ABSTRACT
Coronavirus disease 2019 (COVID-19) pandemic vaccination campaigns globally have been unlike any effort in history. In the United States, the success of these efforts, in part, has hinged on the timely capture and reporting of an unprecedented amount of data from a significantly greater number of administering providers than for routine vaccinations. The pandemic response has highlighted the need to explore the status and value of vaccination data as the critical glue that connects all aspects of the upstream US vaccine development and downstream vaccination delivery system. In this review, we examine immunization information systems and the role that data and staffing play in pandemic responses. We offer three strategic recommendations—regarding funding, expanded provider enrollment, and data reporting—informed by a literature review, a survey and focus group from a convenience sample of 22 immunization jurisdictions, and the vision for enhanced data flow to improve future pandemic responses and routine vaccination.
Introduction
The global coronavirus disease 2019 (COVID-19) vaccination campaigns are unlike any effort in history, characterized by complex storage and handling requirements, tiered rollout (i.e., priority access groups), and vaccination under Emergency Use Authorization.Citation1–3 In the United States, one of the first countries to roll out vaccines (fully vaccinating 62.3% of the population aged 5 years and older as of 6 January 2022),Citation4 success hinged on the timely capture and reporting of an unprecedented amount of data from significantly greater numbers and types of providers administering COVID-19 than routine vaccines.
The evolving pandemic and subsequent response in the United States has highlighted the need to strengthen the vaccination infrastructure and its importance, particularly for adults. An evaluation of the effort provides some insights that may be useful to the United States and other nations in strengthening adult immunization programs.Citation5,Citation6 In this review article, we summarize the current state of understanding of the US National Vaccine Program and its network of IIS in the context of the COVID-19 pandemic response and the policies and practices put into place to respond to the COVID-19 public health emergency. We draw on this experience and characterize lessons learned to date that can inform improvements to the routine vaccination delivery system and support the next pandemic. The outcomes from improvements include improved quality of adult vaccination records being captured in IIS and increased use of such data. Vaccination records are critical to informing clinical decision-making, public health strategies, and program interventions to address disparities in vaccination coverage.
Background
The US National Vaccine Program has long sought a system that provides a comprehensive vaccination record that can be accessed by health-care providers and patients, regardless of where an individual receives vaccines.Citation7–9 Globally, other middle- and high-income countries face this same challenge, as well as developing countries which heavily rely on paper-based systems to register those who have been vaccinated.Citation9–13 Immunization information systems (IIS) are confidential, population-based, computerized databases that record all immunization doses administered by participating providers to persons residing within a given geopolitical area. At the point of clinical care, an IIS can provide consolidated immunization histories for use by a vaccination provider in determining what vaccines people need according to the national vaccination schedule for children, adolescents, and adults. At the population level, an IIS provides aggregate data on vaccinations for use in disease surveillance, program operations, and guiding public health action with the goals of improving vaccination rates and reducing vaccine-preventable disease.
In the United States a national IIS does not exist
Congress opposed a single national IIS in 1994,Citation14 and as a result jurisdictions created their own unique systems whose data were not collated at a national level. These systems are governed by state and local jurisdictions and are designed to solve local challenges.Citation15,Citation16 These IIS emerged within the boundaries of the 64 immunization jurisdictions, comprising all 50 states; the District of Columbia; five cities; and eight islands and territories that make up the US National Immunization Program.Citation17 Prior to broad adoption of electronic health record (EHR) systems, many state and local jurisdictions developed immunization registries to manage patient vaccination data. Over time, standards-based systems evolved that require uniform approaches to data reporting and exchange.Citation18 Before the COVID-19 pandemic, there was no collective way to report de-identified data to the federal level.
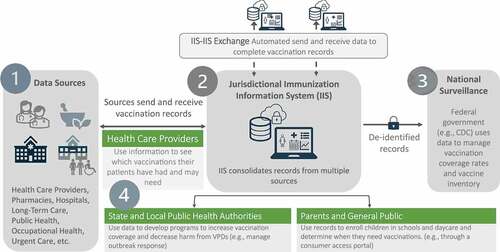
shows the movement of data in COVID-19 vaccination campaigns beginning at various points of care, including health-care providers, pharmacies, hospitals, long-term care facilities (point 1 in ). These sources send vaccination records to the jurisdictional IIS in which doses were administered (point 2 in ), ideally through automated data exchange. These data may be exchanged with other jurisdictional IIS (IIS-IIS Exchange) when individuals cross jurisdictional boundaries. De-identified COVID-19 data are sent to the federal level for national surveillance, in near-real time (point 3 in ). This is not typical for routine vaccinations. However, regardless of vaccine, three types of users typically access vaccination data: health-care providers, state and local public health authorities, and parents and the public who need these vaccination records. Records are used for clinical decision-making, outbreak response, and enrolling children in school, respectively (point 4 in ).
Figure 1. The flow of vaccine information in COVID-19 vaccination campaigns through the immunization information systems to end users: health care providers, state and local public health authorities, parents and the public.
The federal government purchased all COVID-19 vaccines for vaccination programs. As part of the provider enrollment agreements, all vaccinators agreed to report vaccine administration data (within 24–72 hours of administration), vaccine inventory, vaccine wastage, and adverse events to the federal government.Citation19,Citation20 To achieve this reporting requirement, local, state, and federal public health authorities rapidly modified and enhanced existing vaccine delivery and reporting systems that manage vaccine allocation and distribution and monitor vaccine uptake.Citation1,Citation20,Citation21 The COVID-19 vaccination distribution and reporting system was built upon the existing infrastructure of the pre-pandemic adult and childhood vaccination program (e.g., centralized vaccine distribution system used to order, distribute, and manage over $4 billion in vaccines).Citation1–Citation5–22–Citation24 The COVID-19 mass vaccination program leveraged public health partnerships that were created (e.g., linkages with pharmacies) and strengthened (e.g., with professional organizations) over a decade ago when responding to the H1N1 pandemic.Citation22 As in the earlier 2009–2010 H1N1 pandemic, the federal government purchased all COVID-19 vaccine doses for use in vaccination programs at no cost to patients, and providers were able to claim a vaccine administration fee from public and private payors as well as seek payment for uninsured individuals through the federal government’s uninsured adults program.Citation1–Citation22-24
Hundreds of millions of federal dollars have thus far been invested in support of national, state, and local immunization systems in response to the COVID-19 pandemic.Citation25,Citation26 As a result of these significant investments, public health officials were able to increase staff capacity and support for fundamental improvements to immunization infrastructure. The results include enhancing IIS to support more automated bidirectional data exchanges; exchanging data with other jurisdictions (although in its infancy); expanding provider enrollment; improving immunization data reporting and analysis; increasing outreach and education for both consumers and providers; launching long-standing priority initiatives that lacked funding (e.g., consumer access portals); and strengthening partnerships with providers and consumers. Access to near-real-time data allowed jurisdictions and federal agencies to monitor vaccination coverage trends, predict areas of vulnerability to outbreaks, and identify strategies to help mitigate the effects of the pandemic.
Methods
In this review article, we summarize the current state of understanding of the US national vaccine infrastructure and the role of IIS as a tool in the COVID-19 pandemic response. Recommendations were gathered from an analysis of the peer-reviewed and gray literature, as well as from findings from a seven-question on-line survey administered to all 64 immunization jurisdictions from November to December 2021. In late December 2021, a one-hour virtual focus group was conducted with 22 survey respondents. Participants discussed findings from the survey including expanded sites for vaccination; challenges with enrolling providers in the program; ways to increase the number of doctors and health-care providers connected to and reporting data to the IIS; how vaccination records are sent to IIS; and which long-term investments are important to sustain. Findings were validated with the expertise of the authors representing the Association of Immunization Managers, the American Immunization Registry Association, a local health department, and AMDA—The Society for Post-Acute and Long-Term Care Medicine. Resultant lessons learned and recommendations are stratified by vaccination user type (health-care providers, state and local public health authorities, parents and the general public) as described in .
Results
Lessons learned are described alongside associated recommendations to strengthen the US National Vaccination Program. Participants described how mandatory reporting of COVID-19 vaccination data made it possible to strategize and inform decisions on where to focus resources and tailor interventions for unvaccinated individuals. This requirement, a mandatory provision of the federal COVID-19 provider agreement, provided a glimpse into how a centralized system might benefit the three user types (). Three strategic recommendations to sustain this vision are listed in by data user type and include (1) investing financially in the long-term needs of each user, (2) expanding provider electronic data exchange to promote provider connections to the IIS, and (3) conducting a systematic review of IIS reporting mechanisms of vaccination data to the federal level.
Table 1. Recommendations to support vaccination data users under routine administration and public health emergencies.
Recommendation 1: Invest in the financial long-term needs of users: health care providers (in financial outlay costs), public health authorities (in staffing and immunization technology infrastructure), and parents and the public (in access to complete vaccination records)
The implementation of an IIS is a significant commitment of resources at the national and subnational levels and includes investments in human resources, technology, and policies. In the United States, public health vaccination programs and IIS have been chronically underfunded for decades, subjected to boom-and-bust funding cycles. IIS require system enhancements and modifications to meet evolving needs. Technology investments today carry a cost into the future for routine maintenance and staff to operate and maintain these investments. As is the case with one-time funding, funds spent on efforts that offer a temporary benefit are unlikely to improve the long-term stability of IIS. While all jurisdictions currently have some form of an IIS, long-term funding is needed to ensure (1) financial outlays to health-care providers to connect to IIS, (2) trained staff and technology investments, and (3) access to immunization records for parents and the public.
In an optimal system, providers and IIS are connected bidirectionally, meaning providers can query the IIS to receive data on vaccines administered outside of their provider setting and on individual vaccination recommendations. A central role of IIS is consolidating data from a range of sources and ideally exchanging data with other jurisdictions (). In a unidirectional connection, providers only report doses they administer to the IIS. For providers without an electronic connection, they must manually (e.g., through an IIS user interface) report doses administered to the IIS. The bidirectional exchange of data is critical to support providers at the point of care and assists in population health management, including the ability to assess vaccination coverage at the population level to evaluate which communities may be more vulnerable to outbreaks due to low vaccination rates.
Doctors and health-care providers need financial support to connect to IIS
Bidirectional exchange is important to help providers make informed decisions at the point of care. IIS offer clinical decision support functionality, as do many EHRs. Immunization clinical decision support is the system’s (e.g., IIS, EHR) automated review of an individual’s vaccination history compared with vaccination recommendations, and it produces specific vaccine recommendations. IIS data are critical for accurate clinical decision support. In the past decade, the federal government incentivized providers and hospitals to adopt EHR systems to exchange vaccination data between health-care providers, patients, and jurisdictional IIS. Electronic data exchange minimizes the burden to report and allows providers to query the IIS for their patients’ record when vaccines were administered elsewhere (e.g., mass vaccination site).Citation15 Many health-care providers, such as large-chain pharmacies, have been able to adopt similar capabilities without incentive payments; however, smaller independent pharmacies and many nontraditional providers have been only partially able to connect, often, unidirectionally. Because these investments incentivized provider EHR connections to IIS, many providers were already connected to the IIS and able to automatically exchange data during the pandemic. Additional providers who were not traditional vaccinators but who use a certified EHR were better equipped to onboard to the IIS and provide data in an automated way during the pandemic.
State and local public health authorities require long-term investments to support staffing and immunization technology infrastructure
As a result of chronic, unreliable funding, public health departments have been hesitant to support long-term investments both in technology and workforce which has hindered the ability to execute public health functions, including managing outbreaks. shows the requisite expertise and the types of technology investments important to managing a pandemic vaccination program. Currently, many immunization programs are facing decisions about what system modifications or enhancements should be made () with funds available today while being mindful of an anticipated funding cliff in 2024 when the current influx of funds will no longer be available to fund even modest increases in annual maintenance and operations.Citation25–27
Table 2. Needs of state and local public health authority for workforce development and technological systems modifications.
Parents and the general public desire access to vaccination records
The pandemic has increased awareness about not only vaccines for adults but also the existence of IIS and the importance of having data at the local, state, and federal levels (e.g., policy makers, the public). Providing access to vaccination records for individuals and parents through patient portals or mobile applications requires resources. Public health authorities should continue to prioritize modernization of paper-based records to create a more secure standards-based digital record using a trusted exchange framework, such as the SMART Health Card,Citation28 allowing broader global usage.
Recommendation 2: Expand provider electronic data exchange by establishing an incentive program to promote provider connections to the IIS for systems, including with pharmacy and long-term care systems
When launched in December 2020, the primary goal of the US COVID-19 vaccination program was to equitably distribute vaccine doses as quickly as possible across jurisdictions. This was accomplished through mass vaccination drives and partnerships with providers and chain pharmacies that have extensive reach into many communities. In doing so, jurisdictions across the nation galvanized resources (human and financial) toward recruiting as many providers as possible to become vaccinators and connected these providers to IIS or other systems to report vaccine administration data. To help ensure equitable distribution, all providers need access to report doses administered and to be able to review accurate vaccination records and the corresponding clinical decision support.
Traditional vaccinators like pharmacies and hospitals have a far-and-wide reach into the communities they serve.Citation29 These entities were able to store large quantities of vaccine at the ultra-low temperatures required for most of the early COVID-19 vaccine supply; thus, they initially administered and continue to administer a large proportion of vaccinations. However, many new providers were essential to expanding the availability of vaccines and vaccinating the population quickly. These new providers include long-term care facilities (LTCF), specialty health care, urgent care and home health providers, community health centers, federally qualified health centers, rural health centers, organizations serving homeless and incarcerated populations, occupational and employee health clinics, dialysis centers, and dental offices, all of which joined the ranks of existing pre-COVID-19 immunization providers. Participants in our focus group estimated a potential 30% to 35% increase in the number of vaccination providers, with a variety in the types of providers enrolled as new vaccinators. It is unclear if some of these newly enrolled vaccinators will continue to vaccinate for routine vaccines. However, some new providers included temporary sites such as the Federal Emergency Management Agency (FEMA) pop-up mass vaccination sites, which were never meant to be sustained beyond the needs of the emergency surge.
Enrolling new providers expanded access to COVID-19 vaccination. New providers came from many different locations, including those that counsel and recommend vaccines but might not directly administer vaccines, such as LTCF. These sites also need access to IIS to access their patients’ vaccination history and clinical decision support to recommend appropriate vaccines. Leveraging this information, providers could play a critical role in providing counsel about vaccination, particularly as many individuals have had questions about COVID-19 vaccines and were uncomfortable getting vaccinated.Citation30,Citation31 For example, COVID-19 vaccination of those in LTCF primarily occurred, in every jurisdiction, through the COVID-19 federal Pharmacy Partnership for Long-Term Care Program (FPP).Citation32 This federal collaboration was launched to (1) help vaccinate high-risk populations (those who live and work in LTCF) and (2) ensure doses administered are reported to the federal level without undue burden on providers and independent LTCF pharmacies that were not able to connect to IIS.Citation32
Many types of providers, such as small independent pharmacies (e.g., Omnicare versus large pharmacy chains like Walgreens and CVS), schools, and occupational health units of organizations might not be able to connect to IIS via Health Level 7 (HL7), the current standard and format for electronic data exchange. Many of these providers (e.g., pharmacies, LTCF) were not eligible for government incentives prior to the pandemic. For these reasons, standards for all providers’ software might not exist, and costs to develop automated electronic exchange may have inhibited the implementation of automated electronic exchanges. Establishing bidirectional data exchange for all providers is critical for providing access to accurate information and clinical decision support beyond the pandemic.
Recommendation 3: Conduct a systematic review of IIS reporting mechanisms of vaccination data to the federal level to improve the quality, type, and efficiency of reporting by state and local jurisdictions
The COVID-19 response has illustrated how intertwined vaccination rates and population health are in managing vaccine-preventable diseases (VPD). Currently, every jurisdiction must pull daily reports from their reporting systems to manually report to CDC. In addition to IIS data, data from other federal systems that may not feed into jurisdictional IIS, such as the Department of Defense, Department of Veterans Affairs, and Bureau of Prisons, were transmitted into a federal data clearinghouse, thus, in this pandemic response, providing the first comprehensive national picture of any vaccinations in near-real time.
The need to rapidly create a federal reporting system for administered vaccine data has resulted in data issues, such as double reporting of data to the IIS and to federal entities, generating concerns about data quality, given the multiple reporting mechanisms of the same de-identified data. Systems and procedures invoked for the pandemic lack sustainability and highlight the fragmentation of reporting. A thoughtful review of these systems is warranted as the pandemic settles into its endemic state.
Automated electronic data exchange worked for many providers to report COVID-19 vaccines to jurisdictional IIS; however, many providers had to and continue to provide data manually through the submission of flat files (e.g., Excel spreadsheets). The use of an Excel spreadsheet to report daily doses administered is a mechanism offered to provider who are not familiar with or are unable to implement and report data through HL7 connections for COVID-19 vaccine reporting. The use of an Excel spreadsheet is a burden both to providers who must enter data and to public health departments which must reconcile these data into the IIS. Because public health authorities need this data, they have accepted this more burdensome and manual mechanism to receive data, which requires additional resources in public health departments to reconcile and process. These methods also do not allow providers to query and leverage IIS data, patients’ consolidated records, and forecasts for clinical decision support. Unfortunately, some providers have also had to double-enter data into their own facility or organization’s systems as well as into state IIS or federal systems to meet the mandatory COVID-19 reporting requirements.
The pandemic highlighted the mobility of individuals as they received COVID-19 vaccine in multiple jurisdictions, motivating the signing of memorandums of understanding among jurisdictions authorizing data to be exchanged with other IIS. However, ubiquitous data exchange between IIS is still a work in progress.
Transparent and reliable data connections that foster trust in the public health reporting systems from the provider to the IIS, other jurisdictions, and federal agencies are a requisite to fully manage population health. A thoughtful review of these systems is warranted as the pandemic settles into its endemic state, and that review should include a broad stakeholder engagement on strategic ways to improve the quality and efficiency of reporting by state and local jurisdictions.
Looking forward
There has been an increase in the number of facilities (and providers) that provide COVID-19 vaccination services, potentially increasing access for routine immunization. For the first time, there is a near-real-time reporting of vaccination data, creating a national picture of vaccine coverage, and demonstrating what is possible. While these methodological practices have been effective in the current response environment, they are unsustainable in the long term. There are still significant challenges that remain in streamlining and easing the burden of providers reporting data, consolidation of these data, and reporting to the federal level. Toward this end, permanent and sustainable processes need to be put in place and integrated into routine immunization programs. Public health authorities should start with a critical and thoughtful review of the existing local, state, tribal, and federal systems and methods for obtaining and reporting data. Looking beyond the current pandemic, a timely reporting mandate for all routinely recommended vaccines should be in place for the same reasons COVID-19 vaccination data are required: to provide near-real-time visibility to monitor population health and manage outbreaks of VPDs (e.g., measles and hepatitis A) and planning stages of vaccine distribution.
A key limitation of this article is the interpretation by the expertise of the authors without control for bias. Given the recommendations are based on this expertise and the response of 22 participants and one focus group elements of the recommendations are influenced by commentary of the authors.
Conclusion
Fundamentally, the pandemic has increased and escalated the importance of IIS and the perception and value that IIS and the data they capture have in mounting an effective emergency vaccination campaign.
The development of relationships among pharmacies and adult immunization providers—as well as the mandatory nature of provider reporting of doses administered to an IIS—created a national data set of de-identified data to support the management of COVID-19 vaccination programs, the first time such a national database was crafted. How these data streams were collected varies widely (e.g., third-party reporting, manual submission through a flat file rather than an automated HL7 connection). Because there is no single entity responsible for the quality of COVID-19 data—much less routine immunization data—the shared responsibility falls across tens of thousands of providers, systems, and local, state, tribal, and federal levels of government to improve the completeness and accuracy of data.
The recommendations captured in this review propose actions needed (1) to facilitate the capabilities of IIS to exchange data not only with providers but with IIS in other jurisdictions and (2) to support the development and implementation of policies and standards that facilitate complete population-level data capture, consolidation, and access to accurate immunization information. It will be critically important to galvanize political will to ensure sustained, continued investments (IIS maintenance and enhancements) in technological capacity and the recruitment and retention of high numbers of competent staff for the long term. The lessons learned and recommendations amid this pandemic can be used to turn the crisis we are weathering into a stronger and better prepared public health response.
Acknowledgements
The authors wish to thank the members of the Association of Immunization Managers for their thoughtful conversations and to Charlotte Moser, Mary Beth Kurilo, Lonnie Peterson, and Katelyn Wells for their thoughtful review of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kaiser Family Foundation. “Distributing a COVID-19 vaccine across the U.S. – a look at key issues.” https://www.kff.org/report-section/distributing-a-covid-19-vaccine-across-the-u-s-a-look-at-key-issues-issue-brief/.
- U.S. Food & Drug Administration. “Emergency use authorization for vaccines explained.” https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained.
- U.S. Food & Drug Administration. “Vaccines licensed for use in the United States.” https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states.
- USA Facts.c. “US Coronavirus vaccine tracker.” https://usafacts.org/visualizations/covid-vaccine-tracker-states/.
- National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the national vaccine advisory committee, approved by the national vaccine advisory committee on June 14, 2011. Public Health Rep. 2012;127(Suppl 1):1–7. doi:10.1177/00333549121270S101.
- Lu P-J, Hung M-C, Srivastav A, Grohskopf LA, Kobayashi M, Harris AM, Dooling KL, Markowitz LE, Rodriguez-Lainz A, Williams WW. Surveillance of vaccination coverage among adult populations—United States, 2018. MMWR, Surveillance Summaries. 2021;70(3):1–26. doi:10.15585/mmwr.ss7003a1.
- Centers for Disease Control and Prevention. “Immunization information systems.” https://www.cdc.gov/vaccines/programs/iis/about.html.
- National Governors Association. “Modernizing immunization information systems.” Priorities and Considerations for Governors; 2021 Sep. https://www.nga.org/wp-content/uploads/2021/09/NGA_Modernize_ImmunInfoSystems.pdf.
- Atkinson KM, Mithani SS, Bell C, Rubens-Augustson T, Wilson K. The digital immunization system of the future: imagining a patient-centric, interoperable immunization information system. Ther Adv Vaccines Immunother. 2020;8:2515135520967203. doi:10.1177/2515135520967203.
- Derrough T, Olsson K, Gianfredi V, Simondon F, Heijbel H, Danielsson N, Kramarz P, Pastore-Celentano L. Immunisation information systems – useful tools for monitoring vaccination programmes in EU/EEA countries, 2016. Eurosurveillance. 2017;22(17):30519. doi:10.2807/1560-7917.ES.2017.22.17.30519.
- Danovaro-Holliday MC, Contreras MP, Pinto D, Molina-Aguilera IB, Miranda D, García O, Velandia-Gonzalez M. Assessing electronic immunization registries: the Pan American Health Organization experience. Revista Panamericana de Salud Pública. 2019;43:e28. doi:10.26633/RPSP.2019.28.
- Dolan SB, Carnahan E, Shearer JC, Beylerian EN, Thompson J, Gilbert SS, Werner L, Ryman TK. Redefining vaccination coverage and timeliness measures using electronic immunization registry data in low- and middle-income countries. Vaccine. 2019;37(13):1859–1867. doi:10.1016/j.vaccine.2019.02.017.
- Pancholi J, Birdie R, Guerette J, Chritz S, Sampath V, Crawford J “Landscape analysis of electronic immunization registries; lessons learned from a landscape analysis of EIR implementations in low and middle income countries.” https://www.villagereach.org/wp-content/uploads/2020/06/2020-EIR-Landscape-Analysis.pdf.
- Task Force for Global Health and the Public Health Informatics Institute. “Immunization information systems: the first twenty-five years a commemorative history.” https://phii.org/sites/default/files/iis_history_spotlight-_technology.pdf.
- Watson WC, Saarlas KN, Hearn R, Russell R. The all kids count national program: a Robert Wood Johnson Foundation initiative to develop immunization registries. Am J of Prev Med. 1997;13(2):3–6. doi:10.1016/S0749-3797(18)30104-1.
- Inkelas M, Wood D, Borenstein P, Bordley WC, Dixon ML, Lamoureux J. Lessons learned in planning for community child immunization registries. Am J of Prev Med. 1997;13(2):7–11. doi:10.1016/S0749-3797(18)30105-3.
- Hinman AR, Orenstein WA, Schuchat A. Vaccine-Preventable diseases, immunizations and MMWR—1961-2011. MMWR Morb Mortal Wkly Rep. 2011;60:49–57.
- The White House. “Transforming health care: the president’s health information technology plan.” https://georgewbush-whitehouse.archives.gov/infocus/technology/economic_policy200404/chap3.html.
- Centers for Disease Control and Prevention. “CDC COVID-19 vaccination program provider requirements and support.” https://www.cdc.gov/vaccines/covid-19/vaccination-provider-support.html.
- U.S. Indo-Pacific Command. “Tiberius platform Aids COVID-19 logistics, delivery.” https://www.pacom.mil/Media/News/News-Article-View/Article/2449032/tiberius-platform-aids-covid-19-logistics-delivery/#::text=Tiberius%20was%20specifically%20developed%20for%20Operation%20Warp%20Speed,deliver%20safe%20and%20effective%20COVID-19%20vaccines%20to%20Americans.
- Centers for Disease Control and Prevention. “COVID-19 vaccination reporting systems, COVID-19 vaccine IT overview.” https://www.cdc.gov/vaccines/covid-19/reporting/overview/IT-systems.html.
- The National Academies Press. “The 2009 H1N1 influenza vaccination campaign: summary of a workshop series.” https://www.nap.edu/read/12992/chapter/1.
- Centers for Disease Control and Prevention. “COVID-19 vaccines are free to the public.” https://www.cdc.gov/coronavirus/2019-ncov/vaccines/no-cost.html.
- Health Resources & Services Administration. “COVID-19 claims reimbursement to health care providers and facilities for testing, treatment, and vaccine administration for the uninsured.” https://www.hrsa.gov/coviduninsuredclaim.
- FY 2021 coronavirus response and relief supplemental appropriations act of 2021, Pub. L. No.116-260.
- Centers for Disease Control and Prevention. “Administration announces $200 million from CDC to jurisdictions for COVID-19 vaccine preparedness.” https://www.cdc.gov/media/releases/2020/p0924-200-million-jurisdictions-covid-19-preparedness.html#::text=CDC%20is%20awarding%20%24200%20million,for%20the%20COVID%2D19%20vaccine.
- Trust for America’s Health. “The impact of chronic underfunding on America’s public health system: trends, risks, and recommendations, 2020.” https://www.tfah.org/report-details/publichealthfunding2020/.
- The Commons Project. “Manage your COVID-19 health information with SMART® health cards.” https://thecommonsproject.org/shc.
- Shen AK, Tan ASL. Trust, influence, and community: why pharmacists and pharmacies are central for addressing vaccine hesitancy. J Am Pharm Assoc. 2022;62(1):305–308. doi:10.1016/j.japh.2021.10.001.
- AARP. “Vaccine hesitancy among older adults, with implications for COVID-19 vaccination and beyond.” https://www.aarp.org/ppi/info-2021/vaccine-hesitancy-among-older-adults.html.
- Kaiser Family Foundation. “KFF COVID-19 vaccine monitor: November 2021.” https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-november-2021/.
- Centers for Disease Control and Prevention. “COVID-19 vaccine access in long-term care settings.” https://www.cdc.gov/vaccines/covid-19/long-term-care/pharmacy-partnerships.html.
