ABSTRACT
Vaccination offers the best way to prevent invasive meningococcal disease (IMD). As demonstrated in countries with national immunization programs (NIPs) against IMD, meningococcal conjugate vaccines have contributed to significant declines in incidence. Since some meningococcal vaccines are associated with modest immunogenicity in infants, possible immunological interference upon concomitant administration with some pediatric vaccines, and administration errors resulting from improper reconstitution, opportunities for improvement exist. A quadrivalent conjugate vaccine, MenQuadfi® (Meningococcal [Serogroups A, C, Y, and W] Conjugate Vaccine; Sanofi, Swiftwater, Pennsylvania), was approved in 2020 for the prevention of IMD caused by meningococcal serogroups A, C, W, and Y in individuals ≥2 years of age in the United States. Five pivotal studies and one ancillary study supported approval in the United States; clinical trials in infants are ongoing. Data on the immunogenicity and safety of this vaccine are presented, and its potential value in clinical practice is discussed.
Introduction
Invasive meningococcal disease (IMD) is an acute and often fatal infection that occurs globally.Citation1–3 The etiologic agent—Neisseria meningitidis—colonizes the nasopharynx in 8% to 25% of healthy adolescents and adults.Citation4 Invasive meningococcal disease results when an encapsulated pathogenic strain enters the bloodstream of a nonimmune host, causing bacteremia, which can progress to sepsis, purpura fulminans, meningitis, or other focal infections.Citation5 Events that trigger invasion, including bacterial and viral respiratory infections and smoking, tend to involve disruption of the normal upper respiratory mucosal barrier.Citation2–8 Case-fatality rates (CFRs) are as high as 10% to 15% despite appropriate antibiotic therapy, with up to 20% of survivors experiencing permanent sequelae including loss of digits or even limbs, neurologic complications, and/or hearing loss.Citation3,Citation9 Incidence of IMD is highest in children younger than 5 years of age, particularly infants, with a second peak observed during adolescence and young adulthood; CFRs are highest among the elderly (i.e., those ≥65 years of age).Citation10–14
Although 12 serogroups of Neisseria meningitidis (distinguished by their polysaccharide capsule) have been identified, only serogroups A, B, C, W, X, and Y are associated with most cases of IMD. Regional variations in serogroup distribution are frequently observed. For example, serogroups B, C, and Y are frequently isolated in North America, while serogroup A is frequently isolated in parts of Africa and Asia.Citation14 Temporal variations in serogroup prevalence, which are unpredictable, are best exemplified by the increasing reports of IMD caused by serogroups X in Africa and W and/or Y in Australia, Africa, South America, and Europe in recent years.Citation15–19 Serogroup W has been associated with IMD reported during mass gatherings such as the annual Hajj/Umrah pilgrimage.Citation11,Citation20,Citation21 Hypervirulent strains of serogroup W are associated with higher mortality and are known to present with atypical symptoms including diarrhea and myocarditis, confounding diagnosis and leaving open the possibility of delays in treatment.Citation22–26
In the United States, the overall incidence of IMD in 2018, the latest year for which final Active Bacterial Core Surveillance (ABCs) data are available, was 0.10 per 100,000 population. The incidence was highest (0.83/100,000 population) in infants, followed by children 1–4 years of age (0.18/100,000 population), adults ≥65 years of age (0.14/100,000 population), and adolescents and young adults 16–23 years of age (0.10/100,000 population).Citation12 A 2018 meta-analysis documented CFRs associated with laboratory-confirmed IMD cases to be 9% in infants, decreasing to 7% in 7-year-old children before increasing to 15% in young adults, remaining below 20% up to the age of 60 years and increasing to a high above 40% in the mid-eighties.Citation27 The pooled CFR was highest for serogroup W (12.8%), followed by serogroup C (12.0%), Y (10.8%), and B (6.9%).Citation27 It is important to note that incidence of IMD caused by serogroups A, C, W, and Y has declined substantially since 1996; some of that decline, which occurred prior to the introduction of quadrivalent meningococcal vaccine, might have been due to reduction in tobacco use and increased use of fluoroquinolones.Citation28 The additional decline in IMD incidence since 2005 is considered attributable to the introduction of quadrivalent meningococcal conjugate vaccines (MenACWY) (see below for discussion of the vaccines available in the United States). This is evident through annual reductions by 10.6% and 35.6% in IMD caused by serogroups C, W, and Y among adolescents (the focus of the meningococcal immunization program in the US) and young adults following receipt of primary and booster doses, respectively, with reductions in IMD incidence being more pronounced in states with high vaccination coverage.Citation13,Citation29,Citation30
Although antibiotic resistance has been uncommon among meningococcal isolates in the United States,Citation31 the recent detection of serogroup Y isolates resistant to both penicillin and ciprofloxacin is a cause for concern.Citation32 This, in the context of the rapidity of progression of IMD, high fatality rates despite treatment, and the likelihood of debilitating lifelong sequelae,Citation1–3,Citation9 underscores the need for primary prevention through vaccination and secondary prophylaxis. Unconjugated polysaccharide vaccines against serogroups A, C, W, and Y do not induce a memory response, do not reduce nasopharyngeal carriage, may manifest hyporesponsiveness upon repeat dosing, and are weakly immunogenic in infants; all of these limitations are addressed by protein–polysaccharide conjugate vaccines.Citation33–35 Conjugate vaccines using serogroup-specific polysaccharide formulations against serogroups A, C, W, and Y, including monovalent and quadrivalent formulations, have been developed and included in national immunization programs (NIPs) globally.Citation11,Citation36 For serogroup B, the poor immunogenicity of the capsular polysaccharide necessitated the development of vaccines based on subcapsular surface protein antigens.Citation37
Inclusion of meningococcal vaccines in NIPs is based on recommendations from national immunization technical advisory groups (NITAGs), which take into consideration the burden of IMD, epidemiological changes, vaccine availability, vaccine efficacy, and cost-effectiveness of available vaccines.Citation38 Although the vaccines introduced over time have collectively contributed to significant declines in the global incidence of IMD,Citation10,Citation11,Citation13,Citation29,Citation30,Citation34,Citation39 revision of national immunization policies may be necessitated by local changes in meningococcal epidemiology, including serogroup distribution.Citation40
Three MenACWY are licensed and available in the United States ().Citation41 The first, licensed in 2005, is MenACWY-D (Menactra®, Sanofi, Swiftwater, PA, USA), which uses a diphtheria toxoid (D) carrier protein and is approved for use in individuals 9 months through 55 years of age.Citation42 The second, licensed in 2010, is MenACWY-CRM (Menveo®, GSK Vaccines Srl, Sovicille, Italy).Citation43 This vaccine uses a nontoxic variant of diphtheria toxin (CRM197) as the carrier protein and is approved for use in persons 2 months through 55 years of age. The most recently introduced vaccine (licensed in 2020) is MenACWY-TT (MenQuadfi®, Sanofi, Swiftwater, PA, USA),Citation44 which features a tetanus toxoid (TT) carrier protein and is approved for use in individuals ≥2 years of age including adults ≥55 years of age. Studies conducted in the US regarding infants from ≥6 weeks of age are ongoing.
Table 1. Quadrivalent (serogroups A, C, W, and Y) meningococcal vaccines currently licensed and available for use in the United States.
The effectiveness and safety of both MenACWY-D and MenACWY-CRM have been extensively documented;Citation13–29,Citation30–45–Citation47 however, there may be room for improvement. For example, while real-world effectiveness of MenACWY-D in adolescents and young adults has been demonstrated, immunogenicity in infants 2–6 months of age is modest.Citation48 Moreover, a descriptive/exploratory study in adults ≥56 years of age revealed the immunogenicity of MenACWY-D to be comparable but not appreciably better than that of an unconjugated quadrivalent polysaccharide vaccine (MPSV4; Menomune® – A/C/Y/W-135, Meningococcal Polysaccharide Vaccine, Groups A, C, Y, and W-135 Combined [Sanofi, Swiftwater, PA, USA]).Citation49 In the case of MenACWY-CRM, while one study documented immunogenicity in adults 56–65 years of age to be non-inferior compared with MPSV4,Citation50 two other studies on the immunogenicity of MenACWY-CRM in adults ≥56 years of age were inconclusive because the data were not sufficiently granular to assess immunogenicity in the age group of interest.Citation51,Citation52
There appears to be immunological interference upon concomitant administration of MenACWY-D with some routine vaccines administered in childhood (e.g., the pneumococcal conjugate vaccine [PCV] and diphtheria and tetanus toxoids and acellular pertussis vaccine adsorbed [DTaP]),Citation42,Citation53 although the clinical significance of the marginally lower responses against the pertussis antigen (fimbriae) in DTaP is not clear since no correlates of protection against pertussis have been defined.Citation42 Similar reductions of the immune responses to pneumococcal and pertussis antigens have been documented upon concomitant administration of MenACWY-CRM with PCV7 or the 13-valent pneumococcal conjugate vaccine [PCV13]Citation43,Citation54 or with DTaP,Citation55 although the attenuation of responses to pneumococcal antigens was restricted only to pneumococcal serotypes 6B and 23F and seen only after the third dose but not other doses of a four-dose series. Again, the clinical significance of lower responses to pertussis antigens is uncertain due to lack of defined correlates of protection against pertussis.Citation43 Finally, although it does not constitute concomitant administration, use of MenACWY-D 1 month after DTaP has been shown to result in attenuated responses to meningococcal antigens, prompting the suggestion that these two vaccines (MenACWY-D and DTaP) either be administered simultaneously or that MenACWY-D be administered prior to DTaP.
Although a few case reports soon after the introduction of MenACWY-D suggested a possible link between its use and the incidence of Guillain–Barré syndrome (GBS),Citation56 multiple subsequent studies found no association between the two.Citation41–57–Citation61 A cohort study that evaluated the safety of MenACWY-CRM reported a higher risk for Bell’s palsy in individuals receiving concomitant vaccines compared with those receiving MenACWY-CRM alone, but it was not clear whether the observed association was a chance occurrence, due to concomitant vaccination, or due to an underlying predisposition to Bell’s palsy.Citation62 No other significant safety concerns regarding MenACWY-D or MenACWY-CRM have been documented after licensure,Citation45,Citation46,Citation63,Citation64 confirming the excellent safety profile of conjugate ACWY vaccines. However, administration errors are more common with MenACWY-CRM than with other vaccines, presumably due to the need for reconstitution of two components, which are supplied in separate vials.Citation45–Citation65–67 The two components include the lyophilized MenA conjugate component and the liquid MenCWY component,Citation43 leaving open the possibility that reconstitution errors will result in lack of protection against some of the serogroups targeted by the vaccine.Citation65
MenACWY-TT was developed to help address some of the limitations associated with previously approved MenACWY. Preclinical screening studies by Sanofi that employed more than 100 glycoconjugates synthesized on a small scale to evaluate physicochemical properties, stability, ease of manufacturing, and immunogenicity in mouse models identified TT as being the most immunogenic carrier protein.Citation68 This finding led to the choice of TT as the carrier protein for the MenACWY-TT vaccine, with the expectation of improving immunogenicity in infants and the elderly.
The approval of MenACWY-TT in the United States was based on demonstrated noninferior immunogenicity and comparable safety versus licensed comparators in different age groups.Citation69 Salient characteristics of MenACWY-TT are listed in . MenACWY-TT was approved in 2020 for use in individuals ≥2 years of age in the United States and individuals ≥12 months of age as a single dose in the European Union and other countries. Clinical data from five pivotal studies (MET35, MET50, MET43, MET49, and MET56) and one ancillary study (MET44) conducted in the United States and Puerto Rico supported licensure of the vaccine in persons ≥2 years of age ().Citation70–76 Additional data on immunogenicity, safety, and compatibility with concomitantly administered vaccines are available from studies conducted in other countries that supported licensure outside the United States;Citation77–80 these were reviewed by Martinón-Torres et al.Citation81 and included studies in which Nimenrix®, another MenACWY-TT vaccine licensed outside the US, was used as comparator. This manuscript provides an overview of the published data on the immunogenicity and safety of MenACWY-TT in individuals ≥2 years of age, including adults ≥56 years of age – an age group for which an FDA-approved meningococcal conjugate vaccine did not previously exist. We also discuss the vaccine’s potential role in clinical practice in the United States.
Table 2. Salient characteristics of the US-licensed MenACWY-TT conjugate vaccine.
Table 3. Summary of studies that supported the licensure of MenACWY-TT in the United States.
Methods
In this overview of the immunogenicity and safety of MenACWY-TT, the intent was to focus on the clinical studies that supported the licensure of the vaccine in the United States for use in individuals ≥2 years of age. The PubMed/Medline database was searched on July 1, 2021, to retrieve the published peer-reviewed literature on the immunogenicity and safety of MenACWY, with studies focusing on the immunogenicity and safety of MenACWY-TT among participants in the United States being identified for inclusion through an iterative process as depicted in Supplementary Figure S1, which also provides details on the terms used in the literature search.
Results
Literature search
The literature search described above retrieved 106 primary articles. Following review of the titles and abstracts of each retrieved study, 5 primary articles (defined as articles directly reporting the findings of individual clinical studies on the immunogenicity and safety of MenACWY-TT) were retained; see Supplementary Figure S1 for an accounting of articles retrieved, eliminated, and retained. One primary article reporting the findings of a MenACWY-TT study conducted in children 2–9 years of age (MET35)Citation71 was not retrieved through the search (possibly due to non-inclusion of the keyword ‘MenACWY-TT’ in the ‘substances’ section of the PubMed record for this article) but was captured based on the authors’ knowledge of its publication and suitability for inclusion. Accordingly, a total of six primary articles that documented the immunogenicity and safety of MenACWY-TT in individuals ≥2 years of age have been included ().Citation71–76 Study participants included meningococcal vaccine-naïve individuals, individuals primed with licensed MenACWY, and those concomitantly receiving other routinely administered adolescent vaccines. In each active-controlled study, a US-licensed meningococcal vaccine approved for use at the time of conduct of the study was used as the comparator: MenACWY-D, MenACWY-CRM, or the quadrivalent meningococcal polysaccharide vaccine (MPSV4). MPSV4, which was discontinued in 2017, was the only vaccine licensed for use in adults ≥56 years of age in the United States at the time two of the studies of MenACWY-TT in this age group (MET49 and MET44) were conducted.Citation75,Citation76
Immunogenicity
For purposes of licensure, assessment of the immunogenicity of investigational meningococcal vaccines is based on serum bactericidal assays (SBAs) whose results have been shown to correlate with protection against IMD.Citation82 The SBAs are performed either with human complement (hSBA) or with baby rabbit complement (rSBA).Citation83 In April 2011, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the United States Food and Drug Administration (FDA) endorsed the use of hSBA to infer the effectiveness of investigational meningococcal conjugate vaccines.Citation84,Citation85 Assessment of the immunogenicity of MenACWY-TT was therefore based on hSBA, with immunogenicity being defined using the following parameters: seroprotection (hSBA titers ≥1:8 on post-vaccination Day 30); seroresponse (post-vaccination hSBA titers ≥1:8 [for the Phase II US study in adolescents]Citation72 or ≥1:16 [for all other US studies]Citation71–73–Citation76 if pre-vaccination titers were <1:8, or a ≥4-fold increase in hSBA titer by Day 30 following vaccination if pre-vaccination titers were ≥1:8); and geometric mean antibody titers (GMTs). Demonstration of immunological noninferiority required the lower limit of the 2-sided 95% confidence interval (CI) of the difference between proportions of participants in the MenACWY-TT group and the comparator group who achieved a seroresponse to be greater than −10% for all four serogroups. Immunogenicity findings of the studies (i.e., seroprotection rates, seroresponse rates, and hSBA GMTs) are depicted in and summarized in Supplementary Tables S1–3. Data on seroprotection are discussed below.
Figure 1. Immunogenicity findings of pivotal Phase II and Phase III studies of MenACWY-TT: Seroprotection rates based on post-vaccination Day 30 hSBA titers. The seroprotection rate was defined as the proportion of participants with post-vaccination hSBA titers ≥1:8. White bars depict data for MenACWY-TT and gray bars depict data for comparator vaccines.
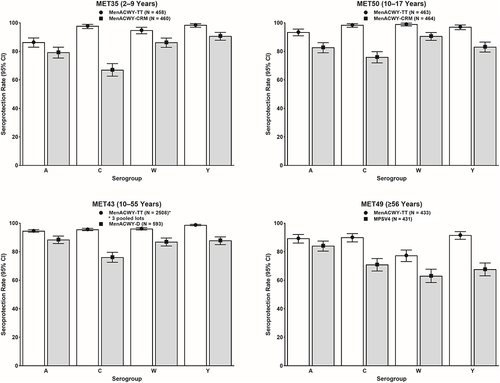
Figure 2. Immunogenicity findings of pivotal Phase II and Phase III studies of MenACWY-TT: Seroresponse rates based on post-vaccination Day 30 hSBA titers. The seroresponse rate was defined as the proportion of participants with post-vaccination hSBA titers ≥1:8 (for study MET50) and ≥1:16 (for all other studies) if pre-vaccination titers were <1:8 or with a ≥4-fold increase if pre-vaccination titers were ≥1:8. White bars depict data for MenACWY-TT and gray bars depict data for comparator vaccines.
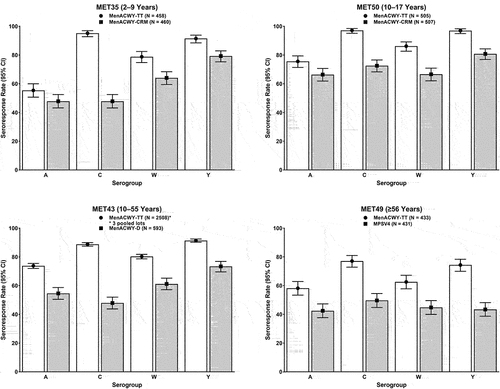
Figure 3. Immunogenicity findings of pivotal Phase II and Phase III studies of MenACWY-TT: Geometric means of hSBA titers on post-vaccination Day 30 in the per-protocol population. White bars depict data for MenACWY-TT and gray bars depict data for comparator vaccines.
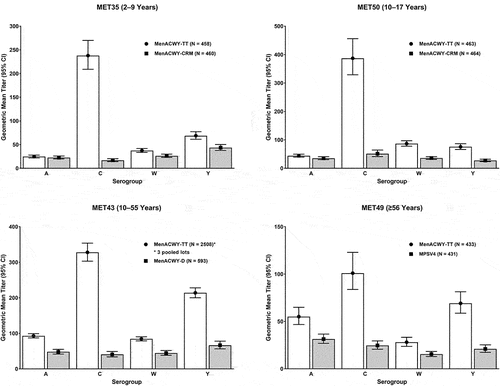
Figure 4. Immunogenicity of booster doses of MenACWY-TT among participants ≥15 years of age in Study MET56: seroprotection rates (top), seroresponse rates (middle), and GMTs (bottom) based on hSBA titers on post-vaccination Days 6 (left) and 30 (right) in the per-protocol population. White bars depict data for MenACWY-TT and gray bars depict data for MenACWY-D.
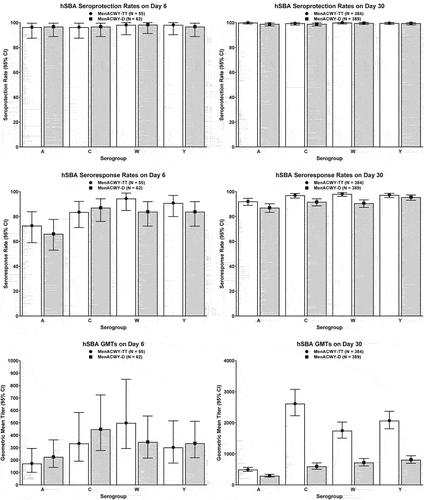
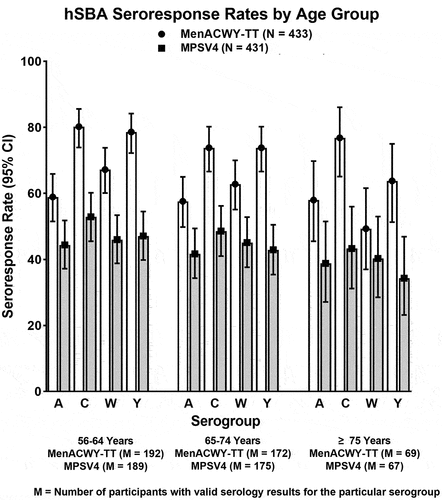
Figure 5. Immunogenicity of MenACWY-TT across age groups among adults ≥56 years of age in Study MET49: hSBA seroresponse rates on post-vaccination Day 30 in the per-protocol population. White bars depict data for MenACWY-TT and gray bars depict data for MPSV4.
Study MET35 (NCT03077438), a Phase III randomized study, compared the immunogenicity of a single dose of MenACWY-TT versus that of MenACWY-CRM in meningococcal vaccine-naïve children 2–9 years of age.Citation71 On post-vaccination Day 30, seroprotection rates for MenACWY-TT were numerically higher than in the MenACWY-CRM group for all four serogroups (Supplementary Table S1 and ).
One Phase II and 2 Phase III studies evaluated the immunogenicity of primary or booster doses of MenACWY-TT in adolescents and adults.Citation72–74 In meningococcal vaccine-naïve adolescents 10–17 years of age, study MET50 (NCT02199691) compared the immunogenicity of a single dose of MenACWY-TT with that of MenACWY-CRM and additionally assessed the immunogenicity of MenACWY-TT when given concomitantly with tetanus, reduced diphtheria, and acellular pertussis vaccine (Tdap) and quadrivalent human papillomavirus vaccine (HPV4).Citation72 Seroprotection rates for all serogroups were numerically higher for MenACWY-TT than for MenACWY-CRM (Supplementary Table S1 and ). Except for the findings noted below, concomitant administration of MenACWY-TT, Tdap, and HPV4 did not adversely affect the immunogenicity of the vaccines. Non-inferiority, as assessed by the geometric mean concentrations (GMCs) of antibodies against pertussis antigens, was met for only one antigen (pertussis toxoid [PT]). Non-inferiority was not met for fimbriae types 2 and 3 (FIM) and marginally missed for both filamentous hemagglutinin (FHA) and pertactin (PRN). Of note, lower anti-pertussis antibodies have been observed following concomitant administration of Tdap and other MenACWY; however, the clinical relevance of these findings, if any, is unknown.Citation86,Citation87
Study MET43 (NCT02842853) evaluated the immunogenicity of three lots of MenACWY-TT versus MenACWY-D in meningococcal vaccine-naïve adolescents and adults 10–55 years of age, demonstrating lot-to-lot consistency and noninferior immunogenicity (pooled lot data)Citation73(Supplementary Table S1). The proportion of MenACWY-TT recipients achieving seroprotection was numerically higher for each serogroup than those receiving MenACWY-D (Supplementary Table S1 and ).
Finally, study MET56 (NCT02752906) compared the immunogenicity of a booster dose of MenACWY-TT with that of MenACWY-D in participants ≥15 years of age who had received a single dose of MenACWY-D or MenACWY-CRM 4–10 years earlier.Citation74 Seroprotection rates were high for both MenACWY-TT and MenACWY-D, ranging between 99.5% and 100% across all 4 serogroups on Day 30 (Supplementary Table S1 and ). The kinetics of the booster response were also evaluated, revealing MenACWY-TT and MenACWY-D each elicited robust immune responses as early as 6 days after booster vaccination, as assessed by seroprotection rates, seroresponse rates, and hSBA GMTs (Supplementary Table S1 and ).
One Phase II study and 1 Phase III study assessed the immunogenicity of a single dose of MenACWY-TT versus MPSV4 in meningococcal vaccine-naïve adults ≥56 years of age.Citation75,Citation76 The Phase II study MET44 (NCT01732627) compared the immunogenicity of a single dose of MenACWY-TT with that of MPSV4,Citation75 demonstrating robust immunogenicity against all four serogroups. These findings were corroborated by the pivotal Phase III study MET49 (NCT02842866),Citation76 which documented numerically higher seroprotection rates in participants receiving a single dose of MenACWY-TT compared with those receiving MPSV4 for all four serogroups, with non-overlapping 95% CIs for serogroups C, W, and Y (Supplementary Table S1 and ). Non-inferiority of the immune responses elicited by MenACWY-TT, as measured by seroresponse rates on Day 30, was also apparent when the analyses were done by age group of the participants (). This study also documented hSBA GMTs that were similar across age subgroups for all four meningococcal serogroups ().Citation76
Safety
Regarding the safety monitoring protocol, participants were monitored for immediate reactions for 30 minutes following vaccination while at the study site. Solicited injection site and systemic reactions were recorded by participants or by parents/guardians in a diary card at home daily for 7 days following vaccination. All unsolicited adverse events that occurred within 30 days following vaccination were recorded by participants or by parents/guardians and collected by the study site at the next visit. Unsolicited adverse events that were medically attended (i.e., visits to an emergency room or an unexpected visit to a health care provider), and all serious adverse events (SAEs) were collected for at least 6 months after vaccination (except for MET44 study, wherein the SAEs were collected through 30 days).
As shown in , MenACWY-TT was well tolerated in all age groups studied, with its reactogenicity being comparable to that of licensed meningococcal vaccines (the sole exception being higher rates of local reactions seen with MenACWY-TT versus MPSV4, which may be a function of differences in reactogenicity between toxoid conjugated versus nonconjugated vaccinesCitation88,Citation89 and routes of administration, with MPSV4 being administered subcutaneously instead of intramuscularly). No safety concerns were apparent whether MenACWY-TT was administered alone or concomitantly with routine licensed vaccines (Tdap or HPV4).
Table 4. Summary of safety findings of phase II and phase III studies of MenACWY-TT, safety analysis population.
Discussion
The data summarized above attest to the immunogenicity of MenACWY-TT in individuals ≥2 years of age, including adults ≥56 years of age. Importantly, immunological non-inferiority of MenACWY-TT in terms of seroresponse rates was demonstrated for all serogroups and against all meningococcal vaccine comparators (MenACWY-CRM, MenACWY-D, and MPSV4). The included studies enrolled 8,076 individuals ≥2 years of age, including 1,207 participants ≥56 years of age and provide compelling evidence that MenACWY-TT is well tolerated and safe. Moreover, co-administration with routinely recommended adolescent vaccines does not appear to materially impact the immunogenicity of either MenACWY-TT or the concomitantly administered vaccines.Citation72 Finally, MenACWY-TT elicits robust booster responses. These findings support the potential for MenACWY-TT to overcome some of the deficiencies of alternative formulations and to be incorporated into NIPs worldwide.
Of note, across studies and age groups (≥2 years of age), MenACWY-TT elicited more robust immune responses versus all comparator formulations against serogroup C,Citation71–76 which accounts for approximately one-third of all IMD cases in the United States.Citation12 Although the clinical significance of higher immune responses is not well understood, these robust serogroup-specific responses are potentially relevant to protection. Recent serogroup C disease outbreaks among men who have sex with men (MSM), and the increased incidence of meningococcal urethritis being reported in the United States,Citation90,Citation91 suggest that MenACWY-TT may be useful in this population. Whether higher antibody levels against serogroup C ultimately translates into improved protection against IMD caused by this serogroup represents an area for further investigation. In addition, given the continued prevalence of serogroup C in locales outside the United States,Citation92 MenACWY-TT may serve as a viable option for travel vaccination.
In the United States, MenACWY-TT offers the opportunity to build upon the successes of MenACWY-D and MenACWY-CRM. Immunogenicity of meningococcal conjugate vaccines is modulated by the carrier protein used: D conjugates seem to be less immunogenic in general, while some TT conjugates may dampen polysaccharide responses, and CRM conjugates may cause bystander interference.Citation93 MenACWY-TT has a novel formulation that involved a serogroup-specific conjugation strategy to link capsular polysaccharides to the carrier protein.Citation68 This strategy may contribute to its robust immunogenicity.
The demonstrated compatibility of MenACWY-TT with routine vaccinesCitation72,Citation94,Citation95 argues well for its potential to be included in national vaccination schedules. Moreover, MenACWY-TT is available as a single-vial liquid formulation,Citation44 facilitating avoidance of reconstitution errors, which can help save time and keep costs down.Citation96–99 Elimination of the need for reconstitution would also help ensure protection against all four meningococcal serogroups targeted by the vaccine.Citation65
MenACWY-TT induces rapid booster responses in adolescents and adults irrespective of the type of priming vaccine (MenACWY-D or MenACWY-CRM).Citation74 Seroprotection rates of >96% in MenACWY-primed recipients by post-vaccination Day 6 suggests the potential value of the vaccine for control of outbreaks in closed communities such as scout camps and educational institutions,Citation100–102 where early protection is crucial to prevent additional cases of IMD. Immunization of vaccine-naïve or primed adolescents with MenACWY-TT may be highly beneficial in disease control given their role in transmission to infants and the elderly, who are more vulnerable.
Although no meningococcal conjugate vaccine was labeled for immunization of adults ≥56 years of age in the US until the licensure of MenACWY-TT,Citation41 the higher incidence and CFRs documented with IMD in older adults in the United StatesCitation12,Citation27supports the case for immunization of persons in this age group who are at increased risk for IMD (e.g., due to complement deficiencies, anatomic, or functional asplenia, HIV positivity, or enhanced exposure resulting from travel to areas where IMD is endemic or work as microbiologists). Although immunization of persons ≥56 years of age at increased risk for IMD is recommended by the ACIP, MenACWY-TT is currently the only quadrivalent conjugate vaccine in the US licensed for use in this age group and as such represents the only quadrivalent conjugate that can be used on-label in such high-risk populations.Citation41,Citation103 Two studies conducted in the United States (MET49 [NCT02842866] and MET44 [NCT01732627])Citation75,Citation76 have documented the immunogenicity and safety of MenACWY-TT in adults ≥56 years of age compared with MPSV4, which was licensed and available at the time of conduct of the studies. Immunogenicity and safety were analyzed by age group in both studies, with immunogenicity and safety being demonstrated even in adults ≥75 years of age. Of note, older adults are known to visit countries where serogroup W and/or Y has increasingly been implicated in IMDCitation17–19or participate in the annual Hajj or Umrah pilgrimage where outbreaks caused by serogroup W have been reported.Citation20,Citation21 This group now has the option for on-label use of MenACWY-TT, enabling them to travel with greater confidence, taking into account the potential advantages of conjugate vaccines over previously available MPSV such as preventing acquisition of virulent meningococci and transmission to vulnerable individuals. Vaccination of such travelers assumes added importance given the sheer number of pilgrims participating in these events (more than 4 million globally annually, with projections of significant increases in the future) and the requirement by the Saudi Arabian government that all Hajj or Umrah pilgrims be immunized.Citation21
The studies reviewed here also underscore the safety of MenACWY-TT across age groups when administered alone or concomitantly with routinely recommended adolescent vaccines. The safety of MenACWY-TT, viewed in conjunction with the previously documented safety of MenACWY-CRMCitation45and MenACWY-D,Citation46 suggests that MenACWY as a class is safe and well tolerated. Although concerns about the risk for GBS were raised shortly after MenACWY-D became available for use, several studies have refuted a heightened risk for GBS.Citation57–61 Evaluations of data from the Vaccine Safety Datalink (VSD) through 2014 and from the Vaccine Adverse Event Reporting System (VAERS) through 2016 have revealed no cause for concern regarding GBS in MenACWY-D recipients.Citation41 Similarly, a comprehensive review of VAERS data collected between 2010 and 2015 revealed no safety concerns with MenACWY-CRM, including no heightened risk of GBS or Bell’s palsy following administration.Citation45
A unique strength of the MenACWY-TT clinical development program is the substantial number of participants enrolled (8,076 individuals ≥2 years of age, of whom 1,207 participants were ≥56 years of age). Another strength is that immunogenicity has been assessed using both SBA types accepted by regulatory authorities in different regions of the world (hSBA and rSBA, the latter of which has been performed in a subset of participants in all studies).Citation104,Citation105 Immunogenicity has been assessed using multiple parameters (seroresponse, seroprotection, and GMTs) to satisfy regulatory requirements, with the primary objective being demonstration of non-inferiority as measured by seroresponse. The immunogenicity data are comparable across studies since they were all generated in the same laboratories for all studies, with all hSBAs being performed by the Sanofi Global Clinical Immunology laboratory in Swiftwater, Pennsylvania, and all rSBAs being performed at the UK Health Security Agency (formerly Public Health England) laboratory in Manchester.
Ongoing efforts to evaluate MenACWY-TT are worth mentioning. For example, although data on immunogenicity and safety of the vaccine in toddlers 12–23 months of age are available,Citation77,Citation79,Citation80 corresponding data in infants <12 months of age are currently restricted to a recently published Phase II study (NCT01049035; MET39) conducted by Cornish et al.Citation106 In this study, MenACWY-TT was evaluated in US infants and toddlers (from 6 weeks to 12 months of age). In this age group, MenACWY-TT induced robust immune responses and was well tolerated. Moreover, immunogenicity profiles of routine pediatric vaccines concomitantly administered with MenACWY-TT were similar whether administered with MenACWY-TT or without it. To further assess the performance of MenACWY-TT in young children, seven studies (Phase II or III) in infants as young as 6 weeks of age are currently ongoing and will shed more light not only on immunogenicity and safety but also help identify optimal vaccination schedules.Citation107–113 The findings of these studies are of particular interest since infants are among the age groups at increased risk of IMD. In addition, data on immunogenicity and safety in populations with high-risk conditions are not available. Finally, data on the persistence of immunity following vaccination with MenACWY-TT are limited. However, preliminary data from two Phase III studies evaluating antibody persistence following primary vaccination with MenACWY-TT, MenACWY-CRM, or MPSV4 are encouraging.Citation114,Citation115 Study MET59 (NCT04084769)Citation116 revealed antibody persistence for 3–6 years in adolescents and adults primed with MenACWY-TT.Citation114 Study MEQ00066 (NCT04142242)Citation117 documented antibody persistence for up to 7 years following primary vaccination with MenACWY-TT or MPSV4, with persistence being higher for serogroups C, W, and Y in patients ≥56 years of age receiving MenACWY-TT.Citation115 However, it is unclear whether the level of antibodies that were observed for up to 7 years after primary vaccination translates into a longer duration of protection.
Conclusions
The comprehensive body of evidence on the immunogenicity and safety of MenACWY-TT summarized in this paper supports its use for the prevention of IMD in individuals ≥2 years of age in the United States, with accruing data on its immunogenicity and safety in infants as young as 6 weeks of age potentially enhancing its utility across the entire age spectrum. Compatibility of the vaccine with routinely recommended vaccines suggests that it can be seamlessly incorporated into NIPs. The use of tetanus toxoid protein as a carrier in MenACWY-TT did not adversely affect its reactogenicity or immunogenicity profile. The vaccine induced robust and rapid booster responses against all four meningococcal serogroups. The summarized data collectively demonstrate that MenACWY-TT is a safe and effective quadrivalent meningococcal conjugate vaccine. It offers convenience as it does not require reconstitution and has the potential to effectively address the burden of IMD globally.
Author’s contributions
GSM, SIP, CAR, and PO contributed to the literature search strategy, drafting, and review of the manuscript, and approval of the final version for submission.
Supplemental Material
Download MS Word (583.1 KB)Acknowledgements
The authors thank Jean-Sébastien Persico, MAS, and Sonal Gawande, MD, of Sanofi, Lyon, France, and Anirban Sanyal, PhD, Alok Vyas, PhD, and Priya Upadhyay of Sanofi, Hyderabad, India, for managing the development of the manuscript. Assistance with the preparation of the manuscript was provided by Prasad S. Kulkarni, PhD, CMPP of Asclepius Medical Communications LLC, Ridgewood. New Jersey, USA, and was funded by Sanofi, Lyon, France.
Disclosure statement
CAR and PO are employees of, and hold stock options in, Sanofi. GSM has been an investigator on clinical trials funded by GlaxoSmithKline, Merck, Novartis, Pfizer, Sanofi, and Seqirus, and has received honoraria from these companies for service on advisory boards. SIP has received honoraria from Sanofi for participation in advisory boards on meningococcal conjugate vaccines, influenza vaccines, and COVID-19 vaccines; grants, consulting fees, and honoraria from Pfizer, Inc. for participation in a Data and Safety Monitoring Board (DSMB) for pneumococcal vaccine studies; and honoraria from Seqirus for participation on advisory committees on influenza and COVID-19 vaccines.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2099142.
Additional information
Funding
References
- Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine. 2012;30(Suppl 2):1–17. doi:10.1016/j.vaccine.2011.12.062.
- Rouphael NG, Stephens DS. Neisseria meningitidis: biology, microbiology, and epidemiology. Methods Mol Biol. 2012;799:1–20.
- Mbaeyi S, Duffy J, and McNamara LA. Meningococcal disease. In: Hamborsky J, Kroger A, Wolfe S, editors. Epidemiology and prevention of vaccine-preventable diseases. Washington DC: Public Health Foundation. 207–224; 2015.
- Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009;27 Suppl 2:B71–7. doi:10.1016/j.vaccine.2009.04.070.
- Dos Santos Souza I, Ziveri J, Bouzinba-Segard H, Morand P, Bourdoulous S. Meningococcus, this famous unknown. C R Biol. 2021;344:127–143.
- Gallacher SD, Seaton A. Meningococcal meningitis and COVID-19 co-infection. BMJ Case Rep. 2020;13(8):e237366. doi:10.1136/bcr-2020-237366.
- Jacobs JH, Viboud C, Tchetgen ET, Schwartz J, Steiner C, Simonsen L, Lipsitch M. The association of meningococcal disease with influenza in the United States, 1989–2009. PLoS One. 2014;9:e107486. doi:10.1371/journal.pone.0107486.
- Siddiqui JA, Ameer MA, Gulick PG. Meningococcemia. StatPearls. Treasure Island (FL): StatPearls Publishing LLC.; 2021.
- World Health Organization (WHO). Meningococcal meningitis - Fact sheet. Geneva, Switzerland: World Health Organization (WHO); 2018.
- Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. 2016;59(2):S3–S11. doi:10.1016/j.jadohealth.2016.04.012.
- Borrow R, Alarcon P, Carlos J, Caugant DA, Christensen H, Debbag R, De Wals P, Echániz-Aviles G, Findlow J, et al. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert Rev Vaccines. 2017;16:313–328. doi:10.1080/14760584.2017.1258308.
- United States Centers for Disease Control and Prevention. Enhanced Meningococcal Disease Surveillance Report, 2018. Atlanta, Georgia: Centers for Disease Control and Prevention, 2018.
- MacNeil JR, Blain AE, Wang X, Cohn AC. Current epidemiology and trends in meningococcal disease-United States, 1996-2015. Clin Infect Dis. 2018;66:1276–1281. doi:10.1093/cid/cix993.
- Peterson ME, Li Y, Bita A, Moureau A, Nair H, Kyaw MH, Abad R, Bailey F, Garcia IDLF, Decheva A, et al. Meningococcal serogroups and surveillance: a systematic review and survey. J Glob Health. 2019;9:010409. doi:10.7189/jogh.09.010409.
- Xie O, Pollard AJ, Mueller JE, Norheim G. Emergence of serogroup X meningococcal disease in Africa: need for a vaccine. Vaccine. 2013;31:2852–2861. doi:10.1016/j.vaccine.2013.04.036.
- European Center for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases - Invasive Meningococcal Disease. Solna, Sweden: European Center for Disease Prevention and Control; 2017.
- Public Health England. Meningococcal group W (MenW) immunisation advised for 14 to 18 year-olds. London, UK: Public Health England; 2015.
- Knol MJ, Ruijs WL, Antonise-Kamp L, de Melker HE, van der Ende A. Implementation of MenACWY vaccination because of ongoing increase in serogroup W invasive meningococcal disease, the Netherlands, 2018. Euro Surveill. 2018;23. doi:10.2807/1560-7917.ES.2018.23.16.18-00158.
- Booy R, Gentile A, Nissen M, Whelan J, Abitbol V. Recent changes in the epidemiology of Neisseria meningitidis serogroup W across the world, current vaccination policy choices and possible future strategies. Hum Vaccin Immunother. 2019;15:470–480. doi:10.1080/21645515.2018.1532248.
- Taha MK, Achtman M, Alonso JM, Greenwood B, Ramsay M, Fox A, Gray S, Kaczmarski E. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet. 2000;356:2159. doi:10.1016/S0140-6736(00)03502-9.
- Yezli S. The threat of meningococcal disease during the Hajj and Umrah mass gatherings: a comprehensive review. Travel Med Infect Dis. 2018;24:51–58. doi:10.1016/j.tmaid.2018.05.003.
- Guiddir T, Gros M, Hong E, Terrade A, Denizon M, Deghmane AE, Taha M-K. Unusual initial abdominal presentations of invasive meningococcal disease. Clin Infect Dis. 2018;67:1220–1227. doi:10.1093/cid/ciy257.
- Houweling BM, van Meurs SJ, Versluis J, Grewal S, Verkaik NJ, van den Akker JPC. Massive diarrhoea and sepsis due to an infection with Neisseria meningitidis serogroup W. Neth J Med. 2019;77:116–118.
- Aung M, Raith E, Williams E, Burrell AJ. Severe meningococcal serogroup W sepsis presenting as myocarditis: a case report and review of literature. J Intensive Care Soc. 2019;20:182–186. doi:10.1177/1751143718794127.
- Stinson C, Burman C, Presa J, Abalos M. Atypical presentation of invasive meningococcal disease caused by serogroup W meningococci. Epidemiol Infect. 2020;148:e12. doi:10.1017/S0950268819002152.
- Loenenbach AD, van der Ende A, de Melker HE, Sanders EAM, Knol MJ. The clinical picture and severity of invasive meningococcal disease serogroup W compared with other serogroups in the Netherlands, 2015-2018. Clin Infect Dis. 2020;70:2036–2044. doi:10.1093/cid/ciz578.
- Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37:2768–2782. doi:10.1016/j.vaccine.2019.04.020.
- Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold K, Baumbach J, Bennett N, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998–2007: Implications for prevention of Meningococcal Disease. Clin Infect Dis. 2010;50:184–191. doi:10.1086/649209.
- Macneil JR, Cohn AC, Zell ER, Schmink S, Miller E, Clark T, Messonnier NE. Early estimate of the effectiveness of quadrivalent meningococcal conjugate vaccine. Pediatr Infect Dis J. 2011;30:451–455. doi:10.1097/INF.0b013e31820a8b3c.
- Mbaeyi S, Pondo T, Blain A, Yankey D, Potts C, Cohn A, Hariri S, Shang N, MacNeil JR. Incidence of meningococcal disease before and after implementation of quadrivalent meningococcal conjugate vaccine in the United States. JAMA Pediatr. 2020;174:843–851. doi:10.1001/jamapediatrics.2020.1990.
- Harcourt BH, Anderson RD, Wu HM, Cohn AC, MacNeil JR, Taylor TH, Wang X, Clark TA, Messonnier NE, Mayer LW, et al. Population-based surveillance of Neisseria meningitidis antimicrobial resistance in the United States. Open Forum Infect Dis. 2015;2:ofv117. doi:10.1093/ofid/ofv117.
- McNamara LA, Potts C, Blain AE, Retchless AC, Reese N, Swint S, Lonsway D, Karlsson M, Lunquest K, Sweitzer JJ et al. Detection of ciprofloxacin-resistant, β-lactamase–producing Neisseria meningitidis serogroup Y isolates — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2020;69(24):735–739. doi:10.15585/mmwr.mm6924a2.
- Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J. 2007;26:716–722. doi:10.1097/INF.0b013e3180cc2c25.
- Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines. 2010;9:285–298. doi:10.1586/erv.10.3.
- Clark SA, Borrow R. Herd protection against Meningococcal disease through vaccination. Microorganisms. 2020;8:1675. doi:10.3390/microorganisms8111675.
- Dretler AW, Rouphael NG, Stephens DS. Progress toward the global control of Neisseria meningitidis: 21st century vaccines, current guidelines, and challenges for future vaccine development. Hum Vaccin Immunother. 2018;14:1146–1160. doi:10.1080/21645515.2018.1451810.
- Christodoulides M, Heckels J. Novel approaches to Neisseria meningitidis vaccine design. Pathog Dis. 2017;75. doi:10.1093/femspd/ftx033.
- Huang L, Mauskopf J, Farkouh R, Masaquel C. Use of cost-effectiveness analyses for decisions about vaccination programs for Meningococcal disease in the United States, United Kingdom, the Netherlands, and Canada. Expert review of vaccines 2021.
- Tin Tin Htar M, Jackson S, Balmer P, Serra LC, Vyse A, Slack M, Riera-Montes M, Swerdlow DL, Findlow J. Systematic literature review of the impact and effectiveness of monovalent meningococcal C conjugated vaccines when used in routine immunization programs. BMC Public Health. 2020;20:1890. doi:10.1186/s12889-020-09946-1.
- Presa J, Findlow J, Vojicic J, Williams S, Serra L. Epidemiologic trends, global shifts in meningococcal vaccination guidelines, and data supporting the use of MenACWY-TT vaccine: a review. Infect Dis Ther. 2019;8:307–333. doi:10.1007/s40121-019-0254-1.
- Mbaeyi SA, Bozio CH, Duffy J, Rubin LG, Hariri S, Stephens DS, MacNeil JR. Meningococcal Vaccination: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1–41. doi:10.15585/mmwr.rr6909a1.
- Sanofi Pasteur. Menactra®, Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine Solution for Intramuscular Injection. Swiftwater, PA: Sanofi Pasteur Inc; 2016.
- GSK Vaccines Srl. MENVEO [Meningococcal (Groups A, C, Y, and W-135) Oligosaccharide Diphtheria CRM197 Conjugate Vaccine]. Bellaria-Rosia, Sovicille, Italy: GSK Vaccines, Srl; 2020.
- Sanofi Pasteur I. MenQuadfi™, Meningococcal (Groups A, C, Y, W) Conjugate Vaccine Solution for Intramuscular Injection. Swiftwater, PA: Sanofi Pasteur, Inc; 2020.
- Myers TR, McNeil MM, Ng CS, Li R, Lewis PW, Cano MV. Adverse events following quadrivalent meningococcal CRM-conjugate vaccine (Menveo®) reported to the Vaccine Adverse Event Reporting system (VAERS), 2010-2015. Vaccine. 2017;35:1758–1763. doi:10.1016/j.vaccine.2017.02.030.
- Myers TR, McNeil MM, Ng CS, Li R, Marquez PL, Moro PL, Omer SB, Cano MV. Adverse events following quadrivalent meningococcal diphtheria toxoid conjugate vaccine (Menactra®) reported to the Vaccine Adverse Event Reporting System (VAERS), 2005–2016. Vaccine. 2020;38:6291–6298. doi:10.1016/j.vaccine.2020.07.039.
- Cohn AC, MacNeil JR, Harrison LH, Lynfield R, Reingold A, Schaffner W, Zell ER, Plikaytis B, Wang X, Messonnier NE, et al. Effectiveness and duration of protection of one dose of a meningococcal conjugate vaccine. Pediatrics. 2017;139:139. doi:10.1542/peds.2016-2193.
- Rennels M, King J Jr., Ryall R, Papa T, Froeschle J. Dosage escalation, safety and immunogenicity study of four dosages of a tetravalent meninogococcal polysaccharide diphtheria toxoid conjugate vaccine in infants. Pediatr Infect Dis J. 2004;23:429–435. doi:10.1097/01.inf.0000126297.28952.f8.
- United States National Library of Medicine. Exploratory Trial to Evaluate the Safety and Immunogenicity of Menactra® and Menomune® Vaccines in Subjects ≥ 56 Years (NCT00874549). Bethesda, MD: National Institutes of Health; 2009.
- Stamboulian D, Lopardo G, Lopez P, Cortes-Barbosa C, Valencia A, Bedell L, Karsten A, Dull PM. Safety and immunogenicity of an investigational quadrivalent meningococcal CRM(197) conjugate vaccine, MenACWY-CRM, compared with licensed vaccines in adults in Latin America. Int J Infect Dis. 2010;14:e868–75. doi:10.1016/j.ijid.2010.03.017.
- Ramasamy MN, Clutterbuck EA, Haworth K, Bowman J, Omar O, Thompson AJ, Blanchard-Rohner G, Yu L-M, Snape MD, Pollard AJ, et al. Randomized clinical trial to evaluate the immunogenicity of quadrivalent meningococcal conjugate and polysaccharide vaccines in adults in the United kingdom. Clin Vaccine Immunol. 2014;21:1164–1168. doi:10.1128/CVI.00099-14.
- Lalwani S, Agarkhedkar S, Gogtay N, Palkar S, Agarkhedkar S, Thatte U, Vakil H, Jonnalagedda R, Pedotti P, Hoyle M, et al. Safety and immunogenicity of an investigational meningococcal ACWY conjugate vaccine (MenACWY-CRM) in healthy Indian subjects aged 2 to 75 years. Int J Infect Dis. 2015;38:36–42. doi:10.1016/j.ijid.2015.07.003.
- Pina LM, Bassily E, Machmer A, Hou V, Reinhardt A. Safety and immunogenicity of a quadrivalent meningococcal polysaccharide diphtheria toxoid conjugate vaccine in infants and toddlers: three multicenter phase III studies. Pediatr Infect Dis J. 2012;31:1173–1183. doi:10.1097/INF.0b013e318268dfe4.
- Klein NP, Reisinger KS, Johnston W, Odrljin T, Gill CJ, Bedell L, Dull P. Safety and immunogenicity of a novel quadrivalent meningococcal CRM-conjugate vaccine given concomitantly with routine vaccinations in infants. Pediatr Infect Dis J. 2012;31:64–71. doi:10.1097/INF.0b013e31823dce5c.
- Gasparini R, Tregnaghi M, Keshavan P, Ypma E, Han L, Smolenov I. Safety and Immunogenicity of a quadrivalent meningococcal conjugate vaccine and commonly administered vaccines after coadministration. Pediatr Infect Dis J. 2016;35:81–93. doi:10.1097/INF.0000000000000930.
- Centers for Disease Control and Prevention. Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine–United States, June-July 2005. MMWR Morb Mortal Wkly Rep 2005;54:1023–1025.
- Cho BH, Clark TA, Messonnier NE, Ortega-Sanchez IR, Weintraub E, Messonnier ML. MCV vaccination in the presence of vaccine-associated Guillain-Barré Syndrome risk: a decision analysis approach. Vaccine. 2010;28:817–822. doi:10.1016/j.vaccine.2009.10.050.
- Velentgas P, Amato AA, Bohn RL, Chan KA, Cochrane T, Funch DP, Dashevsky I, Duddy AL, Gladowski P, Greenberg SA, et al. Risk of Guillain-Barré syndrome after meningococcal conjugate vaccination. Pharmacoepidemiol Drug Saf. 2012;21:1350–1358. doi:10.1002/pds.3321.
- Yih WK, Weintraub E, Kulldorff M. No risk of Guillain-Barré syndrome found after meningococcal conjugate vaccination in two large cohort studies. Pharmacoepidemiol Drug Saf. 2012;21:1359–1360. doi:10.1002/pds.3353.
- Baxter R, Lewis N, Bakshi N, Vellozzi C, Klein NP. Recurrent Guillain-Barre syndrome following vaccination. Clin Infect Dis. 2012;54:800–804. doi:10.1093/cid/cir960.
- Baxter R, Bakshi N, Fireman B, Lewis E, Ray P, Vellozzi C, Klein NP. Lack of association of Guillain-Barré syndrome with vaccinations. Clin Infect Dis. 2013;57:197–204. doi:10.1093/cid/cit222.
- Tseng HF, Sy LS, Ackerson BK, Hechter RC, Tartof SY, Haag M, Slezak JM, Luo Y, Fischetti CA, Takhar HS, et al. Safety of quadrivalent meningococcal conjugate vaccine in 11- to 21-year-olds. Pediatrics. 2017; 139. doi:10.1542/peds.2016-2084
- Hansen J, Zhang L, Klein NP, Robertson CA, Decker MD, Greenberg DP, Bassily E, Baxter R. Post-Licensure safety surveillance study of routine use of quadrivalent meningococcal diphtheria toxoid conjugate vaccine. Vaccine. 2017;35:6879–6884. doi:10.1016/j.vaccine.2017.09.032.
- Hansen J, Zhang L, Eaton A, Baxter R, Robertson CA, Decker MD, Greenberg DP, Bassily E, Klein NP. Post-Licensure safety surveillance study of routine use of quadrivalent meningococcal diphtheria toxoid conjugate vaccine (MenACWY-D) in infants and children. Vaccine. 2018;36:2133–2138. doi:10.1016/j.vaccine.2018.02.107.
- Su JR, Miller ER, Duffy J, Baer BM, Cano MV. Notes from the Field: Administration Error Involving a Meningococcal Conjugate Vaccine–United States, March 1, 2010-September 22, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:161–162. doi:10.15585/mmwr.mm6506a4.
- Samad F, Burton SJ, Kwan D, Porter N, Smetzer J, Cohen MR, Tuttle J, Baker D, Doherty DE. Strategies to Reduce Errors Associated with 2-Component Vaccines. Pharmaceut Med. 2021;35:1–9. doi:10.1007/s40290-020-00362-9.
- Tartof SY, Sy LS, Ackerson BK, Hechter RC, Haag M, Slezak JM, Luo Y, Fischetti CA, Takhar HS, Miao Y, et al. Safety of quadrivalent meningococcal conjugate vaccine in children 2–10 years. Pediatr Infect Dis J. 2017;36:1087–1092. doi:10.1097/INF.0000000000001696.
- Kensinger R, Arunachalam AB. Preclinical development of the quadrivalent meningococcal (ACYW) tetanus toxoid conjugate vaccine, MenQuadfi®. Glycoconj J. 2022;39:381–392. doi:10.1007/s10719-022-10050-2.
- United States Food and Drug Administration (FDA). Meningococcal (Groups A, C, Y, W) Conjugate Vaccine - BLA Approval. Rockville, MD: United States Food and Drug Administration (FDA); 2020.
- United States Food and Drug Administration (FDA). Meningococcal (Groups A, C, Y, W) Conjugate Vaccine - BLA Clinical Review Memorandum. Rockville, MD: United States Food and Drug Administration (FDA); 2020.
- Baccarini CI, Simon MW, Brandon D, Christensen S, Jordanov E, Dhingra MS. Safety and immunogenicity of a quadrivalent meningococcal conjugate vaccine in healthy meningococcal-naïve children 2-9 years of age: a phase III, randomized study. Pediatr Infect Dis J. 2020;39:955–960. doi:10.1097/INF.0000000000002832.
- Chang L-J, Hedrick J, Christensen S, Pan J, Jordanov E, Dhingra MS. A Phase II, randomized, immunogenicity and safety study of a quadrivalent meningococcal conjugate vaccine, MenACYW-TT, in healthy adolescents in the United States. Vaccine. 2020;38:3560–3569. doi:10.1016/j.vaccine.2020.03.017.
- Dhingra MS, Peterson J, Hedrick J, Pan J, Neveu D, Jordanov E. Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study. Vaccine. 2020;38:5194–5201. doi:10.1016/j.vaccine.2020.06.013.
- Áñez G, Hedrick J, Simon MW, Christensen S, Jeanfreau R, Yau E, Pan J, Jordanov E, Dhingra MS. Immunogenicity and safety of a booster dose of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adolescents and adults: a Phase III randomized study. Hum Vaccin Immunother. 2020;16:1292–1298. doi:10.1080/21645515.2020.1733867.
- Kirstein J, Pina M, Pan J, Jordanov E, Dhingra MS. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in adults 56 years of age and older: a Phase II randomized study. Hum Vaccin Immunother. 2020;16:1299–1305. doi:10.1080/21645515.2020.1733868.
- Esteves-Jaramillo A, Koehler T, Jeanfreau R, Neveu D, Jordanov E, Singh Dhingra M. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in >/=56-year-olds: a Phase III randomized study. Vaccine. 2020;38:4405–4411. doi:10.1016/j.vaccine.2020.04.067.
- Dhingra MS, Namazova-Baranova L, Arredondo-Garcia JL, Kim KH, Limkittikul K, Jantarabenjakul W, et al. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) administered concomitantly with other paediatric vaccines in toddlers: a phase III randomized study. Epidemiol Infect. 2021;149:1–10.
- Piazza FM, Virta M, Paassilta M, Ukkonen B, Ahonen A, Esteves-Jaramillo A, Forsten A, Seppa I, Ding J, Neveu D, et al. Immunogenicity and safety of an investigational quadrivalent meningococcal conjugate vaccine administered as a booster dose in children vaccinated against meningococcal disease 3 years earlier as toddlers: a Phase III, open-label, multi-center study. Human Vaccines Immunother. 2022;18:1–10. doi:10.1080/21645515.2021.1902701.
- van der Vliet D, Vesikari T, Sandner B, Martinón-Torres F, Muzsay G, Forsten A, Adelt T, Diaz Gonzalez C, Simko R, B’Chir S, et al. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) vs. A licensed quadrivalent meningococcal tetanus toxoid-conjugate vaccine in meningococcal vaccine-naïve and meningococcal C conjugate vaccine-primed toddlers: a phase III randomised study. Epidemiol Infect. 2021;149:e50. doi:10.1017/S0950268821000261.
- Vesikari T, Borrow R, Forsten A, Findlow H, Dhingra MS, Jordanov E. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (MenACYW-TT) in healthy toddlers: a Phase II randomized study. Hum Vaccin Immunother. 2020;16:1306–1312. doi:10.1080/21645515.2020.1733869.
- Martinón-Torres F, Bertrand-Gerentes I, Oster P. A novel vaccine to prevent meningococcal disease beyond the first year of life: an early review of MenACYW-TT. Expert Rev Vaccines. 2021;20:1123–1146. doi:10.1080/14760584.2021.1964962.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi:10.1084/jem.129.6.1307.
- Findlow J, Balmer P, Borrow R. A review of complement sources used in serum bactericidal assays for evaluating immune responses to meningococcal ACWY conjugate vaccines. Hum Vaccin Immunother. 2019;15:2491–2500. doi:10.1080/21645515.2019.1593082.
- United States Food and Drug Administration (FDA). Vaccines and Related Biological Products Advisory Committee: FDA Briefing Document - Use of Serum Bactericidal Antibody as an Immunological Correlate for Demonstrating Effectiveness of Meningococcal Conjugate Vaccines (Serogroups A, C, Y, W-135) Administered to Children Less Than 2 Years of Age. Rockville, MD: United States Food and Drug Administration (FDA); 2011.
- United States Food and Drug Administration (FDA). MenQuadfi™ - Summary Basis for Regulatory Action, April 22, 2020. Rockville, MD: United States Food and Drug Administration (FDA); 2020.
- Rivera L, Schwarz TF, Kim KH, Kim YK, Behre U, Cha SH, Jo DS, Lee J, Lee J-S, Cheuvart B, et al. Immunogenicity and safety of the quadrivalent meningococcal vaccine MenACWY-TT co-administered with a combined diphtheria-tetanus-acellular pertussis vaccine versus their separate administration in adolescents and young adults: a phase III, randomized study. Vaccine. 2018;36:4750–4758. doi:10.1016/j.vaccine.2018.04.034.
- Arguedas A, Soley C, Loaiza C, Rincon G, Guevara S, Perez A, Porras W, Alvarado O, Aguilar L, Abdelnour A, et al. Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine. 2010;28:3171–3179. doi:10.1016/j.vaccine.2010.02.045.
- Powers DC, Anderson EL, Lottenbach K, Mink CM. Reactogenicity and immunogenicity of a protein-conjugated pneumococcal oligosaccharide vaccine in older adults. J Infect Dis. 1996;173:1014–1018. doi:10.1093/infdis/173.4.1014.
- Juergens C, Trammel J, Shoji Y, Patterson S, Watson W, Webber C, Gruber WC, Scott DA, Schmoele-Thoma B. Late onset of injection site reactions after vaccination with the 13-valent pneumococcal conjugate vaccine in adult study populations. Hum Vaccin Immunother. 2018;14:1948–1956. doi:10.1080/21645515.2018.1452576.
- CDC. Meningococcal Disease in Florida 2022 - Serogroup C outbreak among men who have sex with men. Centers for Disease Control and Prevention (CDC), 2022.
- Oliver SE, Mbaeyi SA. A review of global epidemiology and response to meningococcal disease outbreaks among men who have sex with men, 2001–2018. Current Epidemiol Rep. 2018;5:321–330. doi:10.1007/s40471-018-0170-z.
- Parikh SR, Campbell H, Bettinger JA, Harrison LH, Marshall HS, Martinon-Torres F, Safadi MA, Shao Z, Zhu B, von Gottberg A, et al. The everchanging epidemiology of meningococcal disease worldwide and the potential for prevention through vaccination. J Infect. 2020;81:483–498. doi:10.1016/j.jinf.2020.05.079.
- Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: a review. Vaccine. 2010;28:5513–5523. doi:10.1016/j.vaccine.2010.06.026.
- Dhingra MS, Namazova-Baranova L, Arredondo-Garcia JL, Kim K-H, Limkittikul K, and Jantarabenjakul W, et al. Immunogenicity and Safety of a Quadrivalent Meningococcal Tetanus Toxoid-Conjugate Vaccine (MenACYW-TT) Administered Concomitantly with Other Paediatric Vaccines in Toddlers: a Phase III randomized study. Epidemiol Infect. 149. 2021;E90.
- Cornish M, Pina LM, Jezorwski J, DaCosta X, Rehm C, Dhingra MS Safety and immunogenicity of an investigational quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered to infants and toddlers (Abstract ID: 162). 20th International Pathogenic Neisseria Conference (IPNC). Manchester, United Kingdom, 2016:252.
- Wiedenmayer KA, Weiss S, Chattopadhyay C, Mukherjee A, Kundu R, Ayé R, Tediosi F, Hetzel MW, Tanner M. Simplifying paediatric immunization with a fully liquid DTP–HepB–Hib combination vaccine: Evidence from a comparative time-motion study in India. Vaccine. 2009;27:655–659. doi:10.1016/j.vaccine.2008.11.045.
- De Coster I, Fournie X, Faure C, Ziani E, Nicolas L, Soubeyrand B, Van Damme P. Assessment of preparation time with fully-liquid versus non-fully liquid paediatric hexavalent vaccines. A time and motion study. Vaccine. 2015;33:3976–3982. doi:10.1016/j.vaccine.2015.06.030.
- Orsi A, Azzari C, Bozzola E, Chiamenti G, Chirico G, Esposito S, Francia F, Lopalco P, Prato R, Russo R, et al. Hexavalent vaccines: characteristics of available products and practical considerations from a panel of Italian experts. J Prev Med Hyg. 2018;59:E107–e19.
- Mathijssen DAR, Heisen M, Clark-Wright JF, Wolfson LJ, Lu X, Carrol S, van Dijk BCP, Klijn SL, Alemayehu B. Budget impact analysis of introducing a non-reconstituted, hexavalent vaccine for pediatric immunization in the United Kingdom. Expert Rev Vaccines. 2020;19:1167–1175. doi:10.1080/14760584.2020.1873770.
- Lucidarme J, Scott KJ, Ure R, Smith A, Lindsay D, Stenmark B, Jacobsson S, Fredlund H, Cameron JC, Smith-Palmer A, et al. An international invasive meningococcal disease outbreak due to a novel and rapidly expanding serogroup W strain, Scotland and Sweden, July to August 2015. Euro Surveill. 2016;21. doi:10.2807/1560-7917.ES.2016.21.45.30395
- United States Centers for Disease Control and Prevention (CDC). Outbreak of meningococcal disease associated with an elementary school – Oklahoma, March 2010. MMWR Morb Mortal Wkly Rep 2012;61:217–221.
- Barret AS, Clinard F, Taha MK, Girard I, Hong E, Tessier S, Zurbaran M, de Bort C, Antona D, Deghmane AE, et al. Cluster of serogroup W invasive meningococcal disease in a university campus. Médecine Et Maladies Infectieuses. 2020;50:335–341. doi:10.1016/j.medmal.2019.10.003.
- United States Food and Drug Administration (FDA). MenQuadfi, Meningococcal (Groups A, C, Y, W) Conjugate Vaccine Solution for Intramuscular Injection - Prescribing Information. Rockville, MD: United States Food and Drug Administration (FDA); 2020.
- Keiser PB, Gill CJ. Defining efficacy in meningococcal vaccine trials. Clin Invest. 2012;2:589–601. doi:10.4155/cli.12.52.
- Jodar L, Cartwright K, Feavers IM. Standardisation and validation of serological assays for the evaluation of immune responses to Neisseria meningitidis serogroup a and C vaccines. Biologicals. 2000;28:193–197. doi:10.1006/biol.2000.0253.
- Cornish MJ, Hedrick JA, Gabrielsen AA, Johnson AD, Miriam Pina L, Rehm C, Pan J, Neveu D, Da Costa X, Jordanov E, et al. Safety and immunogenicity of an investigational quadrivalent meningococcal tetanus toxoid conjugate vaccine (MenACYW-TT) co-administered with routine pediatric vaccines in infants and toddlers: a Phase II study. Vaccine. 2022;40:1421–1438. doi:10.1016/j.vaccine.2022.01.050.
- United States National Library of Medicine. A Study of a Quadrivalent Meningococcal Tetanus Protein Conjugate Vaccine in Infants and Toddlers (MET39; NCT01049035). Bethesda, MD: National Institutes of Health; 2020.
- United States National Library of Medicine. Immunogenicity and Safety Study of a Quadrivalent Meningococcal Conjugate Vaccine When Co-administered with Routine Pediatric Vaccines in Healthy Infants and Toddlers in Europe (MET58; NCT03547271). Bethesda, MD: National Institutes of Health; 2020.
- United States National Library of Medicine. Safety of a Quadrivalent Meningococcal Conjugate Vaccine Administered Concomitantly with Routine Pediatric Vaccines in Healthy Infants and Toddlers (MET41; NCT03673462). Bethesda, MD: National Institutes of Health; 2020.
- United States National Library of Medicine. Immunogenicity and Safety of a Quadrivalent Meningococcal Conjugate Vaccine When Administered Concomitantly with Routine Pediatric Vaccines in Healthy Infants and Toddlers in the US (MET42; NCT03537508). Bethesda, MD: National Institutes of Health; 2021.
- United States National Library of Medicine. Immunogenicity and Safety Study of a Quadrivalent Meningococcal Conjugate Vaccine Administered Concomitantly with Routine Pediatric Vaccines in Healthy Infants and Toddlers (MET61; NCT03691610). Bethesda, MD: National Institutes of Health; 2021.
- United States National Library of Medicine. Immunogenicity and Safety of a Quadrivalent Meningococcal Conjugate Vaccine in Infants and Toddlers When Administered Concomitantly with Routine Pediatric Vaccines in the United Kingdom (MET52; NCT03632720). Bethesda, MD: National Institutes of Health; 2021.
- United States National Library of Medicine. Safety and Immunogenicity of a Quadrivalent Meningococcal Conjugate Vaccine When Administered Concomitantly with Routine Pediatric Vaccines in Healthy Infants and Toddlers in the Russian Federation and Mexico (MET33; NCT03630705). Bethesda, MD: National Institutes of Health; 2021.
- Peterson J, Deseda C, Julien K, Zambrano B, Áñez G, Jiayuan S, Pan J, Arroum H, Varghese K, Jordanov E, et al. 03. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster dose in adults and adolescents vaccinated against meningococcal disease 3 - 6 years earlier. Open Forum Infect Dis. 2021;8: S125. doi:10.1093/ofid/ofab466.206.
- Robertson CA, Jacqmein J, Selmani A, Galarza K, Oster P. 1046. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine (MenACYW-TT) administered as a booster to adults ≥ 59 years of age. Open Forum Infect Dis. 2021;8:S614–S5. doi:10.1093/ofid/ofab466.1240.
- United States National Library of Medicine. Study to Evaluate the Immune Response After a Booster Dose of a Quadrivalent Meningococcal (MenACYW) Conjugate Vaccine When Administered Alone or Concomitantly with a Licensed Meningococcal Serogroup B Vaccine, in Participants Who Received Primary Quadrivalent Meningococcal Conjugate Vaccine (MCV4) (MET59; NCT04084769). Bethesda, MD: National Institutes of Health; 2021.
- United States National Library of Medicine. Study to Assess the Safety and Immunogenicity of a Single Dose of a Quadrivalent Meningococcal (MenACYW) Conjugate Vaccine in Older Adults Who Received a Primary Vaccination (3 or More Years Earlier) in Study MET49 (MEQ00066; NCT04142242). Bethesda, MD: National Institutes of Health; 2019.
