ABSTRACT
Rare cases of viral keratitis after coronavirus disease 2019 (COVID-19) vaccination have been reported. Furthermore, to our knowledge, cases of viral keratitis after two rounds of COVID-19 vaccination have not yet been reported. We report the case of a 19-year-old man without a history of keratitis, who developed viral keratitis soon after receiving the second and third doses of inactivated COVID-19 vaccines. Each time after the patient received treatment with topical and systemic drugs, his ocular symptoms were gradually relieved, and corrected visual acuity in both eyes returned to normal. COVID-19 vaccination may be associated with rare cases of the development of keratitis in individuals without a medical history of keratitis. Physicians should be aware of the possible relationship between ocular symptoms and adverse reactions to the COVID-19 vaccination. Despite the potential risks of COVID-19 vaccination, the benefits of immunization against the virus far outweigh these risks.
Introduction
Currently, five coronavirus disease 2019 (COVID-19) vaccines with different mechanisms of action have been widely used in China. Although vaccines have become more effective and safer owing to advancements in biotechnology, none are completely free from adverse effects. Data from the Chinese Center for Disease Control and Prevention (CCDC) showed that the adverse events of COVID-19 vaccines were reported at the rate of 11.86 per 100,000 persons from December 2020 to March 2022 in China. Among these adverse events, 83% were general reactions such as fever, swelling, and subcutaneous nodules at injection sites, while 17% were uncommon adverse reactions such as allergic skin rash, angioedema, and acute severe allergic reaction.Citation1
Many vaccines have been implicated in ocular adverse events, the most common of which are reactions of the eyelids and conjunctiva. Optic neuritis and various patterns of intraocular inflammation are the most frequently implicated serious adverse events. However, viral keratitis is relatively rare.Citation2–5
Patient presentation
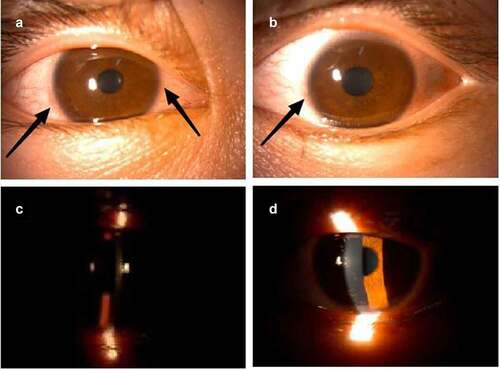
A 19-year-old man without a history of eye disease received the first and second doses of inactivated COVID-19 vaccine (CoronaVac, Sinovac Life Sciences, Beijing, China) on 20 May 2021 and 15 June 2021, respectively. Three weeks after the second dose of COVID-19 vaccination, blurry vision in both eyes was observed, and the patient was diagnosed with viral keratitis in another hospital. He received ganciclovir ophthalmic gel and levofloxacin eye drops without relief of the ocular symptoms. He was admitted later to our hospital. Upon examination, the patient was found to have poor eyesight (best-corrected visual acuity of 20/60 in the right eye and 20/100 in the left eye). Slit-lamp examination revealed conjunctival hyperemia, rough corneal epithelium, irregular patchy infiltration of the entire cornea, and positive corneal fluorescence staining (). As for laboratory blood tests, rheumatoid factor, anti-cyclic citrullinated peptide antibody, human leukocyte antigen-B27, and other examinations for rheumatism were all within normal limits. Multiple enlarged lymph nodes were revealed on cervical ultrasonography on both sides of the neck, which were consistent with the manifestations of reactive hyperplasia.
Figure 1. Slit-lamp examination photos on the twenty-fifth day after the second dose of COVID-19 vaccination.
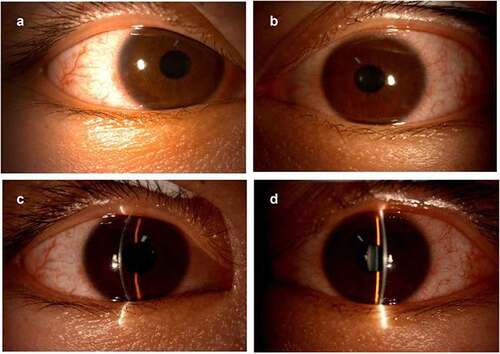
After admission, rubella virus IgG, cytomegalovirus IgG, and herpes simplex virus (HSV) I and II IgG tests were performed, and the results were all positive. The patient received the treatment regimen, including ganciclovir (400 mg, intravenously) every 12 h, ganciclovir eye drops four times daily, and 0.05% cyclosporine eye drops three times daily. One week later, the corrected visual acuity in both eyes returned to 20/20. Small patches of infiltration were observed at the lower center of the cornea in the left eye. Fluorescence staining of both the eyes was negative ().
Figure 2. Slit-lamp examination photos on the thirty-second day after the second dose of COVID-19 vaccination.
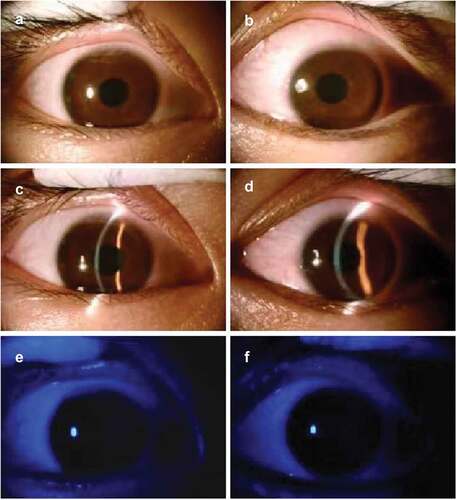
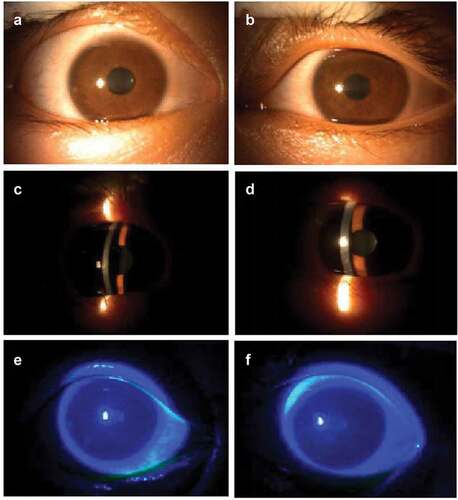
The patient received the third dose of COVID-19 vaccine on 23 December 2022 (CoronaVac, Sinovac Life Sciences, Beijing, China). Seven days after the inoculation, the patient developed the same symptoms in both eyes. He had reduced eyesight (best-corrected visual acuity of 20/30 in the right eye and 20/40 in the left eye). Slit-lamp examination revealed conjunctival hyperemia, rough corneal epithelium, and irregular patchy infiltration of the entire cornea (). After one-week treatment with ganciclovir eye drops, 0.05% cyclosporine eye drops, and acyclovir and ganciclovir administered orally, the corrected visual acuity of both eyes recovered to 20/20. Slit-lamp examination revealed conjunctival hyperemia and neovascularization in the limbus of both eyes ().
Discussion
Viral keratitis is a type of infectious keratitis mainly caused by HSV and varicella-zoster virus (VZV).Citation6 Virus replicates in corneal epithelium and can destroy epithelial cells.Citation7 Patients with epithelial keratitis may present with pain, photophobia, blurred vision, tearing, and redness, which are commonly induced by the exposure to UV light, stress, changes in sex hormones, and immune suppression.Citation8 Keratitis, while uncommon, has been reported after VZV vaccination.Citation9,Citation10 In another study by Bolletta et al., complications such as herpetic keratitis, anterior scleritis, anterior uveitis, toxoplasma retinochoroiditis, Vogt-Koyanagi-Harada disease reactivations, pars planitis, retinal vasculitis, retinal vein occlusions, non-arteritic ischemic optic neuropathy, activations of quiescent choroidal neovascularization secondary to myopia or uveitis, and central serous chorioretinopathy were all reported in 30 days after COVID-19 vaccination.Citation3
In this case, the two doses of COVID-19 vaccine, which is a formalin-inactivated vaccine containing the alum adjuvant, were produced by the same biotechnology company. In a clinical study of this kind of vaccine in 600 healthy adults aged 18–59 years, the most common adverse reactions were injection-site symptoms, such as swelling, erythema, and pain around the injection site, and mild systemic reactions, such as transient fever, tiredness, headache, or chills. However, there are no reports of ocular-related adverse reactions.Citation11 Thus, ocular adverse events related to inactivated COVID-19 vaccination are uncommon.
Keratitis associated with the COVID-19 vaccination is rare. It was reported that ocular herpes virus infection might be activated by the COVID-19 (BNT162b2) mRNA vaccine.Citation12 Cases of viral keratitis reactivated by inactivated COVID-19 vaccination after recovery have also been reported.Citation4 However, in this case, the patient had no previous history of viral keratitis, which oriented us toward the opinion that it was a case of primary viral keratitis related to the COVID-19 vaccination. Similar symptoms were observed after each dose of COVID-19 vaccination. Additionally, the interval between the third dose of vaccination and ocular complications (7 days) was much shorter than that between the second dose of vaccination and the same ocular complications (21 days). This phenomenon was consistent with the study by Zhang,Citation11 which reported a faster and stronger immune response elicited by two doses of vaccine. Although we did not perform PCR for virus testing to confirm the type of viral keratitis, the symptoms were completely relieved by topical application of ganciclovir ophthalmic gel, cyclosporine, and systemic administration of ganciclovir. Although a definitive association between COVID-19 vaccination and viral keratitis is difficult to demonstrate, to some extent, this case further illuminates a probable correlation between inactivated COVID-19 vaccination and viral keratitis.
In conclusion, although this case conveys the possible association between inactivated COVID-19 vaccination and the emergence of viral keratitis, vaccination remains highly effective in the prevention of COVID-19. Moreover, we recommend inactivated COVID-19 vaccination considering the extremely low incidence of viral keratitis. The benefits of immunization against COVID-19 far outweigh these risks.
Acknowledgments
We thank the patient for allowing us to share his details.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- National Health Commission, PRC. Guidelines for coronavirus-19 vaccination (first edition)[EB/OL]. http://www.nhc.gov.cn/xcs/gzzcwj/202103/c2febfd04fc5498f916b1be080905771.shtml.
- Cheng JY, Margo CE. Ocular adverse events following vaccination: overview and update. Surv Ophthalmol. 2022;67(2):1–4. doi:10.1016/j.survophthal.2021.04.001.
- Bolletta E, Iannetta D, Mastrofilippo V, De Simone L, Gozzi F, Croci S, Bonacini M, Belloni L, Zerbini A, Adani C, et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med. 2021;10(24):5960. doi:10.3390/jcm10245960.
- Li S, Jia X, Yu F, Wang Q, Zhang T, Yuan J. Herpetic keratitis preceded by COVID-19 vaccination. Vaccines (Basel). 2021;9(12). doi:10.3390/vaccines9121394.
- Pang K, Pan L, Guo H, Wu X. Case report: associated ocular adverse reactions with inactivated COVID-19 vaccine in China. Front Med (Lausanne). 2021;8:823346. doi:10.3389/fmed.2021.823346.
- Durand ML, Barshak MB, Chodosh J. Infectious keratitis in 2021. Jama. 2021;326(13):1319–1320. doi:10.1001/jama.2021.0424.
- Holland EJ, Schwartz GS. Classification of herpes simplex virus keratitis. Cornea. 1999;18(2):144–154. doi:10.1097/00003226-199903000-00002.
- Vicetti Miguel RD, Sheridan BS, Harvey SAK, Schreiner RS, Hendricks RL, Cherpes TL. 17-Beta estradiol promotion of herpes simplex virus type 1 reactivation is estrogen receptor dependent. J Virol. 2010;84(1):565–572. doi:10.1128/JVI.01374-09.
- Hwang CW, Steigleman WA, Saucedo-Sanchez E, Tuli SS. Reactivation of herpes zoster keratitis in an adult after varicella zoster vaccination. Cornea. 2013;32(4):508–509. doi:10.1097/ICO.0b013e318277acae.
- Jabbour S, Shekhawat NS, Chen A, Woreta FA. Presumed herpes zoster ophthalmicus reactivation following recombinant zoster vaccination. Cornea. 2021;40(2):248–250. doi:10.1097/ICO.0000000000002537.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi:10.1016/S1473-3099(20)30843-4.
- Alkhalifah MI, Alsobki HE, Alwael HM, Al Fawaz AM, Al-Mezaine HS. Herpes simplex virus keratitis reactivation after SARS-CoV-2 BNT162b2 mRNA vaccination: a report of two cases. Ocul Immunol Inflamm. 2021;29(6):1238–1240. doi:10.1080/09273948.2021.1986548.