ABSTRACT
Patients with rheumatic diseases (RD) are considered to be a high-risk population for infection with the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). The effectiveness of inactivated COVID-19 vaccinations (ICVs) was described as more effective than 95%. Despite this, no data on the immunogenicity and safety of the ICV in Han race stable RD patients in China. In this study, we sought to assess the safety and immunogenicity of the ICVs in RD patients in South China. A total of 80 adult stable RD patients were recruited. Following 14–35 days of immunization, cheiluminescence immunoassays (CLIA) were utilized to detect antibodies titers. An investigation into the relative parameters on the immunogenicity response to vaccination was carried out using logistic regression analysis. Compared to the HC group, the positive response of IgG and Nab in RD patients were lower than those in healthy control (HC) (P = .040 and P < .0001, respectively) after two doses of ICV were inoculated. The use of methotrexate (P = .016) and prednisolone (P = .018), and the level of red blood cell distribution width-C (RDW-C) (P = .035) and C-reactive protein (P = .015) were independently associated with lower rises in the magnitude of COVID-19 vaccine antibodies. No vaccine-related serious adverse reactions were observed in either group. After receiving two doses of ICVs, the production of protective antibodies in stable RD patients treated with immunosuppressive agents may decrease. It was discovered that ICVs were safe and well tolerated by RD patients.
Plain Language Summary
What is the context?
There are currently no accessible data on the efficacy and safety of inactivated COVID-19 vaccinations in South China RD patients who are receiving immunosuppressive medications.
What is new?
Inactivated COVID-19 vaccinations were immunogenic in stable RD patients in our investigation. No significant adverse reactions to the vaccination were seen in either group. No disease flares were observed in our study.
What is the impact?
Inactivated COVID-19 vaccinations are immunogenic and safe in stable RD patients in China, according to the findings of this study. The use of methotrexate or prednisolone, the RDW-C level, and the CRP level may all have an effect on the development of protective antibodies following vaccination.
Introduction
The COVID-19 pandemic was triggered by the SARS-Cov-2 virus in 2019, and global demand for safe, effective, and fast-acting protective vaccinations soared.Citation1,Citation2 All eligible individuals should be vaccinated against the COVID-19 virus as soon as feasible, according to the World Health Organization (WHO). Several COVID-19 vaccines have been proven to be efficacious and have been licensed by international health organizations for emergency use.
Rheumatism (RD) patients have a higher risk of contracting COVID-19 than that in the general population.Citation3 Nevertheless, RD patients are excluded from clinical trials for vaccine development. Immune dysregulation increases the likelihood of COVID-19 infection in RD patients, and immunosuppressive drugs may compromise vaccine response.Citation4,Citation5 Serological responses in RD patients following influenza or pneumococcal vaccination were attenuated.Citation6,Citation7 The Korean College of Rheumatology (KCR),Citation8 the German Society of Rheumatology (GSR),Citation9 and the American College of Rheumatology (ACR)Citation10 all highly advocate that the currently licensed COVID-19 vaccinations are safe and effective, and recommend RD patients who are in a stable condition should receive the vaccine, unless they are allergic to any of the vaccine’s ingredients.
Only a few exploratory studies have been conducted in South America (Brazil) and Turkey to assess the immunogenicity and safety of RD patients after receiving two doses of ICV.Citation11,Citation12 There is little information available about whether race has an impact on vaccine immunogenicity. It has been discovered that there are differences in circulating inflammatory cytokine levels as well as genetic variations between ethnic origins.Citation13 A single-center study discovered that serum transformation in RD patients was delayed after the first dose of mRNA vaccine,Citation14 but the response rate of the second dose of vaccine was significantly improved,Citation15 which confirms the importance of the second dose of vaccine. So we focused on immunogenicity following two doses of immunization.
According to published findings,Citation16,Citation17Citation18–28 the use of immunosuppression, such as T-cell consumption or immunosuppressive medication (ie, methotrexate, rituximab, etc.), may alter the antibody response in chronic inflammation and solid organ transplantation patients after receiving mRNA COVID-19 vaccination (The mechanisms of action of immunosuppressive agents implicated in this article are detailed in Table S1).
Numerous clinical trials have provided crucial data for the safety and effectiveness of mRNA vaccines or subunit vaccinations in healthy people.Citation29–31 Nevertheless, the level of immunogenicity of ICVs in RD patients in South China is unknown. Based on the COVID-19 vaccination strategy for RD patients by local government at that time, and the recommendation mentioned above,Citation8–10 only stable stage patients were involved in this study. The goal of this study is to detect antibody response in stable RD patients in South China, as well as to investigate the factors that influence immunogenicity response to inactivated immunization in South China. So far, no data on the ICVs for South China stable RD patients have been published.
Material and methods
Study design and participants
A total of 23 healthy control cases and patients with RD were recruited from the Department of Rheumatology, Futian Rheumatology Hospital, Futian District, Guangdong Province from July 18 to 18 August 2021. For data analysis, the doctor completed a questionnaire of the COVID-19 vaccination status of patients, including clinical information and vaccine acceptability. All participants received two intramuscular injections of 0.5 ml inactivated vaccines licensed for use in China (including CoronaVac, Sinovac Biotech or BBIBP-CorV, Sinopharm Biotech or KCONVAC, Shenzhen Kangtai Biological Products Co., Ltd, China) at a 21-28-day interval according to national guidelines with ICVs. SARS-CoV-2 immunoglobulin G (IgG),neutralizing Antibody (Nab), and IgM were detected by chemiluminescence immunoassay (CLIA) testing (SARS-CoV-2 Nucleic Acid Detection Kit, Maccura) after 14–35 days of immunization using standardized assays and international standards.Citation32 Experimental calibration and control tests and test operating procedures were performed according to the manufacturer’s instructions. The results of sample detection for IgG and IgM were judged by S/CO value. The manufacturer set the cutoff of IgG and IgM at 1.0 index (S/C). The analyzer calculates the Nab concentration from a calibration curve based on the relative luminescence value (RLU) of the sample to be examined. A titer above 6.0 AU/mL was considered positive according to the manufacturer’s instructions. Thirty-two patients were immunized with CoronaVac, twenty-seven patients with BBIBP-CorV, nine patients with KCONVAC, and twelve patients with mixed vaccine of CoronaVac and BBIBP-CorV sequentially.
All patients signed informed consent before the study (FS202101001). The Ethics Committee of Shenzhen Futian Hospital for Rheumatic Diseases (Shenzhen, Guangdong,China) approved the study protocol (FS202101001), and all the study subjects gave written informed consent according to the Declaration of Helsinki. The protocol of this trial was registered in Chinese Clinical-Trials.gov (No.ChiCTR2100049114).
Inclusion criteria
(1) The respondents were confirmed RD patients by a rheumatologist in South China; (2) Their ages ranged from 18 to 59 years old; (3) They know about their diagnosis; (4) Had the basic ability to read; (5) Had completed the COVID-19 vaccination in China within 14 to 35 days.
Exclusion criteria
(1) Refusing to cooperate with investigators; (2) In severe condition and requires immediate medical treatment; (3) Not fulfill basic immunization standards, such as age ≥60 years old; (4) Had a positive history of COVID-19 infection.
Statistical analysis
All the outcomes of our multivariable analyses relied on the imputed datasets, and were united with Rubin’s rules.Citation33 Analysis of the variables consists of three steps. (1) The baseline characteristics of participants were analyzed. RD patients were used as a grouping variable. The results of SARS-CoV-2 IgG and IgM concentration levels were logarithmically converted to normally distributed variables. The continuous results are reported as means±standard deviation, or as medians [interquartile range (IQR)] for skewed distributions.Categorical variables are demonstrated as frequencies or as a percentage.T-test (normal distribution), two-tailed Mann-Whitney U-test (skewed distribution) and chi-square tests (categorical variables) were applied to reveal any statistical differences between the means and proportions of two groups. (2) A multivariable logistic regression model was used to appraise the associations between the non-antibody production and the predictor clinical variable, and adjusted regression coefficient (B) with 95% confidence intervals (CIs) were assessed to evaluate the relationship between them. (3) This work was conducted based on the recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. We simultaneously showed the results of both unadjusted, minimally adjusted analyses, and fully adjusted analyses.Citation34 Whether or not the covariances were adjusted, we followed the principle that when they were added to the model, the matched OR changed by at least 10%. All of the analyses were carried out with the statistical software packages R (http://www.R-project.org; The R Foundation, Vienna, Austria) and EmpowerStats (http://www.empowerstats.com;). P-values less than 0.05 (two-tailed) were considered to be statistically significant.
Results
Patients demographics
The study included 80 Han RD patients (30.00% men and 70.00% women) and 23 HC patients (52.17% men and 47.82% women). The median time after the second vaccine dose for RD patients was 28 days (IQR, 28–35), while the HC was 29 days (IQR, 29–34). Systemic lupus erythematosus (SLE) was diagnosed in 31.20% (25/80) of RD characters, rheumatoid arthritis (RA) was diagnosed in 31.20% (25/80), and axial spondyloarthritis (axSpA) was diagnosed in 23.7% (19/80). The average age of RD patients was 40.85 ± 9.50 years, while the average age of HC patients was 36.56 ± 11.10 years. In the RD group, the mean body mass index (BMI) was 21.883.02 kg/m2, and 15.00% had a history of systemic damage.As for drugs used within three months, 31.20% (25/80) received MTX, 40.00% (32/80) took prednisolone (prednisolone dosage <10 mg qd). The baseline characteristics of all vaccinated RD patients are presented in Table S2.
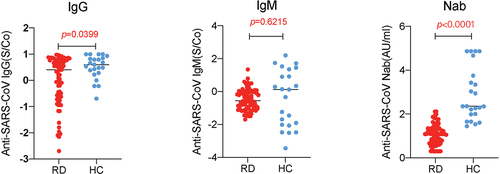
Subsequently, we observed SARS-CoV-2 IgG and Nab in 80 RD patients.
Immunogenicity
Analysis of antibody response and serum conversion rate of inactivated COVID-19 vaccine
Compared with the control group, the level of immunoglobulin G (log-IgG) titer (0.041 ± 0.905) and log-Nab (1.083 ± 0.467) in RD patients decreased significantly (P < .05, P < .01 respectively). However, no significant difference was detected in the log-IgM level (−0.500 ± 0.624) between the two groups (P = .613), for more information, see .
Univariate analysis of related factors of antibody production
The result of univariate analysis of the factors influencing the production of different antibodies was shown in Table S3. As can be seen from this data, usage of MTX (B = 0.30, 95%CI:0.11, 0.80, P = .016) and the level of RDW-C (95%CI:0.39, 0.97, P = .035) were significant determinants of the lack of SARS-CoV-2 IgG response in RD patients.
At the same time, we found that use of prednisolone (95% CI:0.11, 0.81, P = .018) and the level of CRP (95% CI:1.12, 2.78, P = .015) might impact the capacity of SARS-CoV-2 Nab formation in RD patients after the second vaccine dose, see Table S3 for details.
Multivariable analysis of related medication of antibody production
Additionally, we utilized a multivariable logistic regression model to examine the relationships between SARS-CoV-2 antibody response and immunosuppressive use. Table S4 summarizes the non-adjusted and adjusted models. It was discovered that both the use of MTX and the degree of RDW-C had an independent effect on the absence of SARS-CoV-2 IgG production in RD patients following the second vaccine dose, regardless of whether the model was minimally adjusted (adjusted for gender, age) or fully adjusted (adjusted for age; gender; systemic damage; BUN, etc.), the result did not differ significantly (P < .05), as shown in Table S4 and .
Figure 2. Difference of RDW-C in Anti-SARS-CoV-2 IgG group, and CRP in anti-SARS-CoV-2 NaB group. The difference was determined by t test. (a).Logistic regression analysis of the relationship between the level of RDW. C and the production of SARS-CoV-2 IgG antibody (P = .0218). (b).Logistic regression analysis of the relationship between the level of CRP and the production of SARS-CoV-2 NaB antibody (P = .0211).
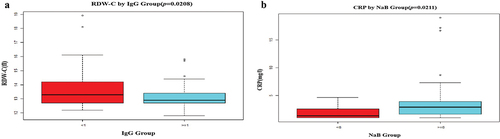
On the other hand, we discovered that the level of CRP and the use of prednisolone medication had an impact on the deficit of SARS-CoV-2 Nab in three models (P < .05), as shown in Table S5 and .
Safety
Our research found ICVs to be safe and well tolerated. Ten patients and one HC occurrence out of 80 reported minor pain. One case had a slight fever, but the other ten had flu-like symptoms such as muscular aches, headaches, and tiredness. Seven patients (7.00%) had adverse responses within seven days of receiving the first dose of vaccination, and three (3.00%) had adverse reactions within seven days of receiving the second dose. Aside from that, all reactions were self-limiting. We did not witness any illness attack during this trial.
Discussion
The COVID-19 pandemic had 240,940,937 confirmed cases and 4,903,911 deaths as of 19 October 2021, with 6,545,309,084 anti-COVID-19 vaccination doses administered.Citation35 Rheumatologists are up against a significant obstacle. The most effective action will be to develop safe and effective vaccinations for group immunity. In addition to the COVID-19 vaccine, many countries, notably China, are working on SARS-CoV-2 inactivated whole viral vaccines due in 2020.Citation36
The inactivated COVID-19 vaccines (ICVs) were found to be safe, immunogenic, and well tolerated by healthy volunteers in clinical trials.Citation37,Citation38 The World Health Organization (WHO) has approved two inactivated vaccines for emergency use (Sinopharm and Sinovac). Simultaneously, China has approved five of its vaccines for emergency use.
Rheumatic disease (RD) is a chronic inflammatory condition that mostly affects the bones, joints, and soft tissues. Immunization with live or attenuated vaccinations is not indicated for RD patients. There is limited evidence of the efficacy of the ICVs in RD individuals; a small amount of research evidence has been focused on mRNA vaccines.Citation39,Citation40 A recent native Chinese study showed that the percentage of inhibition was significantly lower in RA patients receiving two doses of vaccines compared with the HC group. This study enrolled both active and stable patients, and applied percent inhibition measurement.Citation41 A single center study in Hong Kong focused on COVID-19 vaccination and arthritis flare among patients with rheumatoid arthritis (RA), and found that no evidence of increased risk of arthritis flare after fully vaccinated with ICV.Citation42 But they did not study its immunogenicity. Based on the COVID-19 vaccination strategy for RD patients by local government at that time, and the recommendation mentioned above,Citation8–10 only stable stage patients were involved in this study.
Our results indicated that compared with the HC controls, the positive reactions of stable RD patients to IgG and Nab were 60.00% (48/80) and 71.25% (57/80), respectively, whereas those positive response of HC were 82.6% (19/23) and 78.3% (18/23), respectively. It is similar to the conclusion reached in a study of inactivated influenza or pneumonia vaccines.Citation6,Citation7 This outcome was consistent with the findings of the majority of investigations on the response of mRNA vaccines.Citation15,Citation16,Citation43
In addition, we used a logistic regression to investigate the factors that contributed to the reduction in antibody response to ICVs in RD patients, and discovered that the level of RDW-C (P = .035) and the use of MTX (P = .016) were impact factors that affected the production of IgG; Simultaneously, the level of CRP (P = .015) and the use of prednisolone (P = .018) were factors causing reduced Nab production.
The volume distribution width of red blood cells (RDW) is a parameter used to quantify the variability in the size of red blood cells in peripheral blood during standard blood testing. It is a biomarker of inflammatory activity in autoimmune disorders.Citation44,Citation45 The level of CRP increases to a variety of infections and inflammatory conditions, and these immune activation parameters, mediated by the predominance of memory CD4+ T cell phenotypes, have an effect on the immunogenicity and protective efficacy of vaccines available to prevent infections.Citation46 Inflammation is also a factor in the decline in vaccination serum conversion rates.Citation47
In this study, we discovered that treatment with MTX may significantly lower the IgG antibody response, which was in agreement with the findings of earlier studies.Citation11,Citation48 MTX may have a negative impact on immunogenicity in RD patients, and it is possible that this was due to reduced cellular immunity (CD8+ T lymphocyte activation) after vaccination.Citation49
Other DMARDs (leflunomide, hydroxychloroquine, IL-17 inhibitor, and iguratimod) had no effect on the development of SARS-CoV-2 antibodies. The antibody positive rate in RD patients using prednisolone (median dose 7.5 mg/day, P = .018) ranged from 31.58 to 31.42% in our study. Glucocorticoids have been shown to impair the immunological response to vaccinations.Citation50 Prednisolone’s dose-dependent effect (more than 10 mg/day) has been linked to a reduction in the immunological response caused by vaccines in persons with inflammatory diseases.Citation51,Citation52 The outcomes of our investigation corroborated the findings.
Except for MTX and prednisolone, no additional drugs were shown to alter the antibody response in this study. There could be several reasons for this, including:
The recruited vaccinated population had RD patients during the quiescent state of the disease, and they might have used fewer doses or types of drugs than those in the active state, even though their medications were used adequate;
Individual preferences for different therapies may have influenced the results. For example, iguratimod is a rheumatoid arthritis therapy that inhibits nuclear factor (NF-kB) activation approved by the Pharmaceuticals and Medical Devices Agency of Japan (PMDA), and the China Food and Drug Administration (CFDA).Citation53 It is less frequently used in the West than in China;
In our investigation, among those without antibody IgG responses, eight TNF-a inhibitors, one Jak inhibitor, and one IL-17 antagonist were used; while five TNF-a inhibitors were used among non-antibody Nab responses. Surprisingly, all individuals who got belimumab developed antibodies against vaccine. Unfortunately, there is not a single instance of rituximab reported, and we were unable to evaluate the immunogenicity of RD patients while they were in an active state.
The adverse reactions (AR) were mild to moderate in this investigation. No Grade 3 or Grade 4 adverse reactions were observed as to the Advisory Committee on Immunization Practices’ Interim (ACPI) definition.Citation54 A mild reaction occurred in 3.00%-7.00% of RD patients within the first week after receiving ICVs, which is similar to the general population,Citation55 but greater (9.5%) in a cross-sectional research conducted in Mexico. This study did not find thrombocytopenic purpura,Citation56 myocarditis,Citation57 or other disease outbreaks.Citation58 Unlike prior research,Citation48 the AR was not associated with elevated CRP (P = .332).
As previously indicated, patients with weakened immune systems couldn’t produce enough antibodies to fight against SARS-CoV-2 infection.Citation59 In light of COVID-19, it is critical to improve vaccine responses in people with weakened immune systems. Long-term immunization against SARS-CoV-2 necessitates constant monitoring of susceptible patients, including those with RD. The immunological response of RD patients in South China to inactivated vaccines was found to be diminished. RD patients receiving ICVs should not delay.
There are a few limitations in this study, as follows: First, it was conducted at a single center and with a small sample size, and non-randomized design. Second, this study was limited to adult Han ethnicity individuals in China who were between the ages of 18 and 59 years old. As of 21 March, 2021, the government prioritizes early-stage vaccination for healthy citizens or patients with stable chronic diseases aged 18–59 years old in China. Consequently, it is necessary to further evaluate the tolerance, safety, and immunogenicity of ICVs in Asian children, adolescents, and the elderly (over 60 years old). Third, we did not conduct any experiments to assess the cellular immune response following vaccination,because the antibody response correlates well with T-cell mediated immunity.Citation60 Fourth, the persistence of the immune response induced by the ICVs were not observed.in our study, we only detected the immunogenicity of ICVs on 14-35 days after the second injection, and long-term follow-up cohort study need to be explored. We did not inspect peri-vaccination immunosuppression situation in this study. A greater number of enrolled cases should be obtained through multi-center studies.
Conclusion
We first described a glimpse of the safety data regarding the ICVs in stable RD patients in the Han population without serious side effects or disease attacks. In this study, we discovered that the ICVs have high levels of safety and tolerance. It is critical to develop a vaccination portfolio comprised of a variety of techniques in order to provide a more effective defense against the SARS-CoV-2 pandemic.
Author’s contribution
Conception and design: HQ Z, HJ L, Z L, ZB Y, XP L, LP D, ZH Y, and ZH Y.
Data curation: all authors.
Formal analysis: HQ Z, HJ L, ZB Y, XP L, XK Z, Y Z, TT Y, and YS C
Investigation: all authors.
Methodology: all authors.
Project administration: HQ Z, ZH Y, and ZZ Y.
Resources: all authors.
Data analysis: HQ Z, Z L, ZH Y, and HJ L
Supervision: HJ L, ZZ Y, ZH Y and HQ Z.
Writing—original draft: HQ Z, HJ L and Z L.
Writing—review & editing: HJ L, ZB Y, XP L, KX Z, LP D, YS C, and ZH Y.
Approval of final manuscript: all authors.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Shenzhen Futian Hospital for Rheumatic Diseases (No. FS202101002).
Supplemental Material
Download Zip (6.2 KB)Acknowledgements
We would like to express our gratitude to everyone who contributed to this study. I would like to express my heartfelt thanks to: Dr. Zhongyu Xie (Department of Orthopedics, The Eighth Affiliated Hospital of Sun Yat-sen University, China), Dr. Dapeng Li (Department of Pulmonary Medicine & Tuberculosis, The Third People’s Hospital of Shenzhen, China) for management support and invaluable assistance,Dr. Haofei Hu (Department of Nephrology, The First Affiliated Hospital of Shenzhen University, China).Fenlian Guo, Shiyin Yan, Dr. Xinpeng Chen, Weizhen He, Dr. XinMin Huang, Dr. Jianhua Gao, Dr. Jiansong Li (Shenzhen Futian Hospital for Rheumatic Diseases, China) for participate in the collection of samples, which was deeply appreciated by the entire team.
Data availability statement
The datasets analyzed in this manuscript are not publicly available. Requests to access the datasets should be directed to [email protected].
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2090176.
Additional information
Funding
References
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. [ accessed 2021 Oct 16]. https://covid19.who.int/?gclid=EAIaIQobChMI2_CM6eDZ6gIVghh9Ch3nDQm1EAAYASAAEgLqwPD_BwE.
- Li L, Guo P, Zhang X, Yu Z, Zhang W, Sun H. SARS-CoV-2 vaccine candidates in rapid development. Hum Vaccin Immunother. 2021 Mar 4;17(3):1–8. doi:10.1080/21645515.2020.1804777.
- Akiyama S, Hamdeh S, Micic D, et al. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis.2020 Oct 13;80:384–391. annrheumdis-2020-218946
- Friedman MA, Winthrop KL. Vaccines and disease-modifying antirheumatic drugs: practical implications for the rheumatologist. Rheum Dis Clin North Am. 2017;43(1):1–13. doi:10.1016/j.rdc.2016.09.003.
- Sanchez-Piedra C, Diaz-Torne C, Manero J, Pego-Reigosa JM, Rúa-Figueroa Í, Gonzalez-Gay MA, Gomez-Reino J, Alvaro-Gracia JM. Clinical features and outcomes of COVID-19 in patients with rheumatic diseases treated with biological and synthetic targeted therapies. Ann Rheum Dis. 2020;79(7):988–990. doi:10.1136/annrheumdis-2020-217948.
- Holvast A, Huckriede A, Wilschut J, et al. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann Rheum Dis. 2006;65(7):913–918. doi:10.1136/ard.2005.043943.
- van Aalst M, Langedijk AC, Spijker R, et al. The effect of immunosuppressive agents on immunogenicity of pneumococcal vaccination: a systematic review and meta-analysis. Vaccine. 2018 Sep 18;36(39):5832–5845. doi:10.1016/j.vaccine.2018.07.039.
- Park JK, Lee EB, Shin K, et al. Korean college of rheumatology task force for COVID-19 vaccine guidance for patients with autoimmune inflammatory rheumatic diseases. COVID-19 vaccination in patients with autoimmune inflammatory rheumatic diseases: clinical guidance of the Korean college of rheumatology. J Korean Med Sci. 2021 Mar 29;36(12):e95. doi:10.3346/jkms.2021.36.e95.
- Schulze-Koops H, Krüger K, Hoyer BF, Leipe J, Iking-Konert C, Specker C. Commission for pharmacotherapy and the board of directors of the German society for rheumatology. Updated recommendations of the German society for rheumatology for the care of patients with inflammatory rheumatic diseases in times of SARS-CoV-2-methodology, key messages and justifying information. Rheumatology (Oxford). 2021 May 14;60(5):2128–2133. doi:10.1093/rheumatology/keab072.
- Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, Calabrese C, Gravallese EM, Harpaz R, Kroger A, et al. American college of rheumatology guidance for COVID-19 vaccination in patients with rheumatic and Musculoskeletal diseases: version 3. Arthritis Rheumatol. 2021;73(10):e60–e75. doi:10.1002/art.41928.
- Medeiros-Ribeiro AC, Aikawa NE, Saad CGS, Yuki EFN, Pedrosa T, Fusco SRG, Rojo PT, Pereira RMR, Shinjo SK, Andrade DCO, et al. Immunogenicity and safety of the Corona Vac inactivated vaccine in patients with autoimmune rheumatic diseases: a phase 4 trial. Nat Med. 2021;27(10):1744–1751. doi:10.1038/s41591-021-01469-5.
- Seyahi E, Bakhdiyarli G, Oztas M, Kuskucu MA, Tok Y, Sut N, Ozcifci G, Ozcaglayan A, Balkan II, Saltoglu N, et al. Antibody response to inactivated COVID-19 vaccine (CoronaVac) in immune-mediated diseases: a controlled study among hospital workers and elderly. Rheumatol Int. 2021;41(8):1429–1440. doi:10.1007/s00296-021-04910-7.
- Stowe RP, Peek MK, Cutchin MP, Goodwin JS. Plasma cytokine levels in a population-based study: relation to age and ethnicity. J Gerontol a Biol Sci Med Sci. 2010;65(4):429–433. doi:10.1093/gerona/glp198.
- Moyon Q, Sterlin D, Miyara M, Anna F, Mathian A, Lhote R, Ghillani-Dalbin P, Breillat P, Mudumba S, de Alba S, et al. Bnt162b2 vaccine-induced humoral and cellular responses against SARS-CoV-2 variants in systemic lupus erythematosus. Ann Rheum Dis. 2021 Oct 4;81(4):575–583: annrheumdis-2021-221097. doi:10.1136/annrheumdis-2021-221097.
- Ruddy JA, Connolly CM, Boyarsky BJ, Werbel WA, Christopher-Stine L, Garonzik-Wang J, Segev DL, Paik JJ. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80(10):1351–1352. doi:10.1136/annrheumdis-2021-220656.
- Haberman RH, Herati R, Simon D, Samanovic M, Blank RB, Tuen M, Koralov SB, Atreya R, Tascilar K, Allen JR, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80(10):1339–1344. doi:10.1136/annrheumdis-2021-220597. Epub 2021 May 25. PMID: 34035003; PMCID: PMC8219484
- Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2014;66(7):1016–1026. doi:10.1002/acr.22246. PMID: 24339395.
- Chan ES, Cronstein BN. Methotrexate—how does it really work? Nat Rev Rheumatol. 2010;6(3):175–178. doi:10.1038/nrrheum.2010.5. PMID: 20197777.
- Burns CM. The history of cortisone discovery and development. Rheum Dis Clin North Am. 2016;42(1):1–14, vii. doi:10.1016/j.rdc.2015.08.001. PMID: 26611547.
- Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. doi:10.1038/s41584-020-0372-x. Epub 2020 Feb 7. PMID: 32034323.
- Mohammadi O, Ta K. Azathioprine. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing ; 2022. https://www.ncbi.nlm.nih.gov/books/NBK542190/. 2022 Jan. PMID: 31194347
- Prakash A, Jarvis B. Leflunomide: a review of its use in active rheumatoid arthritis. Drugs. 1999;58(6):1137–1164. doi:10.2165/00003495-199958060-00010. PMID: 10651393.
- Mucke HA. Iguratimod: a new disease-modifying antirheumatic drug. Drugs Today (Barc). 2012;48(9):577–586. doi:10.1358/dot.2012.48.9.1855758. PMID: 23032798.
- Prasad N, Manjunath R, Rangaswamy D, Jaiswal A, Agarwal V, Bhadauria D, Kaul A, Sharma R, Gupta A. Efficacy and safety of cyclosporine versus tacrolimus in steroid and cyclophosphamide resistant nephrotic syndrome: a prospective study. Indian J Nephrol. 2018;28(1):46–52. doi:10.4103/ijn.IJN_240_16. PMID: 29515301; PMCID: PMC5830809.
- Gadina M, Le MT, Schwartz DM, Silvennoinen O, Nakayamada S, Yamaoka K, O’-Shea JJ. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019 Feb 1;58(Suppl 1):i4–i16. doi:10.1093/rheumatology/key432; PMID: 30806710; PMCID: PMC6657570
- Gerriets V, Goyal A, Khaddour K. Tumor necrosis factor inhibitors. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Apr 30. https://www.ncbi.nlm.nih.gov/books/NBK482425/. 2022 Jan. PMID: 29494032
- Fobelo Lozano MJ, Serrano Giménez R, Castro Fernández M. Emergence of inflammatory bowel disease during treatment with secukinumab. J Crohns Colitis. 2018 Aug 29;12(9):1131–1133. doi:10.1093/ecco-jcc/jjy063; PMID: 29746636
- Jordan N, D’-Cruz DP. Belimumab for the treatment of systemic lupus erythematosus. Expert Rev Clin Immunol. 2015;11(2):195–204. doi:10.1586/1744666X.2015.996550. Epub 2014 Dec 29. PMID: 25543845.
- Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, van Assen S, Bijl M, Breedveld FC, D’Amelio R, Dougados M, Kapetanovic MC, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(1):39–52. doi:10.1136/annrheumdis-2019-215882.
- Ramasamy MN, Jessop LJ. CoronaVac: more data for regulators and policy makers. Lancet. 2021 Jul 17;398(10296):186–188. doi:10.1016/S0140-6736(21)01543-9.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi:10.1016/S1473-3099(20)30843-4.
- R A, Wang H, Wang W, Tan W. Summary of the detection kits for SARS-CoV-2 approved by the national medical products administration of China and their application for diagnosis of COVID-19. Virol Sin. 2020;35(6):699–712. doi:10.1007/s12250-020-00331-1. Epub 2020 Dec 22. PMID: 33351166; PMCID: PMC7754703.
- Nand IS, Fisher LD, Chiang Y-T, Latini R, Masson S, Maggioni AP, Glazer RD, Tognoni G, Cohn JN. Changes in brain natriuretic peptide and norepinephrine over time and mortality and morbidity in the Valsartan Heart Failure Trial (Val-HeFT). Circulation. 2003;107(9):1278–1283. doi:10.1161/01.CIR.0000054164.99881.00.
- Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology(STROBE) statement: guidelines for reporting observational studies. The The Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X.
- WHO Coronavirus (COVID-19) Dashboard with vaccination data. WHO; 2021. https://covid19.who.int.
- Kumar S, Çalışkan DM, Janowski J, Faist A, Conrad BCG, Lange J, Ludwig S, Brunotte L. Beyond vaccines: clinical status of prospective COVID-19 therapeutics. Front Immunol. 2021 Oct 1;12:752227. doi:10.3389/fimmu.2021.752227.
- Bueno SM, Abarca K, González PA, Gálvez NMS, Soto JA, Duarte LF, Schultz BM, Pacheco GA, González LA, Vázquez Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine in a subgroup of healthy adults in Chile. Clin Infect Dis. 2021 Sep 19: ciab823. doi:10.1093/cid/ciab823.
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39–51. doi:10.1016/S1473-3099(20)30831-8.
- Furer V, Eviatar T, Zisman D, Peleg H, Paran D, Levartovsky D, Zisapel M, Elalouf O, Kaufman I, Meidan R, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis.2021;80(10):1330–1338. doi:10.1136/annrheumdis-2021-220647.
- Boyarsky BJ, Ruddy JA, Connolly CM, Ou MT, Werbel WA, Garonzik-Wang JM, Segev DL, Paik JJ. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021 Mar 23;80(8):1098–1099: annrheumdis-2021-220289. doi:10.1136/annrheumdis-2021-220289.
- Zhao T, Shen J, Zhu Y, Tian X, Wen G, Wei Y, Xu B, Fu C, Xie Z, Xi Y, et al. Immunogenicity of inactivated SARS-CoV-2 vaccines in patients with rheumatoid arthritis: a case series. Front Public Health. 2022 Apr 25;10:875558. doi:10.3389/fpubh.2022.875558. PMID: 35548080; PMCID: PMC9081335
- Li X, Tong X, Yeung WWY, Kuan P, Yum SHH, Chui CSL, Lai FTT, Wan EYF, Wong CKH, Chan EWY, et al. Two-Dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022;81(4):564–568. doi:10.1136/annrheumdis-2021-221571. Epub 2021 Oct 22. PMID: 34686479; PMCID: PMC8550868
- Boekel L, Steenhuis M, Hooijberg F, et al. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. Lancet Rheumatol 2021 Aug 6; 3(11): 778–788. doi:10.1016/S2665-9913(21)00222-8.
- Lee H, Kong SY, Sohn JY, et al. Elevated red blood cell distribution width as a simple prognostic factor in patients with symptomatic multiple myeloma. Biomed Res Int. 2014;2014:145619. doi:10.1155/2014/145619.
- Wang H, Wang J, Huang R, Xia J, Zuo L, Yan X, Yang Y, Wu C. Red blood cell distribution width for predicting significant liver inflammation in patients with autoimmune hepatitis. Eur J Gastroenterol Hepatol. 2019;31(12):1527–1532. doi:10.1097/MEG.0000000000001447.
- Balandya E, Reynolds T, Obaro S, Makani J. Alteration of lymphocyte phenotype and function in sickle cell anemia: implications for vaccine responses. Am J Hematol. 2016;91(9):938–946. doi:10.1002/ajh.24438.
- Frasca D, Blomberg BB. Aging induces B cell defects and decreased antibody responses to influenza infection and vaccination. Immun Ageing. 2020 Nov 19;17(1):37. doi:10.1186/s12979-020-00210-z.
- Deepak P, Kim W, Paley MA, et al. Effect of Immunosuppression on the immunogenicity of mRNA vaccines to SARS-CoV-2 : a prospective cohort study Ann Intern Med2021 Aug 31;174 (11):1572–1585. doi:10.7326/M21-1757.
- Haberman RH, Herati RS, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80(10): 1339–1344. doi:10.1136/annrheumdis-2021-220597.
- Aikawa NE, Campos LM, Silva CA, Carvalho JF, Saad CGS, Trudes G, Duarte A, Miraglia JL, Timenetsky MDCS, Viana VST, et al. Glucocorticoid: major factor for reduced immunogenicity of 2009 influenza a (H1N1) vaccine in patients with juvenile autoimmune rheumatic disease. J Rheumatol. 2012;39(1):167–173. doi:10.3899/jrheum.110721.
- Bugatti S, De Stefano L, Balduzzi S, Greco MI, Luvaro T, Cassaniti I, Bogliolo L, Mazzucchelli I, D’-Onofrio B, di Lernia M, et al. Methotrexate and glucocorticoids, but not anticytokine therapy, impair the immunogenicity of a single dose of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic inflammatory arthritis. Ann Rheum Dis. 2021;80(12):1635–1638. doi:10.1136/annrheumdis-2021-220862.
- Sakuraba A, Luna A, Micic D. Serologic response to coronavirus disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis. Gastroenterology. 2021 Sep 29;162(1): 88–108. doi:10.1053/j.gastro.2021.09.055.
- Nozaki YI. Novel molecular insights and a new csDMARD for rheumatoid arthritis, from Japan to the world. Life (Basel). 2021 May 20;11(5):457.
- Oliver SE, Gargano JW, Scobie H, Wallace M, Hadler SC, Leung J, Blain AE, McClung N, Campos-Outcalt D, Morgan RL, et al. The advisory committee on immunization practices’ interim recommendation for use of Janssen COVID-19 vaccine — United States, February 2021. MMWR Morb Mortal Wkly Rep. 2021 Mar 5;70(9):329–332. doi:10.15585/mmwr.mm7009e4.
- Damasceno DHP, Amaral AA, Silva CA, Simões E, Silva AC. The impact of vaccination worldwide on SARS-CoV-2 infection: A review on vaccine mechanisms, results of clinical trials, vaccinal coverage and interactions with novel variants. Curr Med Chem. 2022 Sep 1; 29(15):2673–2690. doi:10.2174/0929867328666210902094254.
- Shibata K, Tanaka H, Otani A, Kubo M, Hasegawa A, Amano I. Development of thrombocytopenic purpura following BNT162b2 mRNA COVID-19 vaccination. Rinsho Ketsueki. 2021;62(10):1519–1521. doi:10.11406/rinketsu.62.1519.
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021 Aug 10;144(6):471–484. doi:10.1161/CIRCULATIONAHA.121.056135.
- Li X, Ostropolets A, Makadia R, et al. Characterizing the incidence of adverse events of special interest for COVID-19 vaccines across eight countries: a multinational network cohort study. medRxiv[Preprint]. 2021 Apr 17:03.25.21254315. doi:10.1101/2021.03.25.21254315.
- Chiang TP, Connolly CM, Ruddy JA, Boyarsky BJ, Alejo JL, Werbel WA, Massie A, Christopher-Stine L, Garonzik-Wang J, Segev DL, et al. Antibody response to the Janssen/Johnson & Johnson SARS-CoV-2 vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis. 2021;80(10):1365–1366. doi:10.1136/annrheumdis-2021-221145.
- Li D, Sempowski GD, Saunders KO, Acharya P, Haynes BF. SARS-CoV-2 neutralizing antibodies for COVID-19 prevention and treatment. Annu Rev Med. 2022 Jan 27;73(1):1–16. doi:10.1146/annurev-med-042420-113838. Epub 2021 Aug 24. PMID: 34428080