ABSTRACT
Immunization implementation in the community relies upon post-licensure vaccine safety surveillance to maintain safe vaccination programs and to detect rare AEFI not observed in clinical trials. The increasing availability of electronic health-care related data and correspondence from both health-related providers and internet-based media has revolutionized health-care information. Many and varied forms of health information related to adverse event following immunization (AEFI) are potentially suitable for vaccine safety surveillance. The utilization of these media ranges from more efficient use of electronic spontaneous reporting, automated solicited surveillance methods, screening various electronic health record types, and the utilization of natural language processing techniques to scan enormous amounts of internet-based data for AEFI mentions. Each of these surveillance types have advantages and disadvantages and are often complementary to each other. Most are “hypothesis generating,” detecting potential safety signals, where some, such as vaccine safety datalinking, may also serve as “hypothesis testing” to help verify and investigate those potential signals.
Introduction
The rapid development and implementation of effective, lifesaving vaccines COVID-19 is an exciting illustration of the ability of basic science, industry, governmental, and institutional bodies to respond to novel global infectious disease threats. A key challenge that accompanies that same unprecedented rapidity from bench research to widespread field introduction of often novel vaccine designs, is the limited time available to accrue safety information. Despite often large clinical trials, the overlapping nature of these, coupled with shorter initial follow-up for emergency use access or provisional licensure, has increased the focus upon post-implementation safety surveillance systems.Citation1 In addition, the whole of population nature of COVID-19 vaccine programs has appropriately focused those same populations’ attention upon the safety of vaccines being administered to their families or patients.Citation2 This focus has rightly sped up a revolution already quietly taking place in vaccine safety informatics. AEFI may occur as a result of a vaccination, or purely by chance following a vaccination in a coincidental temporal association. Differentiating between these in an era where safety concerns can emerge rapidly from sources as varied as clinical presentations, media concerns, or unvalidated social media posts is increasingly complex. This review will summarize the challenges, existing systems, innovations, and collaborations in vaccine safety that have emerged.
Vaccine safety – why different – why need timely
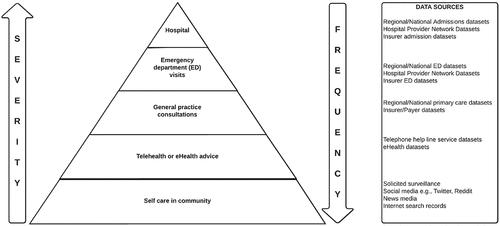
Like medicines, no vaccine is 100% safe. However, as opposed to other medicines, vaccines are primarily used in otherwise healthy recipients for prevention at a population level. This places an even greater responsibility upon the principle of primum non nocere (first do no harm), as no symptoms or underlying pathology are being treated at the time of receipt, with potential future benefit the rationale. Maurice Hilleman, responsible for leading the development of more than 40 vaccines, including many currently used childhood vaccines, is credited as often saying “I never breathe a sigh of relief until the first 3 million doses are out there.”Citation3 Even large clinical trials, with tens of thousands of participants, are unlikely to detect rare or even uncommon adverse events following immunization (AEFI).Citation1 Post-licensure implementation is where we learn most about uncommon and rare adverse events, as well as the safety of vaccines in varied populations, and those who may have been excluded from clinical trials. Even large well-conducted phase III clinical trials are not statistically powered to detect many rare AEFI, with the 44,000 participant Pfizer Cominarty trial able to detect AEFI more common than 1 in 5000.Citation1 For COVID-19 vaccines, where the rapidity and scale of implementation has been unprecedented, it has been critical to detect and investigate AEFI signals rapidly to determine if these are real and, if proven, to inform risk mitigation. Examples of proven signals include Vaccine Induced Thrombosis and Thrombocytopenia (VITT) following some adenoviral vectored vaccines, and myocarditis following mRNA vaccines.Citation4–8 Consideration of where differing AEFI may present or be detected also influences data systems involved in vaccine safety surveillance (). Challenges for safety surveillance following the life-saving implementation of COVID-19 vaccines have included vaccine, pandemic and pandemic response factors. Vaccine factors have included the use of novel technologies such as mRNA vaccines and adenoviral vectored vaccines; novel adjuvants; and prior experience with other (non COVID-19) coronavirus vaccines that raised the possibility of vaccine associated enhanced disease whereby prior vaccination theoretically could increase the severity of subsequent SARS-CoV-2 infection. Pandemic factors included circulating SARS-CoV-2 infection in many settings causing illness in the post-vaccine window that may be interpreted as AEFI. Pandemic response factors include emergency-use authorizations (provisional licensure) enabling initial access for the community with shorter than normal follow-up after all trial participants had completed vaccination; a massive expansion of the vaccinating workforce increasing risk of errors, and; widespread vaccine-related mandates. Even with established vaccines, many AEFI of significance may first be raised by clinicians, prompting subsequent signal confirmation in surveillance systems. VITT and myocarditis are examples of specialist hematologists and cardiologists respectively noting unusual presentation trends, triggering signal confirmation, ongoing surveillance and public health action.Citation5,Citation9,Citation10 In contrast, surveillance systems of large populations have been responsible for identifying an excess of observed Guillain-Barre Syndrome cases following the adenoviral-vector Vaxzevria® vaccine.Citation11
Differentiating signal detection from signal validation and investigation
Vaccine safety concerns may arise due to an apparent increase in the rate of a known AEFI or apparent increased reporting of a presentation not previously described as an AEFI. Even a solitary case of sufficient severity may represent a potential signal. Prior to the introduction of COVID-19 vaccines the Brighton Collaboration in conjunction with the Coalition for Epidemic Preparedness Innovations (CEPI) Safety Platform for Emergency vACcines (SPEAC) Project, developed a list of Adverse Events of Special Interest (AESI).Citation12 These AESI were selected due to previous identification with vaccination in general (e.g. anaphylaxis); experience with similar vaccine platforms, or events that may occur as part of the clinical manifestations or complication of the target pathogen. Most AESI and AEFI may also occur in the absence of vaccination.Citation13 Differentiating between what is likely a true vaccine safety signal and what is a coincidental temporally related event following vaccination relies upon robust surveillance and clinical follow-up.
A safety signal in pharmacovigilance has been defined by the WHO in 2002 as “Reported information on a possible causal relationship between an adverse event and a drug, the relationship being previously unknown or incompletely documented.”Citation14–16 However, it may also occur with an unexpected change in observed rate of an adverse event already established as having a causal relationship. In 2010, a marked increased rate of fever and febrile convulsions occurred following one of the seasonal influenza brands used in Australia, even though both were known AEFI following seasonal influenza vaccines in young children.Citation17,Citation18 In 2010, the Council for International Organizations of Medical Sciences (CIOMS) Working Group on Pharmacovigilance proposed the following modification:Citation14
Information that arises from one or multiple sources (including observations and experiments), which suggests a new potentially causal association, or a new aspect of a known association, between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action.
Signal detection is the step of detecting potential signals. Signal validation (or verification) typically refers to the largely epidemiological process of determining whether the observed events represent a true signal or are within expected reporting rates. Signal investigation often involves combined clinical and epidemiological review to help establish causality and understand potential mechanisms behind why the events may have occurred.
Spurious reports
With the emergence of vaccine hesitancy as a major global health challenge, spurious reports of death or serious illness have also emerged, especially on social media platforms.Citation19–21 Even single spurious reports can harm public confidence and damage vaccine programs. They may also spur reposting or additional similar reports that may be detected as a safety signal.
Politics and social media
Increasing political polarization in many countries has been associated with a trend for politicians from even major political parties to publicly voice anti-vaccine views and rhetoric. Frequently, social media platforms have been used to share these views.Citation22–24
Healthy recipients
Together with the increased burden of proof of safety needed for the immunization of healthy vaccinees, there are special groups not typically studied in pre-licensure clinical trials for whom even greater reassurance is needed. Women are more likely to be willing to receive COVID-19 vaccine when non-pregnant than during pregnancy, with safety concerns the most common reason.Citation25 However, routine pharmacovigilance to reassure safety outcomes for pregnant women and their babies is difficult and often requires bespoke studies. Many vaccine safety surveillance systems and vaccine registries do not reliably capture pregnancy status, and few systems enable linkage of a vaccine receipt in a pregnant mother to perinatal outcomes in an infant. Patients who are immunosuppressed or with chronic diseases, as well as the frail elderly are also hard to identify as a group using typical surveillance systems.
Spontaneous surveillance and background rates
Spontaneous (passive) surveillance forms the mainstay of most national and international pharmacovigilance systems. Spontaneous surveillance relies upon health-care workers (and sometimes community members) to report AEFI. Spontaneous systems have the advantage of drawing from the entire population, with the primary disadvantage being under-reporting is the norm, even for severe AEFI. Mandating reporting of AEFI for health-care providers is common in many countries, however, there is little evidence whether such legislation substantially increases reporting rates, with reasons for under-reporting multiple and often specific to local circumstances.Citation26,Citation27 Many systems allow for only health-care workers to report AEFI, however, consumers appear to have a similar probability of reporting serious AEFI.Citation28
The advent of online reporting has facilitated spontaneous surveillance, with some systems even allowing public searching of de-identified reports.Citation26,Citation29 Globally, national regulatory agencies (NRAs) and the World Health Organization (WHO) contribute to the aggregated database Vigibase at Uppsala in Sweden, allowing for the central team and individual nations to search global data as well as their own. Vigibase is limited by the bias from its major spontaneous surveillance AEFI contributors, the US Vaccine Adverse Event Reporting Scheme (VAERS) and the European Eudravigilance database, which means vaccines not widely deployed in these settings are less likely to be represented. Collaborative networks including the Global Vaccine Data Network have also attempted to combine data from multiple high- and low-income settings to address whether specific AESI are associated with COVID-19 vaccines, as part of a US CDC funded data-linkage project (https://www.globalvaccinedatanetwork.org/global-covid-vaccine-safety-gcovs).
Many AEFI, including severe AEFI, also occur independent of vaccination. In order to determine whether vaccination has increased the risk of events, age adjusted expected “background” rates of events are calculated using hospital, emergency department and/or primary care datasets.Citation13,Citation30 These are helpful to determine whether the reported AEFI are within the number expected to occur in the days or weeks following vaccination by chance alone, or whether they are likely to represent a safety signal.
Spontaneous surveillance remains the primary mechanism for detecting unexpected and rare AEFI worldwide and is primarily “hypothesis generating” in its nature, with more active surveillance often used to confirm and investigate potential safety signals.Citation31,Citation32 Routine automated disproportionality analyses, Bayesian analyses and sequential probability ratio tests such as the MaxSPRT have been employed within spontaneous datasets to reduce the time to detecting safety signals where an AEFI type has been predefined.Citation26,Citation33 Disproportionality signal detection analyses can be further enhanced using complementary time-to-onset (TTO) methods.Citation34
The implementation of a global vaccination program has also resulted in a massive increase in AEFI reports. Improvements in reporting systems, databases, automated analyses, and visualizations have been critical to avoid reporting systems being overwhelmed with the sheer number of reports.Citation35
Active surveillance methodologies
Active surveillance involves actively looking for AEFI, whether events known to occur following vaccination, or adverse events of special interest (AESI) that are considered important to perform surveillance for other reasons. Traditional active surveillance includes hospital-based surveillance for AESI, using manual or electronic searching within the hospital.Citation36 However, the advent of rapid communications, large clinically related datasets, the internet and social media has expanded the range and capacities of active surveillance methodologies. Active surveillance can be both hypothesis generating and allow hypothesis testing (safety signal confirmation and investigation). The attributes of differing surveillance system methodologies are summarized in .
Table 1. Vaccine safety surveillance system types and attributes.
Solicited surveillance
Solicited surveillance has been widely deployed as part of COVID-19 vaccine implementation in multiple countries and settings, including V-SAFE in the United States, the Yellow Card Vaccine Monitor in the United Kingdom and AusVaxSafety in Australia.Citation37,Citation38 In voluntary systems such as V-SAFE, demographic data are collected more completely. Vaccinees receive messages typically by diary card, downloaded apps, SMS or e-mail at discrete time points post-vaccination (such as 1 day, 1 week, and 6 weeks) asking if they have experienced AEFI.Citation39 If they respond in the affirmative, categorical responses such as specific local and systemic reactogenicity questions allow rapid automated calculation of rates in respondents to assess whether these are similar to those observed in clinical trials, or exceed thresholds. Respondents can also be categorized by age and particular risk groups, such as antenatal vaccinees, allowing for rapid accrual of reactogenicity profiles of populations of interest, and publicly available visualization of these.Citation40
Solicited systems play a vital role in rapid characterization and communication of reactogenicity, and common short term AEFI. They can also contribute to vaccine confidence by rapidly profiling reactogenicity in local populations and subgroups.Citation41,Citation42 Due to their sample size, and the reduced compliance with responding to messages over time, they are less well suited to detecting signals relating to uncommon, rare or delayed AEFI. Only data from participants who respond can be included. It is uncertain which contact methodology has the best response rate, although a 2017 review described diary card response rates as having the greatest variability, with SMS and e-mail response rates similar to each other in differing settings.Citation39 SMS-based systems are more expensive, especially at large scale.
Syndromic surveillance
Syndromic surveillance systems utilize diagnoses or diagnosis surrogate terms from de-identified near real-time data systems to detect changes in rates of events of interest. Potentially capable of operating at massive scale, the best known example is the use of Google search terms to detect influenza outbreaks.Citation43 Similar methods have also recently been validated for AEFI detection utilizing historical influenza vaccine AEFI safety signal data from telephone help-line data systems and primary care representation following immunization data.Citation44,Citation45 Social media automated monitoring utilizing machine learning techniques have also been developed to detect mentions of AEFI and differentiate them from other vaccine safety social media messages.Citation46–48 Similar techniques have been developed to monitor media reports of AEFI also.Citation49 By virtue of potentially surveilling data from different acuity levels of health-care advice seeking (from the internet through to hospital presentations) syndromic surveillance may be able to detect changes in rates of “mild” AEFI that may inform more severe outcomes, such as fever rates in young children following vaccination that may predict risks of the rarer outcome of febrile seizures. This is also a potential role for solicited surveillance.
Syndromic systems carry the potential advantages of broad coverage, high sensitivity, and cost-effectiveness but due to potential lack of specificity are likely to have an adjunctive role in AEFI signal detection and characterization.
Data-Linkage
The emergence of large-linked dataset-based surveillance has enabled rapid investigation of emergent potential AEFI signals, for both uncommon and rare AEFI. Vaccine exposure utilizing immunization registries or immunization provider records are linked at an individual level to health outcome datasets (hospital admissions, emergency presentations, primary care consultations, deaths). Rapid-cycle analysis of chosen AESI also allows regular repeat analyses to detect changes in event rates as early as possible.Citation31,Citation50 The inclusion of both vaccinated and non-vaccinated individuals allows comparison of risk with both unvaccinated individuals or with periods where the same individual is not within a putative “risk period” following vaccination, the “self controlled case series” method.Citation50,Citation51
Datalinkage studies have been invaluable in confirming increased rates of rare post vaccine AESI such as Guillain-Barre syndrome following inactivate influenza vaccines as well as allaying concerns regarding other AESI such as spontaneous abortion following influenza vaccines.Citation52,Citation53 Distributed data models allow all linkage to occur within each health-care system, with aggregate de-identified data able to be combined centrally, allowing national networks such as the US Vaccine Safety Datalink (VSD), and international networks like the Global Vaccine Data Network’s Global COVID Vaccine Safety Study to examine even rare AESI across different vaccines and settings.Citation52,Citation53
Like syndromic surveillance of hospital data, datalinkage studies are reliant upon diagnostic codes such as International Classification of Diseases (ICD) coding to classify health outcomes. The accuracy of these codes varies between health systems and between diagnoses.Citation54 In some cases, it is important to confirm from identifiable source notes whether the patient really had the AESI using established case definitions.Citation55 While this is potentially feasible in datalinkage systems at an individual health network level such as with the VSD, it is more complex and often not possible at a jurisdictional or national level.Citation56 Vaccine safety datalinkage has been successfully conducted in settings as diverse as Vietnam and Ecuador.Citation57,Citation58
Vaccine safety datalinkage systems balance the public benefit with potential privacy implications for community members whose data are contained within the system. Consumer surveys, including citizen’s juries, have indicated strong support for datalinkage in this context.Citation15
Safety surveillance in resource poor settings
The first Global Vaccine Safety Blueprint published in 2012 (GVSB1.0) aimed to assist low- and middle-income countries (LMIC) achieve and build upon minimal vaccine safety capacity toward enhanced capacity.Citation59 Recently, GVSB2.0 built upon this concept utilizing maturity levels based upon the World Health Organization Global Benchmarking Tool.Citation60 However, in many LMIC safety surveillance capacity is limited. In these settings, the introduction of vaccines without extensive prior post-licensure experience is becoming more common and poses unique challenges. Vaccines for emerging threats such as Lassa and Nipah viruses are likely to be implemented in resource poor settings where safety surveillance systems may be limited.Citation61 Approaches to this challenge typically involve strengthening of resources in sentinel health-care facilities where vaccinees are likely to present with illness or AEFI, as seen with the successful implementation of novel meningococcal group A conjugate vaccines in Mali and Niger.Citation62,Citation63 This model is also demonstrated in the recent COVID-19-SENT-[Africa-8] project, which will conduct hospital-based sentinel surveillance of adverse events of special interest (AESIs) after vaccination with COVID-19 vaccines in African Advanced Market Commitment-92 eligible countries.
Conclusion
Vaccine safety surveillance and the resultant timely provision of information to policymakers, health-care providers and the community have benefited from the information revolution. The SARS-CoV-2 pandemic and rapid COVID-19 vaccine response have posed unique challenges to vaccine safety surveillance and community confidence. Spontaneous surveillance remains the mainstay of surveillance in most settings and is critical for the detection of rare and unexpected “unknown unknowns.” Web-based reporting, community reporting, and modern business-intelligence systems to automate detection analyses and visualize AEFI data have aided the ability of safety surveillance systems to detect safety signals. Active surveillance systems have augmented signal detection, especially for common and uncommon AEFI, and contributed to vaccine confidence with reactogenicity and safety profiles using real-world data. They have also allowed responsive signal validation and investigation at national and even international levels, with multisite datalinking networks offering the ability to confirm or reject vaccine associations with even very rare events. The adoption of multiple systems across high-income settings and LMIC with differing advantages and disadvantages will allow integrated vaccine safety surveillance to provide optimal protection for communities and vaccine programs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Buttery JP. Developing standard safety outcomes for COVID-19 vaccines. Vaccine. 2021;39(22):1–7. doi:10.1016/j.vaccine.2021.03.004.
- Danchin M, Buttery J. COVID-19 vaccine hesitancy: a unique set of challenges. Intern Med J. 2021;51(12):1987–1989. doi:10.1111/imj.15599.
- Kurth R. [Infectious diseases: how big is the real danger? Interview by Strehlow Karen. Dtsch Med Wochenschr. 2005;Suppl 1:64–65.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic Thrombocytopenia after ChAdox1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi:10.1056/NEJMoa2104840.
- Schultz NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt A-H, Skattør TH, Tjønnfjord GE, et al. Thrombosis and Thrombocytopenia after ChAdox1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi:10.1056/NEJMoa2104882.
- Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med 2021 N Engl J Med; 385:2140–49. doi: 10.1056/NEJMoa2109730.
- Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6(10):1202–1206. doi:10.1001/jamacardio.2021.2833.
- Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi:10.1056/NEJMoa2110475.
- Greinacher A, Selleng K, Palankar R, Wesche J, Handtke S, Wolff M, Aurich K, Lalk M, Methling K, Völker U, et al. Insights in ChAdox1 nCov-19 vaccine-induced immune thrombotic thrombocytopenia. Blood. 2021;138(22):2256–2268. doi:10.1182/blood.2021013231.
- Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, et al. Myocarditis after Covid-19 vaccination in a large health care organization. N Engl J Med. 2021;385(23):2132–2139. doi:10.1056/NEJMoa2110737.
- Patone M, Handunnetthi L, Saatci D, Pan J, Katikireddi SV, Razvi S, Hunt D, Mei XW, Dixon S, Zaccardi F, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144–2153. doi:10.1038/s41591-021-01556-7.
- Lambert PH, Ambrosino DM, Andersen SR, Baric RS, Black SB, Chen RT, Dekker CL, Didierlaurent AM, Graham BS, Martin SD, et al. Consensus summary report for CEPI/BC March 12–13, 2020 meeting: assessment of risk of disease enhancement with COVID-19 vaccines. Vaccine. 2020;38(31):4783–4791. doi:10.1016/j.vaccine.2020.05.064.
- Black S, Eskola J, Siegrist CA, Halsey N, MacDonald N, Law B, Miller E, Andrews N, Stowe J, Salmon D, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009;374(9707):2115–2122. doi:10.1016/S0140-6736(09)61877-8.
- VIII CIOMS Working Group. Practical aspects of signal detection in pharmacovigilance. Geneva: The Council For International Organizations Of Medical Sciences; 2010.
- Berry JG, Gold MS, Ryan P, Duszynski KM, Braunack-Mayer AJ. Vaccine assessment using Linked Data Working G. Public perspectives on consent for the linkage of data to evaluate vaccine safety. Vaccine. 2012;30(28):4167–4174. doi:10.1016/j.vaccine.2012.04.056.
- World HealthOrganization. Safety of medicines : a guide to detecting and reporting adverse drug reactions : why health professionals need to take action. Report No.: WHO/EDM/QSM/2002.2; Geneva; 2002.
- Armstrong PK, Dowse GK, Effler PV, Carcione D, Blyth CC, Richmond PC, Geelhoed GC, Mascaro F, Scully M, Weeramanthri TS, et al. Epidemiological study of severe febrile reactions in young children in Western Australia caused by a 2010 trivalent inactivated influenza vaccine. BMJ Open. 2011;1(1):e000016. doi:10.1136/bmjopen-2010-000016.
- Gold MS, Effler P, Kelly H, Richmond PC, Buttery JP. Febrile convulsions after 2010 seasonal trivalent influenza vaccine: implications for vaccine safety surveillance in Australia. Med J Aust. 2010;193(9):492–493. doi:10.5694/j.1326-5377.2010.tb04029.x.
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–1034. doi:10.1016/S0140-6736(21)00306-8.
- ABCFC. As younger children become eligible for the vaccine, anti-vaxxers spread false information about deaths. ABC News: ABC; 2022 [ accessed 2022 Jan 28].
- Cassidy C ‘Utter fear’: Gold Coast GP receives death threats from ‘anti-vaxxers’ after false claim of child vaccine deaths. The Guardian. 2022 [accessed 2022 Feb 1].
- Sharfstein JM, Callaghan T, Carpiano RM, Sgaier SK, Brewer NT, Galvani AP, Lakshmanan R, McFadden SM, Reiss DR, Salmon DA, et al. Uncoupling vaccination from politics: a call to action. Lancet. 2021;398(10307):1211–1212. doi:10.1016/S0140-6736(21)02099-7.
- Germani F, Biller-Andorno N, Lavorgna L. The anti-vaccination infodemic on social media: a behavioral analysis. PLoS One. 2021;16(3):e0247642. doi:10.1371/journal.pone.0247642.
- Sharfstein JM, Goodman JL, Borio L. The US regulatory system and COVID-19 vaccines: the importance of a strong and capable FDA. Jama. 2021;325(12):1153–1154. doi:10.1001/jama.2021.1961.
- Skirrow H, Barnett S, Bell S, Riaposova L, Mounier-Jack S, Kampmann B, Holder B. Women’s views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. BMC Pregnancy Childbirth. 2022;22(1):33. doi:10.1186/s12884-021-04321-3.
- Clothier HJ, Crawford NW, Russell M, Kelly H, Buttery JP. Evaluation of ‘SAEFVIC’, a pharmacovigilance surveillance scheme for the spontaneous reporting of adverse events following immunisation in Victoria, Australia. Drug Saf. 2017;40(6):483–495. doi:10.1007/s40264-017-0520-7.
- Gidudu JF, Shaum A, Dodoo A, Bosomprah S, Bonsu G, Amponsa-Achiano K, Darko DM, Sabblah G, Opare J, Nyaku M, et al. Barriers to healthcare workers reporting adverse events following immunization in four regions of Ghana. Vaccine. 2020;38(5):1009–1014. doi:10.1016/j.vaccine.2019.11.050.
- Clothier HJ, Selvaraj G, Easton ML, Lewis G, Crawford NW, Buttery JP. Consumer reporting of adverse events following immunization. Hum Vaccin Immunother. 2014;10(12):3726–3730. doi:10.4161/hv.34369.
- Arana JE, Harrington T, Cano M, Lewis P, Mba-Jonas A, Rongxia L, Stewart B, Markowitz LE, Shimabukuro TT. Post-Licensure safety monitoring of quadrivalent human papillomavirus vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2009–2015. Vaccine. 2018;36(13):1781–1788. doi:10.1016/j.vaccine.2018.02.034.
- Siegrist CA, Lewis EM, Eskola J, Evans SJ, Black SB. Human papilloma virus immunization in adolescent and young adults: a cohort study to illustrate what events might be mistaken for adverse reactions. Pediatr Infect Dis J. 2007;26(11):979–984. doi:10.1097/INF.0b013e318149dfea.
- Mesfin YM, Cheng A, Lawrie J, Buttery J. Use of routinely collected electronic healthcare data for postlicensure vaccine safety signal detection: a systematic review. BMJ Glob Health. 2019;4(4):e001065. doi:10.1136/bmjgh-2018-001065.
- Crawford NW, Clothier H, Hodgson K, Selvaraj G, Easton ML, Buttery JP. Active surveillance for adverse events following immunization. Expert Rev Vaccines. 2014;13(2):265–276. doi:10.1586/14760584.2014.866895.
- Costa MA, Huang SS, Moore M, Kulldorff M, Finkelstein JA. New approaches to estimating national rates of invasive pneumococcal disease. Am J Epidemiol. 2011;174(2):234–242. doi:10.1093/aje/kwr058.
- Van Holle L, Zeinoun Z, Bauchau V, Verstraeten T. Using time-to-onset for detecting safety signals in spontaneous reports of adverse events following immunization: a proof of concept study. Pharmacoepidemiol Drug Saf. 2012;21(6):603–610. doi:10.1002/pds.3226.
- Lo Re V 3rd, Klungel OH, Chan KA, Panozzo CA, Zhou W, Winterstein AG. Global covid-19 vaccine rollout and safety surveillance-how to keep pace. Bmj. 2021;373:n1416.
- Deeks SL, Clark M, Scheifele DW, Law BJ, Dawar M, Ahmadipour N, Walop W, Ellis CE, King A. Serious adverse events associated with Bacille Calmette-Guérin vaccine in Canada. Pediatr Infect Dis J. 2005;24(6):538–541. doi:10.1097/01.inf.0000164769.22033.2c.
- Glover C, Crawford N, Leeb A, Wood N, Macartney K. Active SMS-based surveillance of adverse events following immunisation with influenza and pertussis-containing vaccines in Australian pregnant women using AusVaxsafety. Vaccine. 2020;38(31):4892–4900. doi:10.1016/j.vaccine.2020.04.056.
- Chapin-Bardales J, Myers T, Gee J, Shay DK, Marquez P, Baggs J, Zhang B, Licata C, Shimabukuro TT. Reactogenicity within 2 weeks after mRNA COVID-19 vaccines: findings from the CDC v-safe surveillance system. Vaccine. 2021;39(48):7066–7073. doi:10.1016/j.vaccine.2021.10.019.
- Cashman P, Macartney K, Khandaker G, King C, Gold M, Durrheim DN. Participant-Centred active surveillance of adverse events following immunisation: a narrative review. Int Health. 2017;9(3):164–176. doi:10.1093/inthealth/ihx019.
- Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, Marquez PL, Olson CK, Liu R, Chang KT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–2282. doi:10.1056/NEJMoa2104983.
- Pillsbury AJ, Fathima P, Quinn HE, Cashman P, Blyth CC, Leeb A, Macartney KK. Comparative postmarket safety profile of adjuvanted and high-dose influenza vaccines in individuals 65 years or older. JAMA Netw Open. 2020;3(5):e204079. doi:10.1001/jamanetworkopen.2020.4079.
- Pillsbury A, Quinn H, Cashman P, Leeb A, Macartney K. AusVaxsafety c. Active SMS-based influenza vaccine safety surveillance in Australian children. Vaccine. 2017;35(51):7101–7106. doi:10.1016/j.vaccine.2017.10.091.
- Carneiro HA, Mylonakis E. Google trends: a web-based tool for real-time surveillance of disease outbreaks. Clin Infect Dis. 2009;49(10):1557–1564. doi:10.1086/630200.
- Mesfin YM, Cheng AC, Enticott J, Lawrie J, Buttery J. Post-Vaccination healthcare attendance rate as a proxy measure for syndromic surveillance of adverse events following immunisation. Aust N Z J Public Health. 2021;45(2):101–107. doi:10.1111/1753-6405.13052.
- Mesfin YM, Cheng AC, Enticott J, Lawrie J, Buttery JP. Use of telephone helpline data for syndromic surveillance of adverse events following immunization in Australia: a retrospective study, 2009 to 2017. Vaccine. 2020;38(34):5525–5531. doi:10.1016/j.vaccine.2020.05.078.
- Wang J, Zhao L, Ye Y, Zhang Y. Adverse event detection by integrating twitter data and VAERS. J Biomed Semantics. 2018;9(1):19. doi:10.1186/s13326-018-0184-y.
- Habibabadi SKH, Haghighi PD Topic modelling for identification of vaccine reactions in Twitter. In: ACSW, editor. ACSW 2019: Australasian Computer Science Week Multiconference; 2019 January 29; Sydney: Association for Computing Machinery; 2019. p. 1–10.
- Khademi Habibabadi S, Haghighi PD, Burstein F, Buttery J. Vaccine adverse event mining of Twitter conversations: 2-phase classification study. JMIR Medical Informatics. 2022 ;10(6):e34305. doi:10.2196/34305.
- Suppli CH, Hansen ND, Rasmussen M, Valentiner-Branth P, Krause TG, Molbak K. Decline in HPV-vaccination uptake in Denmark - the association between HPV-related media coverage and HPV-vaccination. BMC Public Health. 2018;18(1):1360. doi:10.1186/s12889-018-6268-x.
- Nie X, Xu L, Bai Y, Liu Z, Liu Z, Farrington P, Zhan S. Self-Controlled case series design in vaccine safety: a systematic review. Expert Rev Vaccines. 2022;21(3):313–324. doi:10.1080/14760584.2022.2020108.
- Martin TJ, Fahey M, Easton M, Clothier HJ, Samuel R, Crawford NW, Buttery JP. Acute disseminated encephalomyelitis and routine childhood vaccinations – a self-controlled case series. Hum Vaccin Immunother. 2021;17(8):2578–2585. doi:10.1080/21645515.2021.1901544.
- Donahue JG, Kieke BA, King JP, Mascola MA, Shimabukuro TT, DeStefano F, Hanson KE, McClure DL, Olaiya O, Glanz JM, et al. Inactivated influenza vaccine and spontaneous abortion in the Vaccine Safety Datalink in 2012–13, 2013–14, and 2014–15. Vaccine. 2019;37(44):6673–6681. doi:10.1016/j.vaccine.2019.09.035.
- Dodd CN, Romio SA, Black S, Vellozzi C, Andrews N, Sturkenboom M, Zuber P, Hua W, Bonhoeffer J, Buttery J, et al. International collaboration to assess the risk of Guillain Barré Syndrome following influenza a (H1N1) 2009 monovalent vaccines. Vaccine. 2013;31(40):4448–4458. doi:10.1016/j.vaccine.2013.06.032.
- Burns EM, Rigby E, Mamidanna R, Bottle A, Aylin P, Ziprin P, Faiz OD. Systematic review of discharge coding accuracy. J Public Health (Oxf). 2012;34(1):138–148. doi:10.1093/pubmed/fdr054.
- Yu W, Zheng C, Xie F, Chen W, Mercado C, Sy LS, Qian L, Glenn S, Tseng HF, Lee G, et al. The use of natural language processing to identify vaccine-related anaphylaxis at five health care systems in the Vaccine Safety Datalink. Pharmacoepidemiol Drug Saf. 2020;29(2):182–188. doi:10.1002/pds.4919.
- Hviid A, Hansen JV, Frisch M, Measles MM. Vaccination and autism: a nationwide cohort study. Ann Intern Med. 2019;170(8):513–520. doi:10.7326/M18-2101.
- Ali M, Canh GD, Clemens JD, Park JK, von Seidlein L, Minh TT, Thiem DV, Tho HL, Trach DD. The use of a computerized database to monitor vaccine safety in Vietnam. Bull World Health Organ. 2005;83:604–610.
- Asturias EJ, Contreras-Roldan IL, Ram M, Garcia-Melgar AJ, Morales-Oquendo V, Hartman K, et al. Post-Authorization safety surveillance of a liquid pentavalent vaccine in Guatemalan children. Vaccine. 2013;31(49):5909–5914. doi:10.1016/j.vaccine.2013.09.015.
- Amarasinghe A, Black S, Bonhoeffer J, Carvalho SM, Dodoo A, Eskola J, Larson H, Shin S, Olsson S, Balakrishnan MR, et al. Effective vaccine safety systems in all countries: a challenge for more equitable access to immunization. Vaccine. 2013;31(Suppl 2):B108–14. doi:10.1016/j.vaccine.2012.10.119.
- Organization WH. Global vaccine safety blueprint 2.0 (GVSB2.0) 2021-2023. Geneva; 2021.
- Gouglas D, Christodoulou M, Plotkin SA, Hatchett R. CEPI: driving progress toward epidemic preparedness and response. Epidemiol Rev. 2019;41(1):28–33. doi:10.1093/epirev/mxz012.
- Sow SO, Okoko BJ, Diallo A, Viviani S, Borrow R, Carlone G, Tapia M, Akinsola AK, Arduin P, Findlow H, et al. Immunogenicity and safety of a meningococcal a conjugate vaccine in Africans. N Engl J Med. 2011;364(24):2293–2304. doi:10.1056/NEJMoa1003812.
- Djingarey MH, Diomande FV, Barry R, Kandolo D, Shirehwa F, Lingani C, Novak RT, Tevi-Benissan C, Perea W, Preziosi M-P, et al. Introduction and rollout of a new group a meningococcal conjugate vaccine (PsA-TT) in African Meningitis Belt countries, 2010–2014. Clin Infect Dis. 2015;61(Suppl 5):S434–41. doi:10.1093/cid/civ551.