ABSTRACT
ARCoV is a candidate mRNA vaccine encoding receptor-binding domain of SARS-CoV-2. Its safety, tolerability, and immunogenicity profile have been confirmed in the phase 1 clinical trial in China. A multi-regional phase 3 clinical trial is currently underway to test the efficacy of ARCoV (NCT04847102). Here, we tested the cross-neutralization against SARS-CoV-2 variants of concern (VOCs) of a panel of serum samples from participants in the phase 1 clinical trial of ARCoV by pesudo- and authentic SARS-CoV-2. Our data suggest the immunity induced by the ARCoV vaccine reduced but still has significant neutralization against the Alpha and Delta variants. Moreover, ARCoV maintained activity against the Beta variant, despite of its obvious reduction in neutralizing titers. Our findings further support the solid protective neutralization activity against VOCs induced by ARCoV vaccine.
Dear Editor
ARCoV, which is developed by the Academy of Military Medical Sciences and Suzhou Abogen Biosciences in China, is a candidate mRNA vaccine encoding the receptor binding domain (RBD) of SARS-CoV-2 (Wuhan-Hu-1 strain).Citation1 Its safety, tolerability, and immunogenicity profile have been confirmed in the randomized, double-blind, and placebo-controlled phase 1 clinical trial in healthy adults aged 18–59 years old in China.Citation2 A multi-regional phase 3 clinical trial is currently underway to test the efficacy of ARCoV (NCT04847102).
The continuous global circulation of SARS-CoV-2 has resulted in emergence of SARS-CoV-2 variants of concern (VOCs) including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2). In November 2021, a new VOC Omicron (B.1.1.529) emerged in South Africa with highly transmissibility. These VOCs contain multiple mutations in the spike protein of SARS-CoV-2, especially in neutralizing antibody binding sitesCitation3 (Table S1). These mutations have been reported to confer neutralization resistance to mAbs, a modest loss of susceptibility to convalescent plasma, and sera from vaccinated individuals in comparison with the wild-type Wuhan-01 bearing D614 G.Citation4–6
Herein, a set of sera of 11 participants in the phase 1 clinical trial of ARCoV was tested for neutralization of VOCs. All samples were collected on day 43 post initial immunization with 15 μg of ARCoV.Citation2 The cross-neutralizing activity of these serum samples against all pesudo-SARS-CoV-2 VOCs except the Gamma and Omicron variants was assayed by a 50% neutralization assay. The VSV-based pseudovirus neutralization assay showed that all 11 serum samples still potently neutralized the Alpha variant with geometric mean titer (GMT) of 826.7 (1.7-fold reduction) in comparison with WT with GMT of 1440.9 ( and Tables S1 and S2). We also observed 2.6-fold average reduction of the plasma neutralizing activity against the Delta (GMT: 548.9) variant. However, compared with the WT pseudovirus, the neutralization activity significantly reduced 2.4-fold for the Beta variant (GMT: 610.4) (, Tables S1 and S2, p < .05).
Figure 1. Human serum neutralization of SARS-CoV-2 variant following the second dose of ARCoV.
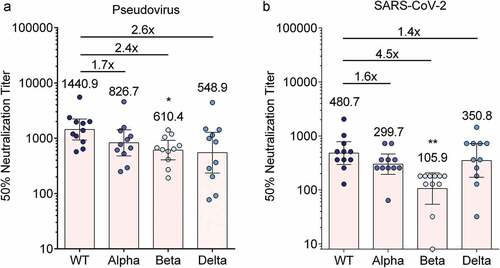
By the authentic virus neutralization assay, we further assessed the cross-neutralizing activity of the serum samples to the VOCs including Alpha (strain BJ202101), Beta (strain GDPCC), and Delta (strain CQ0077). The prototypic SARS-CoV-2 (strain 131, WT) was used as the control.Citation1 As shown in and Table S3, the Alpha and Delta variants were 1.6- and 1.4-fold less sensitive to the sera than the WT, respectively. Notably, the neutralization activity significantly reduced 4.5-fold for the Beta variant (GMT: 105.7) in comparison with the WT strain (, Tables S1 and S3, p < .01).
Our data show the immunity induced by the ARCoV vaccine still has relative potent neutralization against the Alpha and Delta variants. This finding is consistent with the other recent reports assessing neutralization activity of two leading SARS-CoV-2 mRNA vaccines BNT162b2 and mRNA-1273 developed by Pfrzier and Moderna, respectively.Citation7,Citation8 There is growing body of evidence that both humoral and cellular immune responses induced by SARS-CoV-2 mRNA vaccines play an important role in protection against VOCs.Citation9,Citation10 Therefore, the ARCoV-elicited cross-reactive T cell response is likely to be able to provide solid protection against the Beta variant despite of obvious reduction in neutralization activity. Of note, for Delta variant, the pseudovirus was less sensitive to neutralization of serum samples than that of authentic variant in comparison with WT. This discrepancy in neutralization activity is possibly due to the absent of the G142D mutation in the S protein of the authentic virus (Table S2). Despite this, pseudovirus titers still represent a good prediction for the neutralization activity to the authentic viruses. A potential limitation of this study may be absent of data on neutralization activity to the authentic Omicron variant, which will be performed in the future study. Overall, our findings support the solid protective neutralization activity against VOCs induced by ARCoV vaccine, although the vaccine effectiveness against these variants must be validated by the undergoing phase 3 clinical trial.
Supplementary Appendix
Download MS Word (41.7 KB)Disclosure statement
C.F.Q. is an inventor on pending patent applications related to the ARCoV mRNA vaccine. All other authors declare no competing interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2094142.
Additional information
Funding
References
- Zhang N-N, Li X-F, Deng Y-Q, Zhao H, Huang Y-J, Yang G, Huang W-J, Gao P, Zhou C, and Zhang R-R, et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182(5):1–2. doi:10.1016/j.cell.2020.07.024.
- Chen GL, Li X-F, Dai X-H, Li N, Cheng M-L, Huang Z, Shen J, Ge Y-H, Shen Z-W, Deng Y-Q, et al. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Microbe. 2022;3(3):e193–2. doi:10.1016/S2666-5247(21)00280-9.
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Peacock SJ, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. doi:10.1038/s41579-021-00573-0.
- Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD, et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593(7857):130–135. doi:10.1038/s41586-021-03398-2.
- Kuzmina A, Khalaila Y, Voloshin O, Keren-Naus A, Boehm-Cohen L, Raviv Y, Shemer-Avni Y, Rosenberg E, Taube R. SARS-CoV-2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post-vaccination sera. Cell Host Microbe. 2021;29(4):522–528 e522. doi:10.1016/j.chom.2021.03.008.
- Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, Yoon H, Li D, Haynes BF, Sanders KO, et al. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe. 2021;29(4):529–539 e523. doi:10.1016/j.chom.2021.03.002.
- Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021;384(15):1466–1468. doi:10.1056/NEJMc2102017.
- Pegu A, O’-Connell SE, Schmidt SD, O’-Dell S, Talana CA, Lai L, Albert J, Anderson E, Bennett H, Corbett KS, et al. Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science. 2021;373(6561):1372–1377. doi:10.1126/science.abj4176.
- Liu J, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature. 2022. doi:10.1038/s41586-022-04465-y.
- Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, Khan K, Cele S, Bernstein M, Karim F, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603(7901):488–492. doi:10.1038/s41586-022-04460-3.
