ABSTRACT
Vaccines prevent infections in patients with multiple sclerosis (MS). Though recommendations regarding vaccinating patients with MS have been recently published, real-world data regarding vaccines’ planning in patients receiving disease-modifying drugs (DMDs) for MS are missing. Our aim was, therefore, to describe vaccination coverage rates, timing-proposal and safety in real-life vaccinating patients with MS undergoing DMDs before the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination campaign. Patients followed at our MS-center were referred to individualized immunization-programs customized to Italian recommendations, patients’ risks, immunity to exanthematic diseases, ongoing DMDs, or therapy-start urgency. Disease-activity stated the need for an essential immunization-cycle, whose core was composed by four vaccines: meningococcal-B, pneumococcal conjugated, Haemophilus influenzae B, and meningococcal-ACWY vaccines. Vaccines were administered prior to the planned DMD-start when possible, inactivated-vaccines >2 weeks and live-vaccines >4 weeks before treatment-start. Patients received a 6-months clinical-/radiological-follow-up after immunization. One-hundred and ninety-five patients were vaccinated between April 2017 and January 2021. 124/195 (63.6%) started a vaccination-program before therapy-start/-switch and 108/124 (87.1%) effectively completed immunization before new therapy-start without any delay. The time needed for immunization-conclusion reached a median of 27 (confidence interval 22) days in 2020. No increase in clinical-/radiological-activity 3-/6-months after immunization was noted. In conclusion, our study confirmed feasibility and safety of a vaccination-protocol in patients with MS whose duration resulted in a median of 27 days.
Introduction
Despite the fundamental role of vaccines in preventing serious infections in at-risk patientsCitation1, solid data regarding the achievement of a complete immunization in patients with multiple sclerosis (MS) are missingCitation2. A modified Delphi consensus conducted to generate recommendations regarding efficacy, safety, and timing of vaccines (live, inactivated, or recombinant) in MS patients both untreated and receiving disease modifying drugs (DMDs) or glucocorticoids was recently publishedCitation3. Nevertheless, real-life data regarding vaccinations’ planning in patients receiving DMDs for MS are missing, especially in terms of starting a new therapy as soon as possible in presence of disease-activity. Moreover, hesitancy regarding vaccinations still exists among patients with MS due to insufficient knowledge and misconceptions about vaccination and suboptimal vaccine promotion by health-care professionalsCitation4, and this might represent a challenging issue in the context of massive vaccination protocols as those linked to the recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak.
Therefore, we present the results of a real-world, single-center study aimed at describing immunization-program, timing-proposal, safety, and vaccination coverage rates (VCRs) in vaccinating patients with MS undergoing approved DMDs before the start of SARS-CoV-2 vaccination campaign.
Methods
Patients followed at our MS-center were referred to an immunization-program at the vaccination-center of our Hygiene Unit.
Individualized programs were customized according to Italian recommendationsCitation5 and considering patients’ risks, vaccination history, lack of immunity to measles, mumps, rubella (MMR), and varicella zoster virus (VZV), influenza immunization period, ongoing DMDs, or therapy-start urgency. In particular, clinical-relapses (a neurological symptom lasting ≥24 h) and magnetic resonance imaging (MRI) activity (≥1 new lesion, with or without gadolinium-enhancement) within 3-months before vaccination and a therapy-switch defined the need for an essential and shorter cycle. The essential immunization program’s core was composed by four vaccines starting with meningococcal-B vaccine, due to its 1-month lasting cycle, followed by pneumococcal conjugated (PVC-13), Haemophilus influenzae B (HiB) and meningococcal-ACWY vaccines. Other vaccinations, such as human papilloma virus (HPV), hepatitis B/A, MMR, VZV, and diphtheria-tetanus-pertussis (dTap) were considered individually in regard to age, serology, risk-factors, exposure, ongoing DMDs, and therapy-start immediacy.
With regard to the immunization-timing, vaccines were administered prior to the planned DMD-start when possible. In particular, inactivated-vaccines were administered >2 weeks and live-vaccines >4 weeks before treatment-startCitation6, despite product-information requirements that some drugs recommend longer intervals. In case of high-level of immunosuppression (e.g. anti-CD20 therapy), vaccinations other than influenza were started at normalization of CD19+ B-lymphocytes at immunophenotype, if feasible.
Adverse events (AEs), clinically monitored during the vaccination-cycle by questionnaires administered retrospectively at neurological follow-up visits, were described as injection-related (local pain, fever, myalgias, etc.) or disease-related. Clinical relapses and MRI-activity were monitored at 3 and 6 months after the end of it.
Patient’s history was retrieved from clinical-visits records and biochemical/radiological reports.
All patients signed an informed consent form, agreeing to the use of their data for clinical research.
Results
In total, 195 patients with MS were referred for vaccination between April 2017 and January 2021, before the start of SARS-CoV-2 vaccines’ campaign. Their demographic and clinical features are summarized in , section a).
Table 1. (a) Demographic and clinical features of our study population; (b) Clinical details of the patients addressed to a switch.
Out of 195 patients, 124 (63.6%) started a vaccination-schedule before therapy-start/-switch; their treatment-status before vaccination-start is outlined in , section b). Among these patients, 108 (87.1%) effectively completed immunization before the beginning of the new therapy, and even though 38 (35.8%) of them had a relapse within 3 months before immunization, the majority of them (35/38, 92.1%) still received vaccination before treatment-start, without any deferral. Out of the remaining 16 patients that could not proceed on time, 4 (25.0%) could complete immunization because of disease-activity.
The remaining 71 out of 195 patients (36.4%) underwent vaccination during an ongoing therapy, with the purpose of increasing their protection during treatment or to complete the vaccination-schedule while on a first-line therapy, in preparation for a possible switch. We highlight that most patients were on second-line therapy and 41 (57.7%) on anti-CD20 therapy.
shows VCRs of the study population, including documented vaccination previously received, including dTap. Pneumococcal and meningococcal vaccines were often the first administered owing to the longer schedule (specifically, meningococcal-B serotype).
Figure 1. Vaccination coverage rates.
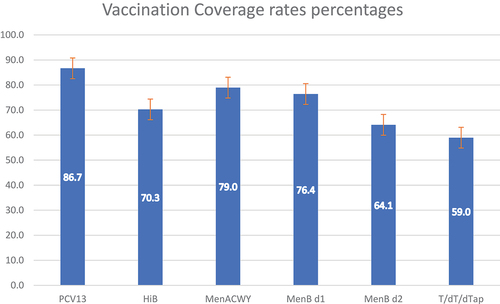
In regard to additional vaccination to our vaccination’s core, 88 (45.1%), 15 (7.7%), 53 (27.2%) and 10 (5.1%) patients received at least one dose of dTap, HPV, and hepatitis-B/A vaccines, respectively. Concerning MMR vaccine, 12 of the 58 (20.7%) who needed vaccination having no therapy or timing contraindications got vaccinated with at least one dose. Sixteen out of 160 (10%) resulted not completely vaccinated or with serological negativity/weak positivity against VZV and 9 (5.6%) patients received at least one VZV vaccine dose.
To evaluate the feasibility of vaccination protocol, we analyzed the therapy-start-/switch and program’s completion timespans. The median time for therapy-switch was 65 with a 95% confidence interval (CI) of 32 days, while the vaccination-program had a core median duration of 40.5 (CI 42) days. Owing to the center’s growing expertise, we observed a reduction in the time needed to complete the program, reaching a median of 27 (CI 22) days in 2020. No delay in therapy start was observed.
Regarding AEs, injection-related reactions were reported in nine patients (4.6%), showing an optimal tolerance profile comparable with other dataCitation7. Referring to the disease-course during the vaccination-cycle, two patients (1.0%) presented a clinical-relapse, while one patient (0.5%) had a radiological activity. Both patients who had clinical-relapse were clinically active within 3 months before immunization. Three and 6 months after the completion of the program, two patients (1, 0.5%, and 1, 0.5%, respectively; 1.0% globally) presented a relapse and eight (4, 2.05%, and 4, 2.05%, respectively; 4.10% globally) had MRI-activity; six out of these last eight patients (75.0%) with MRI-activity were already active 3 months before immunization-start.
Discussion
This real-world study aimed at describing a single-center experience in vaccinating a heterogeneous cohort of patients with MS on DMDs or with scheduled DMDs initiation and before the beginning of the SARS-CoV-2 vaccination campaign started.
We report an excellent tolerability-profile and no increase in the relapse-rate of our patients after any vaccination, confirming their safetyCitation8. Moreover, in our experience, a vaccination-protocol with a median of 27 days (CI 22), in accordance with B meningococcal vaccine schedule timing, was feasible and could allow immunization of MS-patients without delaying therapy-start.
Vaccinations are strongly recommended by both national and international scientific societiesCitation3,Citation8 and timeliness is crucial in those who are about to start long-term immunosuppressive therapy. In a previous studyCitation9 evaluating seroprotection/seropositivity rates for MMR in adults with an acquired immune-deficiency, we found a susceptibility rate of 21.6% in at least one of these infections. Therefore, since most MS-therapies can lower the immunological response to vaccinationsCitation2, a prompt vaccination-evaluation at diagnosis or before a vertical therapy-switch is encouragedCitation10, and this was our approach in the majority (55.8%, 108/195) of our patients. Additionally, considering that most of the studies describing a reduced response to vaccines in patients with MS on DMDs focus on the mere humoral branch of immunityCitation2, we addressed to immunization even for patients already treated and not in need of a switch, wishing to empower a possible, though not completely known yet, cellular responseCitation11.
Vaccines represent an issue of paramount importance, particularly nowadays in relation to the current SARS-CoV-2 pandemic and the novel vaccination strategies. Nevertheless, diffidence regarding vaccination safety among patients still exists and vaccination campaigns promoted by clinicians are suboptimalCitation4, due to the concern of starting a DMD as soon as possible in case of clinical activity. Moreover, practical and real-world-based instruments to support the feasibility of vaccinating fragile patients as those affected by MS do not exist. Thus, our real-world study prompts confidence in health-care professionals in proper individualized vaccination schedule and in increasing awareness on the possibility of vaccinations within a therapy switch in patients with MS. In fact, though the duration of the vaccination-cycle depends also on the number of required vaccines, our data suggest that a complete immunization can be performed in less than a month and does not interfere with clinical management.
Unfortunately, we were not able to characterize the immunological response of our patients. In light of the paramount importance of this issue, further studies are needed to precisely describe all immunological memory arms in response to vaccination and to better define their efficacy within ongoing DMDs for MS.
Acknowledgments
This work was developed within the framework of the DINOGMI Department of Excellence of MIUR 2018–2022 (legge 232 del 2016).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Reyes S, Ramsay M, Ladhani S, Amirthalingam G, Singh N, Cores C, Mathews J, Lambourne J, Marta M, Turner B, et al. Protecting people with multiple sclerosis through vaccination. Pract Neurol. 2020;1–4. doi:10.1136/practneurol-2020-002527.
- Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: a review. Mult Scler Relat Disord. 2020 Oct;45:102439. doi:10.1016/j.msard.2020.102439. Epub 2020 Aug 1.
- Riva A, Barcella V, Benatti SV, Capobianco M, Capra R, Cinque P, Comi G, Fasolo MM, Franzetti F, Galli M, et al. Vaccinations in patients with multiple sclerosis: a Delphi consensus statement. Mult Scler. 2021 Mar;27(3):347–359. doi:10.1177/1352458520952310. Epub 2020 Sep 17.
- Yap SM, Al Hinai M, Gaughan M, Callanan I, Kearney H, Tubridy N, McGuigan C. Vaccine hesitancy among people with multiple sclerosis. Mult Scler Relat Disord. 2021 Nov; 56:103236. doi:10.1016/j.msard.2021.103236. Epub 2021 Sep 2.
- National Plan for Vaccine Prevention 2017-2019; http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf.
- Rubin LG, Levin MJ, Per Ljungman E, Graham D, Robin A, Marcie T, Athos B, Shireesha D, Lillian S, Keyserling H, et al. Infectious diseases society of America. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014 Feb;58(3):309–318. doi:10.1093/cid/cit816.
- Dey A, Wang H, Quinn H, Pillsbury A, Glover C, Hickie M, Wood N, Beard F, Macartney K. Surveillance of adverse events following immunisation in Australia annual report, 2019. Commun Dis Intell (2018). 2021 Apr 30;45. doi:10.33321/cdi.2021.45.23.
- Farez MF, Correale J, Armstrong MJ, Rae-Grant A, Gloss D, Donley D, Holler-Managan Y, Kachuck NJ, Jeffery D, Beilman M, et al. Practice guideline update summary: vaccine-preventable infections and immunization in multiple sclerosis: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2019 Sep;93(13):584–594. doi:10.1212/WNL.0000000000008157.
- Sticchi L, Astengo M, Iavarone IG, Icardi G. Utility of serological screening for measles, mumps and rubella in immunocompromised patients. Hum Vaccin Immunother. 2019;15(12):2854–2855. Published online 2019 Sep 17. doi:10.1080/21645515.2019.1657353.
- Sirbu CA, Florea AA, Ghinescu MC, Docu-Axelerad A, Sirbu AM, Bratu OG, Radu FI. Vaccination in multiple sclerosis - challenging practices (Review). Exp Ther Med. 2020 Dec;20(6):217. doi:10.3892/etm.2020.9347. Epub 2020 Oct 15.
- Mehling M, Hilbert P, Fritz S, Durovic B, Eichin D, Gasser O, Kuhle J, Klimkait T, Lindberg RLP, Kappos L, et al. Antigen-specific adaptive immune responses in fingolimod-treated multiple sclerosis patients. Ann Neurol. 2011 Feb;69(2):408–413. doi:10.1002/ana.22352.
