ABSTRACT
In January 2020, SARS-COV-2 infection spread worldwide and was declared “pandemic” by WHO. Because of the high contagiousness of the virus and devastating effects of the epidemic on public health, numerous efforts have been made to develop suitable vaccines to prevent the infection. Among the side effects developed by patients who undergone vaccination, there are common symptoms but also more serious reactions such as the thrombosis syndromes. This paper presents two cases of thrombosis temporally associated with live-vectored Covid vaccination similar to vaccine-induced thrombocytopenia (VITT) in patients with inherited thrombophilia (respectively, the deficiency of protein S and a Factor II mutation). The clinical manifestation caused by VITT is characterized by widespread thrombosis especially affecting intracranial venous sinus, which may cause massive bleeding and intracranial hemorrhage. Although this condition is widely described in literature, there is no evident correlation between this side effect and inherited condition of thrombophilia. The authors suggest that the presence of inherited thrombophilia should be better investigated and, if necessary, screened during the anamnestic data collection before the vaccine administration, leading the healthcare professional to choose the appropriate vaccine to the patient.
Introduction
In European Union, in 2021, four vaccines were authorized for use with different mechanisms of action to induce the immune response, Vaxzevria and Jansenn are based on a non-replicating viral vector, used as carrier of spike proteins.Citation1 The vaccine development is commonly based on the spike protein of the virus as it is a surface protein accessible by antibodiesCitation2 and viral vector vaccines are based on a genetically engineered so it does not cause disease, but it produces the surface protein in order to generate the immune response.Citation3 In particular, Vaxzevira is based on a modified DNA of a chimpanzee adenovirus that encodes a similar s-peptide to generate the immune response through the activation of T- and B-cells,Citation4 whereas Janssen is based on a harmless cold virus to deliver the gene that encodes the spike protein to induce T- and B-cell production.Citation5
Considering pre-licensure and post-marketing data about vaccine safety, in Italy special anamnestic record has been created by Ministry of HealthCitation6 to be administered to patients before choosing which vaccine to inoculate.
Among the questions administered to patients, there is the reference to the presence of any major hematological disorders, the use of anticoagulant therapy, a previous history of thrombotic episodes, the assumption of oral contraceptives due to the procoagulant action of COVID19 infection.Citation7
Although rare, thrombotic events are among the side effects of COVID19 vaccination, especially related to vaccine based on viral vector,Citation8 including at unusual sites.Citation9 Vessel thrombosisCitation10 is a major side effect also found following administration of ChadOx1 vaccine in subjects with factor XIII deficiency, with an increased Cerebral Venous Sinus Thrombosis (CVST) risk in the presence of heterozygous MTHFR C677T. This side effect has been included in a clinical syndrome called vaccine-induced immune thrombotic thrombocytopenia (VITT), which has specific inclusion criteriaCitation11 (must meet all five criteria):
COVID vaccine 4 to 42 days prior to symptom onset
Any venous or arterial thrombosis (often cerebral or abdominal)
Thrombocytopenia (platelet count <150 × 109/L)
Positive PF4 heparin-induced thrombocytopenia (HIT) ELISA
Markedly elevated D-dimer (>4 times upper limit of normal)
However, minor hematological disorders such as rare deficiency of protein S and coagulation factor II are not mentioned as risk factors of patients undergoing vaccination.
To date, to the best of our knowledge, no cases of severe morbidity and mortality have been reported in subjects with thrombosis temporally associated with live-vectored Covid vaccination in subjects who specifically have protein S or factor II deficiencies and these defects are not currently indicated among the contraindications for the administration of vaccination.
In this case series, we described two cases of thrombosis temporally associated with live-vectored Covid vaccination in a patient with a diagnosed history of factor II deficiency and in a patient with a misdiagnosed protein S deficiency.Citation12
Patients presentation
Case one
In May 2021, a 54-year-old man with a history of vascular pathology (phlebitis and problems of saphenous vein) received the single-dose Johnson COVID-19 vaccine.
Two weeks later, he began to suffer severe pain in the left leg so, he first went to his general practitioner (GP), who prescribed blood tests, D-dimers dosage, and subcutaneous administration of low molecular weight heparin (Enoxaparin 4000 IU).
Three days later, he presented to the hospital emergency room where he was examined by the vascular surgeon who found “thrombosis of large saphenous vein’s anterolateral collateral in patient with varicose vein in large saphenous.” He underwent ecocolordoppler and blood exams which showed thrombocytopenia with platelet count of 36 × 10^3/mL (reference range 150–400) and increased level of D-dimers at 63830 (reference range 45–498), and he was discharged with the prescription of anticoagulant.
The next day, due to persistent pain associated with paresthesia of the left half of face and upper limb, the patient came back to the emergency room where no focal neurological signs and a Glasgow Come Scale of 15 were found, and head CT and angio-TC showed thrombosis of the superior sagittal sinus and hypodensity brain area suggestive for acute/subacute ischemic vascular injury.
During the hospitalization, a deficiency of protein S of coagulation was discovered (activity of 40%, normal range between 62 and 145).
Despite the endovascular procedures and the drug treatment (IVIG: Intra Venous Immunoglobulin), his clinical conditions progressively worsened until encephalic death was declared.
Following the complaint by relatives, a judicial autopsy was performed.
Autopsy findings
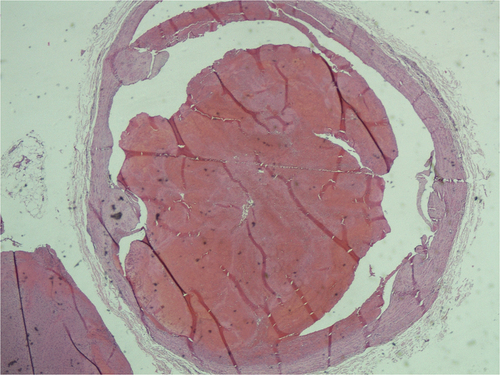
Autopsy revealed no alterations in thoracic and abdominal organs, whereas skull dissection revealed a marked and widespread congestion and cerebral edema. The vascular structures revealed thrombotic-like material within the superior sagittal sinus was found as well as within the transverse sinus, the sigmoid sinuses and the large saphenous vein in the proximal tract of left thigh.
Histopathological findings
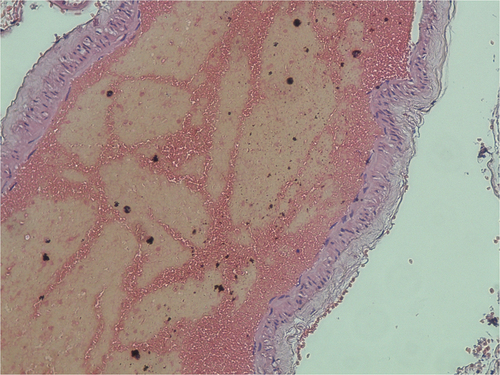
The microscopic examination of the thrombotic-like material revealed consolidated agglomerations of platelets and red blood cells, which can be attributed to recent thrombus (without evidence of re-handling). In the large saphenous vein’s thrombotic material was also found neocapillarization and moderate intra-lesional fibroblastic proliferation ().
In this case, applying the Causality Assessment algorithm, the cause/effect relationship is indeterminate.
Case two
A 42-year-old woman carrier of factor II mutation with no previous history of relevant morbidity presented to the emergency department referring difficulty in walking, lack of strength and confusion. The patient reporting being in her usual state of health until approximately ten days prior to presentation of symptoms at which time she received the first dose of Vaxzevria COVID-19 vaccination. The day after the vaccination she complained persistence of arthralgia, fever, and headache, even after the administration of corticosteroids, antibiotics, and paracetamol, so she went to the emergency room. At the emergency room, suddenly the patient had a seizure, so a CT scan was performed and a frontal cerebral infarction and an extensive thrombosis of the sinuses were found. Routine labs demonstrated thrombocytopenia to 59,000 platelets/mcL (reference range 172,000–440,000 platelets/mcL) and D-dimer elevation to 31458 mcg/L (reference range <500 mcg/L). She was admitted to Neurosurgery Department and underwent mechanical thrombectomy with complete revascularization of the sinuses. After this clinical management, the patient remained hospitalized for one month and then was discharged in stable condition. On follow-up, the patient continued to remain asymptomatic without further episodes.
Performing the Causality Assessment algorithm, the cause/effect relationship between vaccination and adverse events is indeterminate.
Discussion
According to current evidences, a rare complication related to the administration of the adenovirus-based coronavirus vaccines is represented by the thrombosis with thrombocytopenia syndrome (TTS). It is a pathological condition in which patients develop thromboembolic episodes affecting uncommon vascular districts but the most frequent localization of the thrombotic material is the brain vessels, with phenomena of cerebral venous thrombosis (CVT).Citation13,Citation14,Citation15
On the other hand, there are no reported episodes of TTS secondary to the administration of messenger RNA-based vaccinesCitation16 and the analysis of the Centers for Disease Control (CDC) in the USA reported an incidence of one patient vaccinated with AD26.COV2.S in nearly 500.000.Citation17
Furthermore, the European Medicine Agency (EMA) concluded that the CVT following ChAdOx1nCoV-19 have a rate of 1/200.000 administrations with a risk of CVT occurrence after administration of SARS-CoV-2 vaccines comparable to the risk of the general population.Citation19
In this case series, we described two cases of thrombosis temporally associated with live-vectored Covid vaccination. Both patients were also affected by inherited thrombophilia: Prothrombin G20210A and Protein S deficiency.
Protein S deficiency is an autosomal dominant condition due to mutation in the PROS1 gene on chromosome 3Citation18 associated with an increased risk of thromboembolism. Venous thromboembolism including deep vein thrombosis (DVT) and pulmonary embolism (PE) is the major clinical manifestation of protein S deficiency, although thrombosis of the axillary, mesenteric and cerebral veins has also been reported.
Prothrombin G20210A mutation is the second most common inherited variant and results from a substitution of adenine (A) for guanine (G) at position 20210 in a non-coding region of the gene.Citation20 Prothrombin G20210A confers an increased risk of CVT: in 2016, a meta-analysisCitation21 found a modest association (OR 5.8; 95% CI 4.0–8.6). The combination of this variant and other acquired risk factors, such as the administration of drugs with increased thrombogenic effect, further increase this risk; for example, it is well known in the literature that oral contraceptives represent a prothrombotic risk factor especially in subjects with G20210A mutation where this category of drugs exponentially increases the risk of thrombosis.Citation22
To the best of our knowledge, in the literature, there are no reported cases of TTS in subjects with inherited thrombophilia (Prothrombin G20210A and Protein S deficiency), however, in our experience, we reported two cases in which thrombosis temporally associated with live-vectored Covid vaccination has determined, respectively, severe outcome and death of the patient.
Furthermore, adenovirus-based vaccines could increase the thrombotic risk as well as other drugs categories such as oral contraceptives.13,14
In pre-licensure life of the vaccine, it is very hard to assess the safety profile of the products in specific subgroups, which can show a risk-benefit ratio of the immunization intervention different than the general population.
The assessment of a single event is not consistent with the purpose of identifying a definite causal association or the absence of association; the association between vaccine/AEFI in our cases can be considered indeterminate because of the absence of the clear evidence for a causal link, conflicting trends or confounding elements (history of factor II deficiency and misdiagnosed protein S deficiency are important risk factors) or inconsistency with causal association to immunization.
Our case series seem to suggest the opportunity that future studies about pharmacovigilance of vaccines should include a sample of subjects presenting genetic hematological disorders in order to evaluate the incidence of this complication in this specific study population.
Moreover, the positivity to these minor hematological disorders is not sought during the anamnestic history to which the patients are subjected before the administration of SARS-CoV-2 vaccines. In our opinion, it would be appropriate to expand the studies focusing on the possible correlation with genetic hematological disorders. Based on any possible new and broader evidence, scientific community might consider including the Protein S deficiency and Factor II deficiency among the contraindications for the administration of adenovirus-based vaccines in order to reduce the risk of occurrence of TTS. In this eventuality, the presence of these types of inherited thrombophilia should lead the healthcare professional to provide for the administration of an alternative type of vaccine, such as messenger RNA-based vaccines.
Furthermore, it should be considered that the main focus of vaccination is the prevention of severe form of COVID-19, rather than the prevention of infection, in order to achieve a lower risk of hospitalization and death compared to non-vaccinated patients.Citation23,Citation24,Citation25,Citation27 Thrombotic and thromboembolic syndrome represent also one of the main risks related to COVID-19 with a global prevalence of approximately 20%.Citation28,Citation26 Moreover, the risk of thrombotic events related to vaccination is much lower and rated as 10 times lower than that from COVID-19Citation30 and, although the risk is greater in case of adenovirus-vector vaccines,Citation29 the benefits of vaccination continue to be more than the risks.
Conclusion
We describe two rare cases of thrombosis temporally associated with live-vectored Covid vaccination in patients with Protein S deficiency and Prothrombin G20210A. Minor inherited thrombophilia should be screened during the medical history sheets and thrombotic adverse events following immunization in this subgroup of patients must be a signal to consider in pharmacovigilance activities. Finally, it is relevant to underline that the benefit-risk ratio of Covid vaccination remains highly favorable, even more so if certain high-risk groups such as those with hematological disorders are excluded and steered to other vaccine types.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Han X, Xu P, Ye Q. Analysis of COVID-19 vaccines: types, thoughts, and application. J Clin Lab Anal [Internet]. 2021;35:1. https://pubmed.ncbi.nlm.nih.gov/34396586
- Ravichandran S, Coyle E M, Klenow L, Tang J, Grubbs G, Liu S, Wang T, Golding H, and Khurana S. (2020). Antibody signature induced by SARS-CoV-2 spike protein immunogens in rabbits. Sci Transl Med, 12(550),– 10.1126/scitranslmed.abc3539
- Mascellino M Teresa, Di Timoteo F, De Angelis M, and Oliva A. (2021). Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect Drug Resist, 14 3459–5. 10.2147/IDR.S315727
- Sette A, and Crotty S. (2021). Adaptive immunity to SARS-CoV-2 and COVID-19. Cell, 184(4), 861–880. 10.1016/j.cell.2021.01.007
- Grifoni A et al . (2020). Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell, 181(7), 1489–1501.e15. 10.1016/j.cell.2020.05.015
- ASL Foggia Vaccinazioni modulistica https://www.sanita.puglia.it/web/asl-foggia/vaccini-modulistica2021
- Ferrari E et al . (2020). High Prevalence of Acquired Thrombophilia Without Prognosis Value in Patients With Coronavirus Disease 2019. J Am Heart Assoc, 9(21), e017773 10.1161/JAHA.120.017773
- Gómez-Mesa J Esteban, Galindo-Coral S, Montes M Claudia, and Muñoz Martin A J. (2021). Thrombosis and Coagulopathy in COVID-19. Curr Probl Cardiol, 46(3), 100742 10.1016/j.cpcardiol.2020.100742
- Oldenburg J, Klamroth R, Langer F, Albisetti M, von Auer C, Ay C, Korte W, Scharf R E, Pötzsch B, and Greinacher A. (2021). Diagnosis and Management of Vaccine-Related Thrombosis following AstraZeneca COVID-19 Vaccination: Guidance Statement from the GTH. Hamostaseologie, 41(3), 184–189. 10.1055/a-1469-7481
- Cines D B, and Bussel J B. (2021). SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med, 384(23), 2254–2256. 10.1056/NEJMe2106315
- Franchini M, Liumbruno G Maria, and Pezzo M. (2021). COVID-19 vaccine-associated immune thrombosis and thrombocytopenia (VITT): Diagnostic and therapeutic recommendations for a new syndrome. Eur J Haematol, 107(2), 173–180. 10.1111/ejh.13665
- Long B, Bridwell R, and Gottlieb M. (2021). Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am J Emerg Med, 49 58–61. 10.1016/j.ajem.2021.05.054
- Schultz N H et al . (2021). Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med, 384(22), 2124–2130. 10.1056/NEJMoa2104882
- Greinacher A, Thiele T, Warkentin T E, Weisser K, Kyrle P A, and Eichinger S. (2021). Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med, 384(22), 2092–2101. 10.1056/NEJMoa2104840
- Scully M, Singh D, Lown R, Poles A, Solomon T, Levi M, Goldblatt D, Kotoucek P, Thomas W, and Lester W. (2021). Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N Engl J Med, 384(23), 2202–2211. 10.1056/NEJMoa2105385
- American Society of Hematology Thrombosis with Thrombocytopenia Syndrome (also termed Vaccine-induced Thrombotic Thrombocytopenia http://www.hematology.org/covid-19/vaccine-induced-immune-thrombotic-thrombocytopenia
- CDC Advisory Committee on Immunization Practices (ACIP) Thrombosis with thrombocytopenia syndrome (TTS) following Janssen COVID-19 vaccine https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-04-23/03-COVID-Shimabukuro-508.pdf
- Borgel D, Gandrille S, and Aiach M. (1997). Protein S deficiency Thrombosis and haemostasis, 78, 351–6.
- EMA - European Medicines Agency AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood#:~:text=EMA%20confirms%20overall%20benefit%2Drisk,COVID%2D19%20Vaccine%20AstraZeneca
- Poort S R, Rosendaal F R, Reitsma P H, and Bertina R M. (1996). A common genetic variation in the 3'-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood, 88(10), 3698–703.
- Gonzalez J V, Barboza A G, Vazquez F J, and Gándara E. (2016). Prevalence and Geographical Variation of Prothrombin G20210A Mutation in Patients with Cerebral Vein Thrombosis: A Systematic Review and Meta-Analysis. PLoS One, 11(3), e0151607 10.1371/journal.pone.0151607
- Martinelli I, Sacchi E, Landi G, Taioli E, Duca F, and Mannucci P M. (1998). High risk of cerebral-vein thrombosis in carriers of a prothrombin-gene mutation and in users of oral contraceptives. N Engl J Med, 338(25), 1793–7. 10.1056/NEJM199806183382502
- Voysey M et al . (2021). Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet, 397(10269), 99–111. 10.1016/S0140-6736(20)32661-1
- Lopez Bernal J et al . (2021). Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ, 373 n1088 10.1136/bmj.n1088
- Haas E J et al . (2021). Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet, 397(10287), 1819–1829. 10.1016/S0140-6736(21)00947-8
- Spyropoulos A C and Weitz J I. (2020). Hospitalized COVID-19 Patients and Venous Thromboembolism. Circulation, 142(2), 129–132. 10.1161/CIRCULATIONAHA.120.048020
- Thompson M G et al . (2021). Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med, 385(4), 320–329. 10.1056/NEJMoa2107058
- Driggin E et al . (2020). Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J Am Coll Cardiol, 75(18), 2352–2371. 10.1016/j.jacc.2020.03.031
- Andrews N J, Stowe J, Ramsay M Eb, and Miller E. (2022). Risk of venous thrombotic events and thrombocytopenia in sequential time periods after ChAdOx1 and BNT162b2 COVID-19 vaccines: A national cohort study in England. Lancet Reg Health Eur, 13 100260 10.1016/j.lanepe.2021.100260
- Torjesen I. (2021). Covid-19: Risk of cerebral blood clots from disease is 10 times that from vaccination, study finds. BMJ, n1005 10.1136/bmj.n1005