ABSTRACT
Metastasis of head and neck squamous cell carcinoma rarely occurs in hepatic cancer and has a poor prognosis (median survival of 4 months). The efficacy of immunotherapy for these patients remains unknown. Herein, we present a patient with hypopharyngeal carcinoma metastasis to the liver with TERT and TP53 mutations together with a combined positive score of 70. The tumor invaded the abdominal wall, liver, inferior vena cava and retroperitoneal lymph nodes. The patient was treated with pembrolizumab combined with cisplatin and 5-FU for four cycles and has been maintained on pembrolizumab monotherapy until now. The patient achieved a near complete response of hepatic and subcutaneous metastases, and the tumor thrombus disappeared completely. The patient developed grade I rashes on the trunk, which were considered immune-related adverse events; thus, the patient presented a significant tumor response and good tolerance to the therapeutic strategy. On the basis of this observation, pembrolizumab-based therapeutic strategies may be an effective alternative for metastatic hypopharyngeal carcinoma and may prolong overall survival and progression-free survival, which should be confirmed by more patients in the future. Immune-related adverse events also need attention.
Introduction
Head and neck squamous cell carcinoma (HNSCC) accounts for approximately 90% of head and neck cancers, and there are approximately 830,000 new cases annually, representing the sixth most common cancer worldwide.Citation1 Most cases are initially diagnosed as advanced stages (stage III or IV), and surgery and radiotherapy with/without chemotherapy are considered the main therapeutic strategies for advanced HNSCC according to the primary tumor site and stage.Citation2 Hypopharyngeal cancer accounts for 2% of head and neck cancers and usually spreads to local lymph nodes or metastases when confirmed; the 5-year overall survival (OS) rate is only 30–35%.Citation3 Therefore, new therapeutic strategies are urgently needed. Immunotherapy may be a novel and promising treatment. As a result of several clinical trials for recurrent/metastatic HNSCC (R/M HNSCC), programmed cell death-1 (PD-1) inhibitors such as nivolumab and pembrolizumab demonstrated better anticancer efficacy and tolerability than conventional chemotherapeutic agents.Citation4 Here, we report a case of hypopharyngeal carcinoma metastasis to the abdomen that achieved a near complete response to pembrolizumab-based therapeutic strategies.
Case presentation
A 51-year-old male with a sore throat was admitted to the Department of Otolaryngology, Head and Neck Surgery of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, in January 2020, with a 20-year history of smoking 1 pack per day and drinking three times per month.
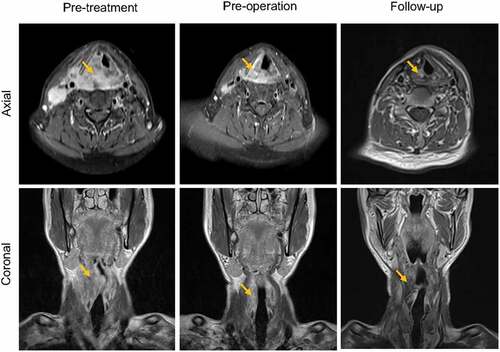
A cauliflower-like mass was found on the right piriform fossa with bilateral cervical lymphadenopathy on physical examination. The pathologic results confirmed poorly differentiated squamous cell carcinoma of hypopharyngeal carcinoma. Further magnetic resonance imaging (MRI) () and positron emission tomography-computed tomography (PET-CT) (data not shown) showed that the tumor stage was cT4aN2cM0 (AJCC2008). The patient underwent neoadjuvant chemotherapy (paclitaxel at 260 mg/m2/d1, cisplatin at 75 mg/m2/d1 and fluorouracil at 750 mg/m2/d1–5) for three cycles followed by radical surgical treatment (including tracheotomy, vertical hemilaryngectomy and bilateral cervical lymph node dissection) plus postoperative concurrent chemoradiotherapy. His eating and respiration were satisfactory. No evidence of recurrence or metastasis was found during normative follow-up until April 2021 ().
Figure 1. MRI T1 sequence of the head and neck region.
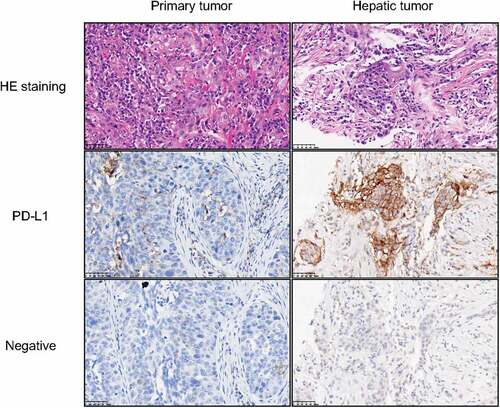
A bump was observed subcutaneously on the right chest wall with a mild and recurrent backache. By abdominal CT and ultrasonography, a round mass of approximately 49 × 36 × 28 mm was found in hepatic S7/8 segments together with multiple enlarged retroperitoneal lymph nodes and tumor thrombus in the inferior vena cava. There were no signs of tumor recurrence on head and neck MRI. The hepatic and chest wall mass was diagnosed as poorly differentiated squamous cell carcinoma by pathology, and the immunohistochemical results included CK (+), CK5/6 (+), P63 (+), Ki67 approximately 55% (+), P40 (+), CK7 (+), CK19 (+), Muc-1 (+), GPC3 (-), Muc-5AC (-), Hep (-) and in situ hybridization: EBers (-). The combined positive score (CPS) of the hepatic metastasis tumor was 75 (). A PET-CT scan of the right posterior lobe of the liver indicated abnormal blood perfusion. Next-generation sequencing (NGS) indicated a low tumor mutational burden (8.0 mut/Mb), microsatellite stability, TERT -443 C to -443 G, and amino acid 176 of TP53 was mutated from cysteine to the terminator. Multiple abdominal metastases occurred after comprehensive treatment of hypopharyngeal carcinoma with stage rT0N0M1 based on the medical history.
Figure 2. Hematoxylin-Eosin (HE) staining and immunohistochemistry of tumors.
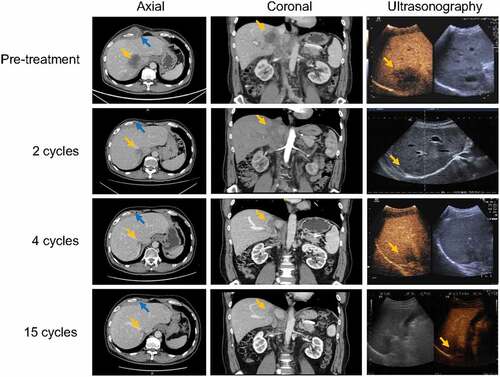
According to the National Comprehensive Cancer Network (NCCN) Guideline Version 1.2021 (section Very Advanced Head and Neck Cancer), the patient was treated with pembrolizumab 200 mg/d1 followed by cisplatin 75 mg/m2/d1 and 5-FU 750 mg/m2/d1–5 after written informed consent was obtained. After two cycles of treatment, the subcutaneous nodule disappeared, and the volume of the hepatic metastatic tumor shrank by 46%. After four cycles of pembrolizumab and chemotherapy, the patient received pembrolizumab maintenance monotherapy. As a result, the hepatic metastatic tumor maintained near complete remission on CT, while the echogenicity was homogeneous on conventional ultrasound, and there were no malignant signs on contrast-enhanced ultrasound. In addition, the subcutaneous metastasis and tumor thrombus disappeared completely on CT ().
Figure 3. Contrast-enhanced CT and ultrasonography of the abdomen.
Immune-related adverse events (irAEs): The patient had grade I rashes on the trunk during immunotherapy and recovered after treatment with glucocorticoids, antiallergic drugs and other symptomatic treatments. The examination and treatment procedures were approved by the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University, and performed in accordance with the Declaration of Helsinki.
Discussion
With the advancement of surgical techniques and chemoradiotherapy, locoregional control of HNSCC has significantly improved. However, at least 50% of patients with advanced HNSCC experience locoregional or distant relapses, which are frequently diagnosed within the first 2 years after treatment.Citation2 The site of the primary tumor (hypopharynx in particular), advanced T and N classification, locoregional control and histologic grade were identified as the most relevant predictors of distant metastasis.Citation5 Lung metastasis accounts for 66%, and the lung is considered the most common distant metastatic site for HNSCC patients, followed by the bone (22%) and the liver (10%).Citation6 Unfortunately, if HNSCC has spread outside of the primary tumor and neck region, the median OS of patients might be only 5 months.Citation7
In this case of advanced hypopharyngeal cancer, the patient accepted a pembrolizumab-based therapeutic strategy and achieved a successful objective response. The size of hepatic metastasis and lymph nodes, together with subcutaneous nodules in the chest wall, decreased drastically and maintained a response for more than 12 months. The patient had microsatellite stability; however, a better response and longer survival have been reported to correlate with microsatellite instability in HNSCC.Citation8 In addition, the patient carried TERT and TP53 mutations, while TERT mutations are associated with the recurrence of primary tumorsCitation9 and worse survival of laryngeal carcinoma patients.Citation10 Patients with TP53 mutations usually have a poorer survival outcome and shorter time to the development of distant metastases,Citation11 which were confirmed in our case. However, D.T. Le et al. discovered that a higher tumor mutation load was associated with prolonged progression-free survival.Citation12
High programmed cell death ligand-1 (PD-L1) expression and CD8+ lymphocyte infiltration provide a suitable microenvironment for the application of immune checkpoint inhibitors (ICIs) in a subset of hepatocellular carcinoma (HCC) cases.Citation13 A high density of CD8+ T-cell infiltration in tumors is associated with a prominently better prognosis for immunotherapy.Citation14 However, cancer metastasis frequently occurs in the liver, which could result in limited benefit from immunotherapy, influencing the objective response rate and overall survival.Citation15 Distinct from other tissues or organs, the liver is an immunoprivileged organ capable of tolerating antigens and promoting an immune response due to the density of T-cell colonies and diversity of tumor-infiltrating immune cells.Citation16 Thus, anti-PD-1/PD-L1 therapy alone may have limited clinical efficacy. Recently, Yu et al. discovered that intrahepatic monocyte-derived macrophages could mediate T-cell depletion and lead to systemic immunosuppression.Citation17 Lee et al. discovered that Tregs in liver tumors play a key role in reducing systemic reactions to anti-PD-1 immunotherapy.Citation18 It is worth noting that the CPS of the hepatic metastatic tumor was 75, while the CPS of the primary hypopharyngeal carcinoma was 10, indicating that PD-L1 expression may vary in different stages of tumor development. Thus, the complex tumor microenvironment could determine the therapeutic efficacy of tumor immunotherapy, and it is necessary to further investigate its inner mechanism in the future.
In addition to tumor response and good tolerance, irAEs cannot be overlooked. irAEs are reported to be related to the suppression of the PD-1/PD-L1 pathway. The incidence of irAEs has been reported to be 54–76%, and they usually occur in the skin, lung and gastrointestinal system.Citation19 A series of AEs, such as myocarditis, could lead to death. In our case, the patient had a recurrent slight rash during immunotherapy and recovered after treatment. Therefore, irAEs should be carefully monitored when using ICIs.
In conclusion, this is a preliminarily successful case of hypopharyngeal carcinoma metastasis to the abdomen with a 12-month near complete response to a pembrolizumab-based therapeutic strategy, which should be confirmed by more patients in the future. In addition, careful attention should be given to related adverse reactions during treatment.
Disclosure statement
The authors report that there are no competing interests to declare.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):1–5. doi:10.3322/caac.21492.
- Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695–1709. doi:10.1016/S0140-6736(08)60728-X.
- Newman JR, Connolly TM, Illing EA, Kilgore ML, Locher JL, Carroll WR. Survival trends in hypopharyngeal cancer: a population-based review. Laryngoscope. 2015;125:624–629.
- Ferris R, Licitra L. PD-1 immunotherapy for recurrent or metastatic HNSCC. Lancet (London, England). 2019;394(10212):1882–1884. doi:10.1016/S0140-6736(19)32539-5.
- Takes RP, Rinaldo A, Silver CE, Haigentz M Jr., Woolgar JA, Triantafyllou A, Mondin V, Paccagnella D, de Bree R, Shaha AR, et al. Distant metastases from head and neck squamous cell carcinoma. Part I. Basic aspects. Oral Oncol. 2012;48(9):775–779. doi:10.1016/j.oraloncology.2012.03.013.
- Marioni G, Blandamura S, Calgaro N, Ferraro SM, Stramare R, Staffieri A, Filippis CD. Distant muscular (gluteus maximus muscle) metastasis from laryngeal squamous cell carcinoma. Acta Otolaryngol. 2005;125(6):678–682. doi:10.1080/00016480410024613.
- Alvi A, Johnson JT. Development of distant metastasis after treatment of advanced-stage head and neck cancer. Head Neck. 1997;19:500–505.
- Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191.
- Morris LGT, Chandramohan R, West L, Zehir A, Chakravarty D, Pfister DG, et al. The molecular landscape of recurrent and metastatic head and neck cancers: insights from a precision oncology sequencing platform. JAMA Oncol. 2017;3:244–255.
- Qu Y, Dang S, Wu K, Shao Y, Yang Q, Ji M, et al. TERT promoter mutations predict worse survival in laryngeal cancer patients. Int J Cancer. 2014;135:1008–1010.
- Neskey DM, Osman AA, Ow TJ, Katsonis P, McDonald T, Hicks SC, et al. Evolutionary action score of TP53 identifies high-risk mutations associated with decreased survival and increased distant metastases in head and neck cancer. Cancer Res. 2015;75:1527–1536.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520.
- Calderaro J, Rousseau B, Amaddeo G, Mercey M, Charpy C, Costentin C, et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64:2038–2046.
- Huang CY, Wang Y, Luo GY, Han F, Li YQ, Zhou ZG, et al. Relationship between PD-L1 expression and CD8+ T-cell immune responses in hepatocellular carcinoma. J Immunother (1991). 2017;40:323–333.
- Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol. 2013;10:292–302.
- O’-Leary K. Liver metastases cultivate an immune desert. Nat Rev Cancer. 2021;21:143.
- Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated Tcell elimination. Nat Med. 2021;27:152–164.
- Lee JC, Mehdizadeh S, Smith J, Young A, Mufazalov IA, Mowery CT, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. Sci Immunol. 2020;5:eaba0759.
- Ramos-Casals M, Brahmer J, Callahan M, Flores-Chávez A, Keegan N, Khamashta M, et al. Immune-related adverse events of checkpoint inhibitors. Nature Reviews Disease Primers. 2020;6:38.
