose vaccine coverage (C2) (d,e).
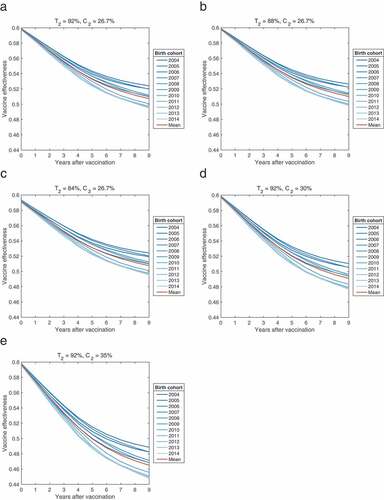
Table 1. Estimated initial efficacy and primary failure of single-dose varicella vaccine with 95% confidence intervals under the base scenario, and sensitivity analyses with different levels of two-dose efficacy and coverage.
Discussion
In this mathematical modeling study, the initial single-dose vaccine efficacy and primary vaccine failure remained stable with changes in the second dose vaccine efficacy and coverage. Although the average immunity period was 21.4 years in the base scenario, it was reduced to 13.8 years when the second dose coverage increased to 35%. This result suggests that the high primary vaccine failure rate is a major cause of breakthrough infections among children.
It is well-established that the primary vaccine failure rate was relatively high after a single dose, regardless of the vaccine strain used. The failure rate after the Oka strain vaccine was reported to be as high as 24% when measured by fluorescent antibody to membrane antigen (FAMA) assay. In South Korea, the MAV strain (SuduVax, Green Cross, Yongin, Korea) accounted for more than 50% of varicella vaccines, followed by the Oka strain (Vari-L, Changchun Institute of Biological Products, Changchun, China).Citation17 Prior studies found that the seroconversion rate by FAMA assay after administration of the MAV strain was 76.7% among 120 children in 2008–2009. In a study performed in 2015, the seropositivity, defined as anti-VZV IgG >100 mIU/mL, was found to be 66% and 64% among 1-year-old children receiving the MAV and Oka vaccines, respectively. These figures decreased to 51% and 46% by the age of 4 years. These results correlate with the estimated values found in our study.
In previous case-control studies, the overall effectiveness of the single-dose vaccination program in South Korea was reported to range from 13% to 40%. However, the cumulative varicella incidence was reduced by >60% among children born after the NIP program,Citation10,Citation18 which does not correspond to the VE estimated through case-control studies. These studies could have potential limitations that fail to account for the indirect effects of vaccination and age-specific contact patterns. In our study, VE was estimated to be higher than in observational studies, highlighting the adjunctive role of mathematical modeling by incorporating the complexity of VZV transmission dynamics and the indirect effects of vaccination. Nonetheless, VE was far below the average for a single dose (81%) against any varicella estimated in a systematic review.
Despite the contending issues described above, the decline in the number of severe varicella cases demonstrates the beneficial impact of universal varicella vaccinations in children.Citation19 However, to further reduce varicella incidences, outbreaks, morbidity, and mortality, further measures need to be considered. Possible solutions include using more efficacious vaccine strains in the Korean NIP and implementing a two-dose varicella vaccination program.
n clinical fields, VE is affected by the characteristics of the vaccine and its recipients.Citation20 Factors such as vaccine storage and handling, cold chain maintenance, and appropriateness of vaccine administration are critical in maintaining VE and may increase the primary vaccine failure rate in field studies. The government and healthcare sectors should take continuous efforts to maintain the cold chain in clinics and educate healthcare workers to comply with the vaccination protocol to improve the benefits of the vaccination program.
Our study has several limitations in that there are uncertainties in parameter estimation. First, data concerning the second dose’s efficacy and coverage in South Korea are limited. Therefore, we used the second-dose vaccine efficacy from a systematic review and assumed that the second dose coverage of the 2011 birth cohort was equal to that of the other birth cohorts. In our study, the influence of second-dose vaccine efficacy on the estimates was not significant, whereas the duration of protection was considerably reduced with an increase in the two-dose vaccine coverage. We also used POLYMOD contact data owing to the lack of Korean contact data, and the contact patterns in European countries may differ from those in South Korea. However, the age contact patterns in the overall POLYMOD data can represent the general contact patterns among age groups. Despite these limitations, our study results correlated well with epidemiologic data and serosurvey.
Conclusions
This mathematical modeling study demonstrated that the South Korean single-dose varicella vaccination program yields a moderate VE, and primary vaccine failure may be a major cause of breakthrough infections. Therefore, introducing more efficacious vaccine strains and a two-dose vaccination strategy can prevent varicella transmission more efficiently among children.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data can be shared upon request to the corresponding authors in a collaborative research approach.
Additional information
Funding
References
- Oh SH, Choi EH, Shin SH, Kim YK, Chang JK, Choi KM, Hur JK, Kim KH, Kim JY, Chung EH, et al. Varicella and varicella vaccination in South Korea. Clin Vaccine Immunol. 2014;21(5):1–5. doi:10.1128/CVI.00645-13.
- WHO. Systematic review of available evidence on effectiveness and duration of protection of varicella vaccines. World Health Organization; 2014 [accessed 2018 Sept 1]. https://www.who.int/immunization/sage/meetings/2014/april/4_Systematic_review_on_effectiveness_and_duration_of_protection_of_varicella_vaccines.pdf.
- Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics. 2016;137(3):e20153741. doi:10.1542/peds.2015-3741.
- Wutzler P, Bonanni P, Burgess M, Gershon A, Safadi MA, Casabona G. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017;16(8):833–843. doi:10.1080/14760584.2017.1343669.
- Lee SG. Korean national immunization survey, 2011. Korea Centers for Disease Control and Prevention; 2011.
- Lee SG. Korean national immunization survey, 2013. Osong (Republic of Korea): Korea Centers for Disease Control and Prevention; 2013 [accessed 2018 Oct 1]. https://nih.go.kr/board/board.es?mid=a40802000000&bid=0071&act=view&list_no=715421.
- National childhood vaccination coverage among children aged 3 years in Korea, 2015. Osong (Republic of Korea): Korea Centers for Disease Prevention and Control; 2016 [accessed 2018 Oct 1]. https://nip.kdca.go.kr/irgd/reference.do?MnLv1=7.
- Shin E, Lee M, Kown S, Ki M, Kim K, Na B, et al. Development of vaccination coverage estimation methods and evaluation indicators of national immunization program in Korea. SEOUL: Korea Center for Disease Control and Prevention; 2005.
- Lee J, Jeoung H, Kim S, Kim J, Lee S. National childhood vaccination coverage among children aged 1-3 and 6 years in Korea, 2019. Public Health Weekly Rep. 2021;13:3549–3559.
- Choi JK, Park SH, Park S, Choi SM, Kim SH, Lee DG, Yoo JH, Choi JH, Kang JH. Trends in varicella and herpes zoster epidemiology before and after the implementation of universal one-dose varicella vaccination over one decade in South Korea, 2003-2015. Hum Vaccin Immunother. 2019;15(11):2554–2560. doi:10.1080/21645515.2019.1603985.
- KDCA. Infectious diseases surveillance yearbook, 2019. Osong (Chungchungbook-do): Korea Disease Control and Prevention Agency; 2019. http://www.kdca.go.kr/npt/biz/npp/ist/simple/simplePdStatsMain.do.
- Lee YH, Choe YJ, Cho SI, Kang CR, Bang JH, Oh MD, Lee JK. Effectiveness of varicella vaccination program in preventing laboratory-confirmed cases in children in Seoul, Korea. J Korean Med Sci. 2016;31(12):1897–1901. doi:10.3346/jkms.2016.31.12.1897.
- Choi EH. Effectiveness of varicella immunization in Korea. Korea Centers for Disease Control and Prevention; 2019.
- Suh J, Lee T, Choi JK, Lee J, Park SH. The impact of two-dose varicella vaccination on varicella and herpes zoster incidence in South Korea using a mathematical model with changing population demographics. Vaccine. 2021;39(18):2575–2583. doi:10.1016/j.vaccine.2021.03.056.
- Mossong J, Hens N, Jit M, Beutels P, Auranen K, Mikolajczyk R, Massari M, Salmaso S, Tomba GS, Wallinga J, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74. doi:10.1371/journal.pmed.0050074.
- Lee SG. Korea national immunization survey. Osong: Korea Centers for Disease Control and Prevention; 2012. Prevention KCfDCa. https://nih.go.kr/board/board.es?mid=a40802000000&bid=0071&act=view&list_no=715227.
- Choi UY, Huh DH, Kim JH, Kang JH. Seropositivity of Varicella zoster virus in vaccinated Korean children and MAV vaccine group. Hum Vaccin Immunother. 2016;12(10):2560–2564. doi:10.1080/21645515.2016.1190056.
- Jung J, Ko YJ, Kim YE, Huh K, Park BJ, Yoon SJ. Epidemiological impact of the Korean national immunization program on Varicella incidence. J Korean Med Sci. 2019;34(7):e53. doi:10.3346/jkms.2019.34.e53.
- Lee YH, Choe YJ, Cho SI, Park H, Bang JH, Lee JK. Effects of One-dose Varicella Vaccination on Disease Severity in Children during Outbreaks in Seoul, Korea. J Korean Med Sci. 2019;34(10):e83. doi:10.3346/jkms.2019.34.e83.
- Crowcroft NS, Klein NP. A framework for research on vaccine effectiveness. Vaccine. 2018;36(48):7286–7293. doi:10.1016/j.vaccine.2018.04.016.
