?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Mumps cases were reported frequently when a routine dose measles–mumps–rubella(MMR) achieved high coverage in Quzhou. The supplementary immunization activities (SIA) using measles mumps (MM) was conducted to control mumps outbreaks. The effectiveness of one and two doses of mumps-containing vaccine (MuCV) was assessed using surveillance data in this study. Mumps cases and immunization information were retrieved from the National Notifiable Disease Reporting System (NNDRS) and the Zhejiang Provincial Immunization Information System (ZJIIS), respectively. Mumps cases of children born from 2006 to 2010 were included. Vaccine effectiveness by dose was calculated using the screening method. A total of 956 mumps cases were identified, of whom 754 (78.9%) had received one dose of MuCV; 108 (11.3%) had received two doses; 94 (9.8%) were unvaccinated. The coverage of one-dose MuCV in the 2006–2010 birth cohorts ranged from 91.6% to 98.9%. Except the 2009 birth cohort in which the coverage of two doses of MuCV was 55.1%, the others were less than 10%. Vaccine effectiveness (VE) of one dose ranged from 47.4% to 86.0%, while VE of two doses ranged from 64.0% to 92.4%. The VE of one and two doses of MuCV waned over time, but the VE of two doses was consistently higher than that of one dose in the same period. The vaccine schedule with two-dose MMR should be implemented among children in Quzhou. The optimal age for the second dose needs to be further evaluated.
Introduction
Mumps is known as infectious parotitis or epidemic parotitis and is caused by mumps virus (MuV). Although patients’ typical facial swelling gave mumps its name, not all patients have swollen parotid glands. Some have mild or no symptoms.Citation1 The best way to protect against mumps is to vaccinate most of the population. In China, mumps-containing vaccine (MuCV) was a self-paid vaccine before 2007. In 2007, the domestic measles–mumps–rubella (MMR) (S79 vaccine strain) was introduced into the Expanded Program on Immunization (EPI) for children who were born after 1 January 2006 and aged 18–24 months. The administration of MuCV in children has been proven highly effective in reducing the incidence of mumps. Mumps incidence declined following widespread vaccination, and many developed countries have experienced a resurgence of mumps cases over the past decade.Citation2–4 There were 893 mumps outbreak cases reported between 1 March 2015 and 31 December 2016 among the highly vaccinated populations in northern Western Australia.Citation5 The surveillance data showed that mumps was one of the most common infectious diseases among children aged 0–14 years in Zhejiang Province in 2008–2017.Citation6 Despite the high vaccination rate of MMR in Quzhou, 8,525 mumps cases were reported from 2005 to 2016, with more than 1,839 mumps cases reported in 2009.Citation7 From September to December in 2010, supplementary immunization activity (SIA) using measles mumps (MM) was performed in Zhejiang province. The children born from 1 October 2005 to 31 December 2009 would receive one dose of MM free of charge, whether they were local or migrant children, and with or without a history of MuCV. The cumulative hazard of mumps after SIA was a broadly linear decrease.Citation8
The increasing incidence of mumps made it necessary to research the effect of the mumps vaccine and explain why mumps outbreaks continue to occur in healthy, highly vaccinated populations. Research suggests that the increasing incidence trend is likely due to a combination of low or incomplete vaccine coverage, primary vaccine failure, and secondary vaccine failure.Citation9 Studies of outbreaks in Canada demonstrated that the chance of developing mumps increased by 10–27% every year after vaccination, implying a waning of immunity in vaccinated individuals to subprotective levels.Citation10 Therefore, rapid assessment of vaccine effectiveness is an important component of management for vaccine-preventable diseases, and perfecting the vaccination program is necessary for interrupting community transmission of mumps. Because of SIA using MM in 2010, children born from 2006 to 2010 in Quzhou may be vaccinated with different doses of MuCV. There was less information available about vaccine effectiveness after the SIA. The objectives of our study were to assess the vaccine effectiveness of MuCV by dose and by birth cohort using surveillance data and explore a better immunization strategy to interrupt community transmission of mumps.
Materials and methods
Surveilliance system
Quzhou is a prefecture-level city located in Zhejiang province in eastern China and includes two districts and four counties; the resident population is about 2,218,000, with an annual birth cohort of approximately 24,000. Quzhou reported the notifiable infectious disease via the National Notifiable Disease Reporting System (NNDRS) and registered the vaccination information via the Zhejiang Provincial Immunization Information System (ZJIIS). The NNDRS is a web-based computerized reporting system, which has been in operation since 2004 in China. When a physician encounters a statutorily reported infectious disease in the course of his or her career, he or she must report it within a specified time limit via the NNDRS. In China, mumps was classified as a category C notifiable infectious disease in 1990 and was mandatorily reported within 24 hours in the NNDRS since 2004. The ZJIIS was a passive surveillance system, which registered the vaccination information for children aged less than 15 years residing in the local areas, regardless of whether they were locally born or migrated. Since 2005, each person in the ZJIIS was assigned a unique identification code when they first contact the vaccination clinic, which recorded the child’s demographic information, historical immunization data, and current immunization status.
Case definition
The mumps cases were diagnosed according to the diagnostic criteria for mumps approved by the Ministry of Health of China in 2007. A clinical case of mumps was defined as a person of acute onset of unilateral or bilateral swelling of the parotid gland or other salivary glands characterized by any of the following, which could not be explained by another more likely diagnosis: (1) fever, headache, weakness, loss of appetite; (2) orchitis; (3) pancreatitis; (4) encephalitis and/or aseptic meningitis. A laboratory-confirmed case was defined as a clinical case with one of the following laboratory evidence: (1) positive for IgM antibodies and no mumps vaccine within 1 month; (2) the titer of serum IgG increased by 4 times or more; and (3) isolated mumps virus in throat swab, urine, or cerebrospinal fluid. A breakthrough case was defined as a clinical case or a laboratory-confirmed case that had received one or more doses of MuCV, and the onset time of symptoms since the last dose vaccination was more than 42 days. An excluded case was defined as a clinical case or a laboratory-confirmed case who had received one dose of MuCV within 42 days before the onset of illness.
Data resources
Eight thousand three hundred eighty-four mumps cases residing in Quzhou were reported in the NNDRS during 2006–2020, 978 of which were children born from 2006 to 2010. Basic information of the 978 mumps cases was obtained from the NNDRS on 10 January 2021, including name, parents’ name, gender, birthday, ID number, telephone, occupation, current address, date of onset, date of diagnosis, date of report, and case classification (clinical or laboratory confirmed). Cases were matched according to name, gender, birthday, ID number, and parents’ name in the ZJIIS to get their immunity dose and vaccination data of MuCV.
After information comparison, five of the 978 mumps cases were reported repeatedly. Two had their year of birth registered incorrectly, one was born in 2005 and the other was born in 2011. Eleven cases were unmatched in the ZJIIS, their ID numbers were listed as non-local personnel, and the vaccination information was not registered in the ZJIIS. Four cases were excluded based on case definition. Finally, 956 cases were included in the study. The vaccination information of children born from 2006 to 2010 was obtained from the ZJIIS on 31 December 2020. The number of children in the 2006–2010 birth cohorts was based on the number of children assigned in the ZJIIS, which included all children residing in Quzhou, regardless of whether they were locally born or migrated.
Statistical analysis
The simple life table method was used to calculate person-year, which stipulated that individuals entering the cohort in the observed year will be calculated as 1/2 person-year and those who are lost to follow-up or have an end result will also be calculated as 1/2 person-year. Assume that the onset of mumps is an end result.
Formula to calculate person-year using the simple life table method is
Lx is the number of years of exposure at time x, Ix is the number of observation at the beginning of time X, Nx is the number of entry into the queue at time X, Dx is the number of end results at time x, and Wx is the number of lost follow-up at time X.
According to the Immunization Procedures and Instructions for Children of China Immunization Program (version 2021), timely vaccination was defined as one dose of MuCV completed within 2 years, the number of cumulative vaccination was defined as the cumulative number of one or two doses of MuCV completed before the statistical deadline.
Case and coverage data by dose were used to calculate vaccine effectiveness using the screening method.Citation11 When estimating the effectiveness of one dose, people who had received two doses were excluded from the calculations of the proportions of cases and the population vaccinated. Similarly, people who had received one dose were excluded from calculations that estimated the effectiveness of two doses.
Where VEi is the vaccine effectiveness of i doses, PCVi is the proportion of cases vaccinated with i doses, PPVi is the proportion of the population vaccinated with i doses, and i is 1 or 2.
Data were collected using Microsoft Office Excel (version 2007) and were analyzed using SPSS for Windows, version 16.0 (SPSS Inc., USA). Differences between proportions were calculated using Chi-square tests and the onset time was compared by t test or analysis of variance and at a significance level of 0.05.
Ethical considerations
Strict regulations were established and supervised by the China CDC to protect patients’ privacy. The local CDCs were given access to the surveillance data for the purpose of research. Personal data was anonymized by deleting the personal identifiers (such as patient name, address, and telephone number) and determined as exempt from ethical review by the ethics committee of the Quzhou Centers for Disease Control and Prevention (QZCDC).
Results
Mumps case characteristics
The 956 mumps cases were all clinically diagnosed, 94 (9.8%) of which were unvaccinated, 754 (78.9%) had received one dose of MuCV, and 108 (11.3%) had received two doses. The mumps incidence was higher in boys than in girls (622 boys vs. 334 girls, χ2 = 75.7, p < 0.001). The median age of the included cases at the time of diagnosis was 5.5 y (range: 5.0 months to 14.2 years). The mean age of the cases with no MuCV, with one dose of MuCV, with two doses of MuCV were 4.1 y, 6.1 y, and 6.5 y, respectively (F = 26.0, p < 0.001). showed that the average incidence of mumps cases was 81.9 per 100,000 person-years, ranging from 73.6 to 95.7 per 100,000 person-years by the 2006–2010 birth cohorts during 2006 to 2020, and the lowest was the 2009 birth cohort. Except for the 2006 birth cohort, the incidence by the other four birth cohorts showed an overall decreasing trend.
Table 1. The incidence of mumps cases by the birth cohorts during 2006 to 2020 in Quzhou.
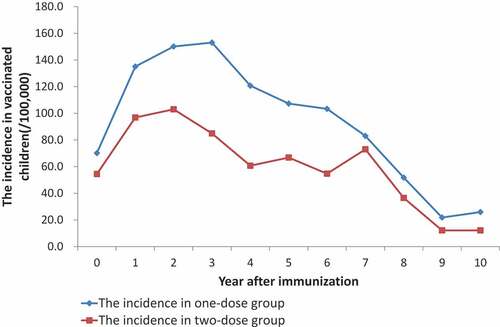
The mumps incidence in the one-dose group increased within 4 years after immunization, then went down year by year. In the two-doses group, the incidence ascended within 3 years since the second dose vaccination and then gradually descended, whereas it was lower than that of the same period in the one-dose group ().
MuCV vaccination coverage
As of the time of statistics, 93,651 children born from 2006 to 2010 were obtained from the ZJIIS, 81,116 of whom had received one dose of MuCV within 2 years, 90,673 had received one dose of MuCV, and 16,553 had received two doses of MuCV. The coverage of timely vaccination with one-dose MuCV ranged from 80.2% to 92.7%, and the cumulative coverage ranged from 91.6% to 98.9%, which showed the increasing trend by the birth cohorts. Except for the 2009 birth cohort, where two-dose MuCV coverage was 55.1%, the other four birth cohorts were all below 10% ().
Table 2. The coverage of one and two doses of MuCV by the birth cohorts during 2006 to 2020 in Quzhou.
VEs of MuCV by dose
The VE of one dose ranged from 47.4% to 86.0%, whereas the effectiveness of two doses ranged from 64.0% to 92.4%. The VE of two doses of MuCV was consistently higher than the effectiveness of one dose for each of the cohorts ().
Table 3. Estimates of VE for one and two doses of MuCV by the birth cohorts during 2006 to 2020 in Quzhou.
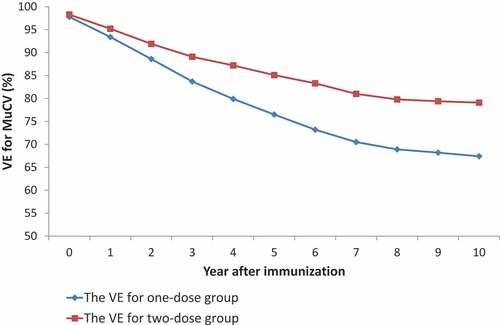
The VE for the one-dose group was 97.8% (95% CI: 96.8%–98.4%) within 1 year after immunization, and it was down to 67.4% (95% CI: 59.5%–73.8%) within 10 years after immunization. The VE for the two-dose group was 98.3% (95% CI: 96.5%–99.1%) within 1 year after immunization, and it was down to 79.1% (95% CI: 72.3%–84.2%) within 10 years after immunization. The VE for the two-doses group was higher than that for the one-dose group in the same period ().
Discussion
Mumps vaccine effectiveness, children who were only eligible to receive one dose of vaccine, and potential waning immunity have been cited as possible explanations for the resurgence of mumps in vaccinated individuals.Citation12,Citation13 Domestic MMR (S79 strain) was introduced into the EPI in Zhejiang since 2007, but the EPI only supported one-dose mumps vaccination strategy. The incidence in the 2009 birth cohort was the lowest, and its cumulative coverage of MuCV was the highest. Except for the 2006 birth cohort, the incidence of mumps cases was inversely proportional to the timely coverage and the cumulative coverage of one-dose MuCV by birth cohort. The low incidence in the 2006 birth cohort may be related to long observation years and the peak age of mumps. In order to control mumps and speed up the process of measles elimination, SIA using domestic MM (S79 strain) was launched throughout Zhejiang from September to December 2010. But the coverage of two-dose MuCV was only 17.7%, the other four birth cohorts were all less than 10% except for the 2009 birth cohort. The high vaccination rate in the 2009 birth cohort may be explained by the fact that children born in 2009 were the target population and under 2 years, who have better vaccination compliance. We found that the mumps cases persisted after immunization, whereas it was lower in the two-dose group than that of the same period in the one-dose group. The overall VE estimates of MuCV against mumps were 67.4% (95% CI: 59.5–73.8%) for one dose and 79.1% (95% CI: 72.3–84.2%) for two doses, which were similar to those of the study in Beijing.Citation14 The VE of two doses was consistently higher than that of one dose for each cohort. The qualitative determination of IgG mumps manifested that single dose of MMR vaccine does not provide the effective (>90%) seroconversion required for successful herd immunity to prevent mumps outbreak.Citation15 Therefore, it is necessary to add a second dose of MuCV to the routine immunization schedule to control mumps epidemics in China.Citation16
showed that the VE after immunization went down every year. The estimated overall annual waning rate of MMR vaccine was 0.024 (95% CI: 0.016–0.039) for mumps,Citation17 and the VEs waned within 10 years since vaccination. Our study showed that the VE after immunization dropped more quickly in the one-dose group than in the two-dose group, and it went down below 70% after 10 years in the one-dose group. A French study concluded that the effectiveness of mumps vaccine waned with time and suggested boosting for individuals whose last dose was more than 10 years ago.Citation18 The mean time of waning in vaccine immunity was 14.3 years from the last dose of the mumps vaccine, which encouraged to have two doses MMR and be avoided for mumps infection.Citation19 In our research, the coverages of one and two doses of MuCV were high in the 2009 birth cohort, but the VEs of the 2009 birth cohort were lower than the other birth cohorts. The reason may be related to the interval between two doses of MuCV. The interval of two doses of MuCV in the 2009 birth cohort was less than 1 year. Exploring the benefit of increasing the intervals between vaccine doses to strengthen the persistence of vaccine protection was needed. Our study also demonstrated that boys were more susceptible than girls, and the median age was 5.5 years old, which was consistent with other studies.Citation20 Shanghai’s study showed that the incidence of mumps has significantly declined with high coverage of two-doses MMR, and children in kindergartens and schools are still the most affected populations.Citation21 Therefore, the second dose of MuCV may be administered for preschooler but need to be further studied in the future.
Twelve genotypes of mumps virus are currently recognized by the World Health Organization. Researchers considered that the genotype of the vaccine was significantly different against genotypes of mumps virus, which highlighted the possibility of a recurrence of mumps in vaccinated populations depending on the degree of genetic consistency.Citation22 The mumps vaccine used in the EPI of Zhejiang included MM and MMR, and they were all domestic live-attenuated vaccines with S79 vaccine strain and produced by China National Biotec Group. The MMR vaccine using Jery1 Lynn strain produced by Merk was available for private purchase but was not widely used. Waning humoral immunity and antigenic variation of circulating wild-type mumps strains may play a role in the mumps resurgence.Citation23 Research works have shown the effectiveness of the current MuCV in preventing infection wane over time, which may be less effective against some mumps strains.Citation24 We did not carry out the study of the genotype of the local mumps viruses in the past, and the surveillance need to assess the similarity between the vaccine strain and the virus strain. The MMR contained three virus types and protected against measles, rubella, and mumps, which was better than monovalent mumps vaccine or MM against one or two diseases. ResearchCitation25showed that infants vaccinated with MMR generated more health economic effects than monovalent or bivalent vaccine. Therefore, using MMR in two doses of MuCV was the best choice over monovalent mumps vaccine and MM.Citation26
There were several limitations in our study. First, all mumps cases were clinically diagnosed without laboratory confirmation, which lead to bias. Reverse transcription polymerase chain reaction (RT-PCR) was confirmed as the preferred testing method,Citation27 especially in highly vaccinated populations.Citation28 It will be increasingly used to diagnose vaccine-preventable diseases in the future. Second, serological data of IgG mumps were not available on the effects of different doses of MuCV, and surveillance data for the virus strain was lacking, and more research about MMR will be needed in the future.
In conclusion, SIA was an important vaccination policy and can improve mumps antibody levels of the target population in the short term, but the uptake was poor. The effectiveness of one and two doses of MuCV wanes over time, but the VEs of two doses were superior than that of one dose. The vaccine schedule with two-dose MMR should be implemented among children in Quzhou, but the optimal age for the second dose needs to be further researched.
Authors’ contributions
Zhiying Yin conceived and designed the study. Canjie Zheng, Xiaoying Gong, and Junji Li obtained and organized the data. Tingcui Wen and Ziling Xiang analyzed the data. Quanjun Fang and Shuangqing Wang contributed reagents/materials/analysis tools. Zhiying Yin wrote the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Grennan D. Mumps. Jama. 2019;322:1. doi:10.1001/jama.2019.10982.
- Moghe CS, Goel P, Singh J, Nayak NR, Dhuria M, Jain R, Yadav R, Saroha E, Sodha SV, Aggarwal CS, et al. Mumps outbreak investigation in Jaisalmer, Rajasthan, India, June-r̥september 2016. J Med Virol. 2019;91:347–6. doi:10.1002/jmv.25324.
- Marx GE, Burakoff A, Barnes M, Hite D, Metz A, Miller K, Davizon ES, Chase J, McDonald C, McClean M, et al. Mumps outbreak in a Marshallese community — Denver metropolitan area, Colorado, 2016–2017. MMWR Morb Mortal Wkly Rep. 2018;67:1143–1146. doi:10.15585/mmwr.mm6741a2.
- Lewnard JA, Grad YH. Vaccine waning and mumps re-emergence in the United States. Sci Transl Med. 2018;10. doi:10.1126/scitranslmed.aao5945.
- Westphal DW, Eastwood A, Levy A, Davies J, Huppatz C, Gilles M, Lyttle H, Williams SA, Dowse GK. A protracted mumps outbreak in Western Australia despite high vaccine coverage: a population-based surveillance study. Lancet Infect Dis. 2019;19:177–184. doi:10.1016/S1473-3099(18)30498-5.
- Lu Q, Ding Z, Wu C, Wu H, Lin J. Analysis of epidemiological characteristics of notifiable diseases reported in children aged 0–14 years from 2008 to 2017 in Zhejiang Province, China. Int J Environ Res Public Health. 2019;16:168. doi:10.3390/ijerph16020168.
- Zhou C, Song W, Yin Z, Li S, Gong X, Fang Q, et al. Assessing the changes of mumps characteristics with different vaccination strategies using surveillance data: importance to introduce the 2-dose schedule in Quzhou of China. J Immunol Res. 2020;2020:8130760. doi:10.1155/2020/8130760.
- Yin Z, Zheng C, Fang Q, Gong X, Cao G, Li J, Xiang Z, Song W. Introduction of two-dose mumps-containing vaccine into routine immunization schedule in quzhou, china, using cox-proportional hazard model. J Immunol Res. 2021;2021:5990417. doi:10.1155/2021/5990417.
- Ramanathan R, Voigt EA, Kennedy RB, Poland GA. Knowledge gaps persist and hinder progress in eliminating mumps. Vaccine. 2018;36:3721–3726. doi:10.1016/j.vaccine.2018.05.067.
- Gu XX, Plotkin SA, Edwards KM, Sette A, Mills KHG, Levy O, Sant AJ, Mo A, Alexander W, Lu KT, et al. Waning immunity and microbial vaccines—Workshop of the National Institute of allergy and infectious diseases. Clin Vaccine Immunol. 2017;24. doi:10.1128/CVI.00034-17
- Deeks SL, Lim GH, Simpson MA, Gagne L, Gubbay J, Kristjanson E, Fung C, Crowcroft NS. An assessment of mumps vaccine effectiveness by dose during an outbreak in Canada. Cmaj. 2011;183:1014–1020. doi:10.1503/cmaj.101371.
- Fields VS, Safi H, Waters C, Dillaha J, Capelle L, Riklon S, Wheeler JG, Haselow DT. Mumps in a highly vaccinated Marshallese community in Arkansas, USA: an outbreak report. Lancet Infect Dis. 2019;19:185–192. doi:10.1016/S1473-3099(18)30607-8.
- Liu Y, Xiong Y, Liang Y, Deng X, Hu Y, Hu R, Chen Q, Tang F, Wang Z, Sun X, et al. Waning immunity and potential asymptomatic infection in 3–7 years old children who received one dose of measles-mumps-rubella vaccine: a 4-year prospective study. Vaccine. 2021;39:3509–3515. doi:10.1016/j.vaccine.2021.05.008.
- Ma R, Lu L, Zhou T, Pan J, Chen M, Pang X. Mumps disease in Beijing in the era of two-dose vaccination policy, 2005-2016. Vaccine. 2018;36:2589–2595. doi:10.1016/j.vaccine.2018.03.074.
- Saxena B, Ramachandran VG, Saha R, Shah D. Mumps antibody titer in MMR-vaccinated and vaccine naive children at a public hospital in Delhi. Indian Pediatr. 2021;58:137–139. doi:10.1007/s13312-021-2129-2.
- Qin W, Wang Y, Yang T, Xu XK, Meng XM, Zhao CJ, Li S-Y, Xie S-Y, Li K-C, Su H, et al. Outbreak of mumps in a student population with high vaccination coverage in China: time for two-dose vaccination. Human Vacc Immunother. 2019;15:2106–2111. doi:10.1080/21645515.2019.1581526.
- Schenk J, Abrams S, Theeten H, Van Damme P, Beutels P, Hens N. Immunogenicity and persistence of trivalent measles, mumps, and rubella vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2021;21:286–295. doi:10.1016/S1473-3099(20)30442-4.
- Vygen S, Fischer A, Meurice L, Mounchetrou Njoya I, Gregoris M, Ndiaye B, Ghenassia A, Poujol I, Stahl JP, Antona D, et al. Waning immunity against mumps in vaccinated young adults, France 2013. Eurosurveillance. 2016;21:30156. doi:10.2807/1560-7917.ES.2016.21.10.30156.
- Waugh CJ, Willocks LJ, Templeton K, Stevenson J. Recurrent outbreaks of mumps in Lothian and the impact of waning immunity. Epidemiol Infect. 2020;148:e131. doi:10.1017/S0950268820001296.
- El Zarif T, Kassir MF, Bizri N, Kassir G, Musharrafieh U, Bizri AR. Measles and mumps outbreaks in Lebanon: trends and links. BMC Infect Dis. 2020;20:244. doi:10.1186/s12879-020-04956-1.
- Pang H, Zhou Y, Zhao W, Jiang Q. Epidemiological changes in mumps infections between 1990 and 2017 in urban area of Shanghai, China. Human Vacc Immunother. 2021;17:1358–1365. doi:10.1080/21645515.2020.1827610.
- Won H, Kim AR, Yoo JS, Chung GT, Kang HJ, Kim SJ, Kim SS, Lee J-W. Cross-Neutralization between vaccine and circulating wild-type mumps viruses in Korea. Vaccine. 2021;39:1870–1876. doi:10.1016/j.vaccine.2021.01.039.
- Bankamp B, Hickman C, Icenogle JP, Rota PA. Successes and challenges for preventing measles, mumps and rubella by vaccination. Curr Opin Virol. 2019;34:110–116. doi:10.1016/j.coviro.2019.01.002.
- Willocks LJ, Guerendiain D, Austin HI, Morrison KE, Cameron RL, Templeton KE, et al. An outbreak of mumps with genetic strain variation in a highly vaccinated student population in Scotland. Epidemiol Infect. 2017;145:3219–3225. doi:10.1017/S0950268817002102.
- He HQ, Zhang B, Yan R, Li Q, Fu J, Tang XW, et al. Economic evaluation on different two-dose-vaccination-strategies related to measles, mumps and rubella combined attenuated live vaccine. Chin J Epidemiol. 2016;37:1121–1126.
- Wang D, Nie T, Pan F, Wang Y, Wang J, Qin W. Loss of protective immunity of two-dose mumps-containing vaccine over time: concerns with the new strategy of the mumps immunization program in China. Human Vacc Immunother. 2021;17:2072–2077. doi:10.1080/21645515.2020.1861877.
- Patel LN, Arciuolo RJ, Fu J, Giancotti FR, Zucker JR, Rakeman JL, Rosen JB. Mumps Outbreak Among a Highly Vaccinated University Community-New York City, January-April 2014. Clin Infect Dis. 2017;64:408–412. doi:10.1093/cid/ciw762.
- Trotz-Williams LA, Mercer NJ, Paphitis K, Walters JM, Wallace D, Kristjanson E, Gubbay J, Mazzulli T. Challenges in interpretation of diagnostic test results in a mumps outbreak in a highly vaccinated population. Clin Vaccine Immunol. 2017;24. doi:10.1128/CVI.00542-16.