?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Vaccination coverage worldwide fell from 86% in 2019 to 83% in 2020. The purpose of this research was to determine the level of full immunization coverage among children aged 12 to 23 months in both urban and rural Southwest Ethiopia. A comparative cross-sectional study of sampled 644 children aged 12 to 23 months was conducted in the community (296 from urban areas and 348 from rural areas). Chi-square testing was used to determine whether there was a significant difference in full immunization coverage between urban and rural children, and binary logistic regression was used to identify predictors of full immunization. This study included 635 caregivers of children aged 12–23 months (292 from urban areas and 343 from rural areas), yielding a 98.4% response rate. The overall, full immunization coverage among the whole children was 66.1%. There was a significant difference in fully immunization coverage between urban 74.3% and rural 59.2% of children (χ2 = 16.126, P = .000). Residence, wealth index, ANC follow up and fear of COVID 19 infection at health institutions were predictor variables for overall full vaccination. Knowledge and place of delivery were predictor variables for full vaccination in the urban area whereas distance and male partner involvement in the rural area. Vaccination coverage was higher in urban compared to rural areas but it is still far below the WHO recommended target. Promoting male involvement, health education, and communication are crucial for alleviating poor knowledge about child immunization.
Introduction
While the world was gripped by the COVID-19 pandemic in 2020, children faced the same crisis they had been facing for decades. In 2020, more than 5.0 million children under the age of five died, including 2.4 million newborns. These deaths are not evenly distributed around the globe; children in Sub-Saharan Africa and Southern Asia continue to face the highest risk of death and endure the most of the child mortality burden, with a 14-fold higher risk than children in Europe and North America. This tragic and massive loss of life, the majority of which was caused by preventable or treatable causes, serves as a stark reminder of the urgent need to put an end to preventable child deaths.Citation1
Although vaccination is one of the most effective public health interventions for preventing vaccine-preventable disease, global coverage has dropped from 86% in 2019 to 83% in 2020. The COVID-19 pandemic and its aftermath have strained health-care systems, with 23 million children missing vaccines in 2020, an increase of 3.7 million from 2019 and the highest number since 2009.Citation2,Citation3
With the support of countries and partners, the World Health Assembly has endorsed the Immunization Agenda 2030 (IA2030), a new global vision and strategy to address these challenges over the next decade and save over 50 million lives. The goal of the strategy is to motivate and align the efforts of the community, national, regional, and global stakeholders to achieve a world where everyone, everywhere benefits fully from vaccines for good health and wellbeing.Citation4
Ethiopia adheres to WHO immunization schedules and offers the following vaccines according to the schedules: At birth, one dose of Bacillus Calmette-Guerin (BCG) and an initial dose of oral polio vaccine (OPV0) are given; three doses of each Pentavalent (DPT-HepB-Hib),OPV,and Pneumococcal Conjugate Vaccine (PCV) are given at the 6th,10th, and 14th weeks; two doses of Rotavirus vaccine are given at the 6th and 10th weeks.Citation5–7
Ethiopian governments have made a tremendous effort to improve child immunization coverage, with the help of other nongovernmental organizations.Citation8 Although full immunization coverage has increased from 24% in 2011 to 39% in 2016, according to the EDHS report,Citation9,Citation10 this achievement falls far short of the goal set in the 4th Health Sector Development Plan (HSDP-IV) and the GVAP target plan to achieve 90% coverage nationally and 80% in every district for all vaccines by 2020.Citation5,Citation11
In Ethiopia, the prevalence of fully immunization coverage varies between 20.6% −91.7%.Citation5–8−Citation10–12–Citation37
There is also a high variation of complete vaccination coverage in urban to rural setups; in Ethiopia in 2019, 57.3% in urban and 36.9% in rural areas, 20.4% difference.Citation13
According to studies, place of residence, ANC visit, absence of vaccinator, mothers or caregivers workload, mothers employment status, PNC visit by health extension workers, distance to the immunization site, sex of the child, wanted pregnancy, maternal health care utilization, partners awareness about immunization, knowledge of the schedule and place of immunization, mothers TT vaccination status, mothers age, child vitamin A supplementation, family size, father educational status, child age, occupation, income, being a member of health development army are factors affecting fully immunization coverage.Citation5–7−Citation12–14–Citation43
Male involvement in child immunization and fear of COVID 19 at health institutions are also factors affecting full immunization coverage.Citation8,Citation29 Urban to rural immunization coverage difference is still challenging to achieve the immunization agenda 2030. Therefore, the objectives of this study were to determine the proportion and discrepancies of full immunization coverage between children in the urban and rural areas and to identify predictors of full immunization coverage among children aged 12 to 23 months in both urban and rural areas of Southwest Ethiopia.
Methods and materials
Study design, setting, period, and population
In the Wolaita zone of southwest Ethiopia, a community-based comparative cross-sectional study design was conducted from January 30 to 2 March 2021. Wolaita zone is 328 kilometers south of Addis Ababa, Ethiopia’s capital. In 2021, the zone has a total population of 2,114,379 people, with 1,639,139 (77.5%) living in rural areas and 475,240 (22.5%) living in urban areas. There are 16 districts in the zone, each with six town administrations. In total, there were 483,156 households in the zone. There are 9 hospitals and 68 health centers in the community that provide health care. Mothers/caregivers who had at least one child aged between 12 and 23 months during the study period in a selected district of the zone were included in the study, whereas mothers/caregivers who were unable to respond and had not resided in the study area for at least six months before her child reached the age of 12 months were excluded (Wolaita Zone Health Department Annual Report, 2021).
Sample size and sampling procedure
The required sample size was determined by using two-population proportion formula, with a 95% confidence interval, a power of 80%, and a proportion of full immunized coverage in the urban area (P1) of 72.3% and a proportion of full immunized coverage in the rural area (P2) of 27.7% (15).
Where P = (P1 + P2)/2
P = (0.723 + 0.227)/2 = 0.5
Z a/2 at 95% CI = 1.96, P2 = 0.723, P1 = 0.227
Considering design effect of 2 and 10% non-response rate, n = 40 caregivers for each.
As a result, each group had a sample size of 40 people (80 total). This sample was compared to the sample that was calculated using StatCal of the Epi Info utility with the following assumptions: 95% CI, power 80%, AOR, and percentage of outcome in an unexposed group for each predictor variable. Fully immunization coverage was influenced by ANC visits,Citation15 distance to the health facility,Citation16 and residence.Citation17 The residence had a larger sample size of 292, according to Epi info’s output. The final sample size was 644, after taking into account the design effect of 2 and a 10% non-response rate. Therefore, the required sample size for this particular study was determined by taking the maximum sample size from the second objective of 644.
The study participants were chosen using a multistage stratified sampling technique. Three districts (Sodo Zuriya, Offa, and Damot Gale) were chosen by simple random sampling (SRS) method in the first stage from the five districts of the Woliata Zone. Based on a residence, each district was divided into urban and rural kebeles. In the second stage, SRS method was used to select 7 urban kebeles and 10 rural kebeles from the three districts proportionally. Following the selection of study kebeles, a list of caregivers for children aged 12 to 23 months was obtained from a family folder with the assistance of health extension workers. Then, for each caregiver of a child aged 12–23 months who met the eligibility criteria, an identification number was assigned. According to data from the zonal health office, 54% children aged 12 to 23 months lived in rural areas, while 46% of them lived in towns. Thus, 348 and 296 caregivers of children aged 12 to 23 months were proportionally assigned to randomly selected kebeles in the urban and rural areas, respectively. Finally, the SRS method (computer-generated random numbers) was used to select eligible caregivers of children aged 12–23 months from each sampled kebeles’ family folder.
Data collection tools, procedures, measurements, and quality control
A structured interviewer-administered questionnaire was used to collect data. The questionnaire was adapted and modified from various sources.Citation13–20–Citation38 Before it was distributed, a panel of experts in the field validated it, and its content validity was confirmed. A pretest was conducted on 5% of the sample size in the Boloso Sore district. It was then revised in light of the experts’ recommendations and the pretest results. According to the reliability analysis, Cronbach’s Alpha was greater than 0.7, indicating good internal consistency in the responses. To maintain consistency, it was written in English, then translated into a local language before being rewritten in English.
The questionnaire was divided into seven sections. The first part included socio-demographic questions about caregivers and children, the second part included wealth index-related questions for both urban and rural settings, the third part included previous obstetric history, the fourth part included knowledge-related items, the fifth part covered attitude-related variables, the sixth part covered access to vaccination services, and the seventh part covered the child’s immunization status. Two-degree nurse supervisors and five diploma-nurse data collectors were assigned. Data collectors and supervisors received two days of training on how to administer and collect the questionnaire. Before entering the data, the questionnaire was double-checked for accuracy.
Operational definition
The data about immunization coverage have been obtained both from history and vaccination cards. Immunization coverage by cards: The immunization coverage was calculated with a numerator based only on card documentation, excluding those vaccinated by history from the numerator. Immunization coverage by history: The vaccination coverage was calculated with a numerator based only on the mother/caregiver’s recall report.
Full immunized: A child aged 12 to 23 months who received one dose of BCG and one dose of measles, as well as three doses of pentavalent, three doses of PCV, three doses of OPV, two doses of Rota, and one dose of IPV before his or her first birthday. Not fully immunized: A combination of partially vaccinated and unvaccinated children of 12–23 months old receive one dose of the above six vaccines. Partially immunized: A Child 12‒23 month old who has received at least one vaccine, but not all the EPI vaccines. Unimmunized: A child 12‒23 months old who did not receive EPI vaccinesCitation20
The sum of all 14 knowledge-related questions was used to calculate comprehensive immunization knowledge. For each item, the correct answer received a “1,” while the incorrect answer received a “0.” Respondents with scores greater than or equal to the mean value of the sum of knowledge assessment questions were considered to have good knowledge, while those with scores less than the mean value of the sum of knowledge assessment questions were considered to have poor knowledge.Citation18,Citation19
The attitude questions were graded on a scale of one to seven (with a minimum score of 7 and a maximum score of 35). The attitude was graded on a 5-point scale ranging from strongly disagree to strongly agree, with a score ranging from 1 to 5. Respondents with scores greater than or equal to the mean value of the sum of attitude-related questions were considered to have a positive attitude, while those with scores less than the mean value of the sum of attitude-related questions were considered to have a negative attitude.Citation18 Male involvement in immunization: Males who ordered the mother to take the child for immunization, provided money for transportation for the child to access immunization services, or accompanied the mother when the child accessed immunization services. Male partners or fathers of children who participated in at least one of the above three activities were coded as 1, while those who did not participate in at least one of the three activities were coded as 0.Citation8
Data processing and analysis
The information was coded and entered into Epi Data version 3.1 before being exported to SPSS version 23.0 for analysis. The results of descriptive statistics were presented in tables and graphs after computing summary statistics such as frequency, mean, percentages, and standard deviations. Chi-square testing was used to see if there was a statistically significant difference in full vaccination coverage between urban and rural children. The analysis was done separately for urban and rural areas. The relationship between the full immunization coverage and independent variables like sociodemographic variables, knowledge, attitude, service availability, reproductive – related variables and male involvement was investigated using binary logistic regression. The independent effect of each variable on the dependent variable was determined using multivariable logistic regression. The association’s final results were presented using the AOR with a 95% level of confidence and a p-value <0.05.
Results
Socio-demographic characteristics of caregivers and children
This study included 635 caregivers of children aged 12 to 23 months (292 from the town and 343 from the rural areas), yielding a response rate of 98.4%. The mean (± SD) age of respondents was 29.38 ± 7.25 years. The majority of respondents were between the ages of 35 and 49, accounting for 258 (40.6%) of total respondents and 187 (54.5%) of rural residents, respectively. The majority of respondents (272(79.3%) were farmers in rural areas, compared to 188 (64.2%) who were government employees in urban areas. Five hundred ten (80.3%) of respondents were married (87.8% in rural and 71.6%) in urban areas. In both rural and urban respondents, the majority of respondents had less than five children, accounting for 415 (65.4%), 186 (54.2%), and 229 (78.4%) respectively. A total of 396 (62.4%) of caregivers’ partner participated in the EPI service ().
Table 1. Sociodemographic characteristics of caregivers and children with Chi Square test result in southwest Ethiopia (N = 635).
Knowledge and attitude of caregivers of the child aged 12–23 months toward immunization
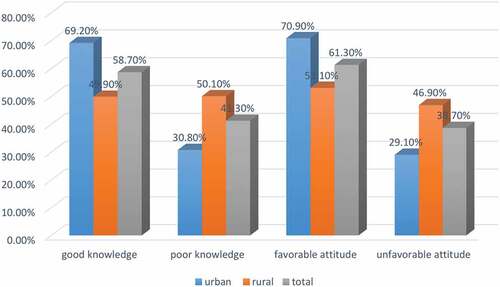
More than half of the total respondents and those living in urban areas had good knowledge and a favorable attitude toward the immunization of their child ().
Reproductive history characteristics of caregivers of children
Overall, 483 (76.1%) of mothers of the child had an ANC visit during their previous pregnancy, with 79.8% and 72.9% being urban and rural residents, respectively. Four hundred and seventy-nine (75.4%) of mothers had their last pregnancy delivered in a health facility, with 87.3% and 65.3% of those being urban and rural residents, respectively ().
Table 2. Reproductive history characteristics of caregivers of the child aged 12–23 months with Chi Square test result in southwest Ethiopia (N = 635).
Vaccination service availability and access
Four hundred sixty-four caregivers (73.1%) were able to access a health facility for immunization services, with 387 (60.9%), 42 (6.6%), 124 (19.5%), and 82 (12.9%) being health center, hospital, health post, and private clinic, respectively. Two hundred ninety-seven caregivers (46.8%) walked to the health facility in less than 30 minutes, while two hundred and two (31.8%) walked for more than 60 minutes. There were differences in health facility accessibility between urban and rural residents: 257 (88%) of urban residents accessed in less than 30 minutes on foot, whereas 202 (58.9%) of rural residents accessed after walking for more than 60 minutes. COVID 19 infection is feared by more than half of all respondents (56.4%) and the majority of urban residents (87%) at health institutions ().
Table 3. Vaccination service availability and accessibility with Chi Square test result in southwest Ethiopia (N = 635).
Vaccination coverage of children aged 12–23 months
Full vaccination, partial vaccination, and non-vaccination coverage were 66.1%, 24.1%, and 9.8%, respectively, among the 635 respondents. About 74.3%, 19.9%, 5.8% in urban and 59.2%, 27.7%, and 13.1% of children in rural areas were fully, partially, and not vaccinated, respectively. Overall, 66.1% (95% CI: 62.4% −69.8%) of children aged 12 to 23 months were fully vaccinated, while 33.9% were not fully vaccinated. The full vaccination coverage documented by card, however, was only 162 (25.5%). On the other hand, 74.5% of full-immunized children had evidence of vaccination supported by the card plus recall. Between urban and rural children aged 12–23 months, there was a significant difference in full vaccination coverage (χ2 = 16.126, P = .000). The most common reason for not fully vaccinating was a lack of awareness of the vaccination time and site, which accounted 52 (24.2%), and the vaccination site being too far away, which accounted 70 (32.6%) ().
Table 4. Urban, rural fully vaccination coverage of children aged 12–23 months and reasons for not fully vaccination with Chi Square test result in southwest Ethiopia (N = 635).
Vaccination coverage for each vaccine type
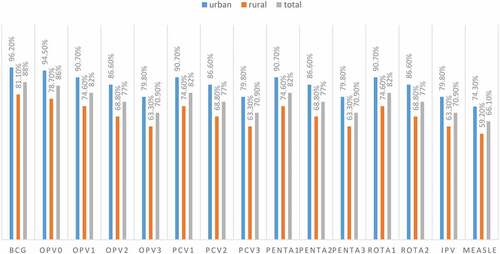
Around 559 (88%) of all children received BCG, and the majority of them (86%) received OPV0, with the percentage of all other vaccines decreasing. PCV1, PENTA2, OPV3, and measles vaccination rates were 521 (82%), 489 (77%), 450 (70.9%), and 420 (66.1%), respectively ().
Determinants of fully vaccination coverage
Three different models were fitted to determine the determinants of full vaccination coverage. The first model was used to evaluate the general factors that influence vaccination coverage. Residence, wealth index, ANC follow-up, and fear of COVID 19 infection at the health institution were identified as significant determinants of full vaccination coverage among the entire children in multiple logistic regression analyses.
In comparison to rural children, urban children had a 3.102 times higher chance of being fully vaccinated [AOR = 3.102; 95% CI: 2.004, 6.865]. Families with a child aged 12–23 months who scored high on the wealth index were 1.102 times more likely than families with a low wealth index score to fully vaccinate their child [AOR = 1.102;95% CI: 1.006, 3.340]. Caregivers of children aged 12 to 23 months who did not fear COVID 19 infection at a health facility were 2.170 times more likely than those who were afraid of the infection to fully vaccinate their children[AOR = 2.170; 95%CI: 1.897, 5.880]. Caregivers of children aged 12–23 months who had an ANC visit during their previous pregnancy were 1.230 times more likely than those who did not have an ANC visit to fully vaccinate their child[AOR = 1.230; 95%CI: 1.056, 2.287] ().
Table 5. Determinants of fully vaccination coverage among urban and rural children aged 12–23 months in southwest Ethiopia (N = 635).
Only urban children were included in the second model, and two variables were identified as significant determinants of full vaccination coverage: knowledge and delivery location.
Caregivers of children aged 12 to 23 months with good knowledge of child vaccination services were 2.501 times more likely than those with poor knowledge to fully vaccinate their child [AOR = 2.501; 95% CI: 1.890, 5.024]. Caregivers of children aged 12–23 months who had their current child delivered in a health facility were 1.430 times more likely than home deliveries to fully vaccinate their child [AOR = 1.430; 95% CI: 1.140, 4 .061] ().
Table 6. Determinants of fully vaccination coverage among urban children aged 12–23 months in southwest Ethiopia (N = 292).
The third model was created with rural children in mind, and two variables were found to be important determinants of full vaccination coverage: distance and male partner involvement.
Caregivers of children aged 12 to 23 months who expected to walk less than 30 minutes to reach a vaccination service were 2.440 times more likely to fully vaccinate their child than those who expected to walk more than 60 minutes [AOR = 2.440; 95% CI: 1.490, 5.688]. Caregivers who had a partner who was involved in child vaccination were 1.820 times more likely to fully vaccinate their children than those who had a partner who was not involved [AOR = 1.820; 95% CI: 1.192, 3 .103] ().
Table 7. Determinants of fully vaccination coverage among rural children aged 12–23 months in southwest Ethiopia (N = 343).
Discussions
In Ethiopia, there is a significant difference in the use of modern health services between urban and rural residents.Citation44 Inequalities in access to health services are a major source of vulnerability for residents, resulting in a variety of health issues.Citation45 The placement of one’s residence also appeared to be an important factor influencing healthcare-seeking behavior, which has a significant impact on community healthcare utilization.Citation46 The goal of this study was to compare fully vaccinated children aged 12–23 months in urban and rural settings, as well as their determinants.
Overall 66.1% (95%CI: 62.4%-69.8%) of children aged 12–23 months were fully vaccinated. This finding was comparable with studies done in Wonago (63.4%, Dessie (65.2%), and Pawi (65%).Citation21–23 The finding of this study was lower than WHO recommended 90% coverage and other studies done in South-south Nigeria (80.3%), Southwestern Nigeria(81.3%), Sekota (77.4%), Debre Markos (91.7%), Adis Ketema (72.4%), Sinana (76.8%), Dabat (81.7%), Areka (75.1%), Lay Armachiho (76%), Minjar Shenkora (75.6%), Arba Minch (73.2%), Tehuldere (83.1%), Wogera (76.1%), Asosa (71.7%).Citation5–8−Citation11–12-15–16-20–24-29–39-40 This disparity in immunization coverage could be due to disparities in access to vaccination services and community awareness of the importance of child immunization. The quality of vaccination services may influence the likelihood of immunization service use and, as a result, vaccination completion rates. It could also be due to differences in study periods and the number of vaccines included in Ethiopia’s current expanded immunization program, such as PCV, Rota, IPV, and measles 2. Differences in health-care systems between countries could also explain the observed disparity. However it was higher than studies done in Sub Saharan Africa (59.4%), Burkina Faso(50.2%), Ethiopia national level (24%-60%), Mecha (49.3%), Jigjiga (36.4%), East Gojjam (58.4%), Ambo (36%), Bench Maji (42.2%), Hosana (30.5%), East Harege(22.9%), Mekelle (51%), Afar zone 3(20.6%), Somalia region (41.1%).Citation9–10-13–14-17–19-Citation30–37-41–Citation42 This could be due to the government’s tireless efforts to achieve the sustainable development goal of lowering child mortality from vaccine-preventable diseases. Furthermore, most areas with low full immunization coverage may have weakened healthcare systems, resulting in low vaccine uptake. Furthermore, some regions, such as Afar and Somalia, have difficult-to-reach areas with nomadic and pastoralist residents who do not have permanent residences.
Prevalence of full vaccination was higher among children of urban residents than in rural children with a 15.10% (95%CI; 0.102–0.192) point estimate for the difference. The finding was consistent with studies done in Zimbabwe, South Nigeria, Sindh Pakistan, Peshawar, the national level of Ethiopia, Arba Minch, Jigjiga, Somalia region, and Lay Armachiho.Citation5,Citation14,Citation17,Citation27,Citation36,Citation39,Citation43,Citation47 However, the finding was in contrast to studies done in Pawi, Tehuledere, Ambo, and Mecha.Citation23,Citation28,Citation31 The higher coverage in urban areas could be attributed to the greater accessibility of health facilities to EPI, as well as differences in immunization awareness and health-seeking behavior.
In this study, residence, wealth index, ANC follows up and fear of COVID 19 infection at health institutions were found to be significantly associated with overall full vaccination at p < .05.
Urban children had a 3.102 times greater chance of being fully vaccinated than rural children. The finding was consistent with studies done at a national level in Ethiopia, Somalia region, Jigijiga, Mecha, and Lay Armachiho,Citation5,Citation14,Citation17,Citation18,Citation37 but in contrast to studies done in Pawi and Tehuledere.Citation23,Citation28 The disparities may be due to differences in modern health service utilization between urban and rural residents, with urban residents using modern health services more than rural residents.Citation45
Families of the child aged 12–23 months who had a score of high wealth index were 1.102 times more likely to fully vaccinate their child than a score of low wealth index families. The finding was consistent with studies done in SSA, Zimbabwe, the national level of Ethiopia, the Somalia region, and Sinana.Citation14,Citation15,Citation37,Citation41,Citation43 This could be attributed to differences in childcare practices, improved health-seeking behavior, and improved health-care access.
Caregivers of the child aged 12–23 months who did not fear COVID 19 infection at a health institution were 2.170 times more likely to fully vaccinate their child than those who had fear of the infection. The finding was consistent with studies done in Assosa town.Citation29 In fact, in the early stages of the COVID-19 pandemic, this could be due to the risk of infection and the need to keep a physical distance. Stay-at-home and social distancing strategies reduce children’s access to routine immunization services, putting them at risk for vaccine-preventable diseases and their complications.Citation48
Caregivers of the child aged 12–23 months who had an ANC visit for the last pregnancy were 1.230 times more likely to fully vaccinate their child than those who did not have an ANC visit. The finding was consistent with studies done in Zimbabwe, the national level of Ethiopia, Wogera, Ambo, Sekota, Wonago, Dessie, and Asossa.Citation8,Citation12,Citation21,Citation22,Citation29,Citation31,Citation37,Citation43 This could be due to the fact that women who attend ANC follow-up may receive postnatal counseling on child immunization.
In this study, knowledge, and place of delivery were found to be significantly associated with full vaccination in an urban area at p < 0.05.
Caregivers of the child aged 12–23 months who had good knowledge about child vaccination services were 2.501 times more likely to fully vaccinate their child than those who had poor knowledge. The finding was consistent with studies done in Southern Nigeria, Sindh Pakistan, Sekota, Somalia region, Ambo, Arba Minch, Bench Maji, Wonago, Mecha, Lay Armachiho, and Dessie.Citation5,Citation12,Citation14,Citation18,Citation19,Citation21,Citation22,Citation27,Citation31,Citation39,Citation43 This could be because mothers have a better understanding of vaccine-preventable diseases, immunization schedules, and the importance of vaccination, which may increase their motivation to immunize their children.
Caregivers of the child aged 12–23 months who delivered their current child in a health institution were 1.430 times more likely to fully vaccinate their child than home deliveries. The finding was consistent with studies done in Zimbabwe, Southern Nigeria, Somalia region, Ambo, Arba Minch, Jigijiga, Bench Maji, Mecha, and Dessie.Citation12–14–Citation17–19–Citation22–27–Citation31–36–Citation39–43 This could be because some vaccines, such as BCG and OPV 0, are frequently administered immediately after birth in health care facilities.
In this study, distance and male partner involvement were found to be significantly associated with full vaccination in a rural area at p < 0.05.
Caregivers of the child aged 12–23 months who expected to walk on foot for less than 30 minutes to access vaccination service were 2.440 times more likely to full vaccinate their child than walked on foot for more than 60 minutes. The finding was in line with studies done in Sekota, Somalia region, Dabat, Debre Markos, Sinana and Minjar Shenkora.Citation12,Citation14–16,Citation20,Citation25 This is because the time spent traveling to the vaccination site costs mothers/caregivers a high opportunity cost by necessitating multiple visits, particularly when vaccine vials for a small number of children were not opened.Citation49 If mothers/caregivers traveled a long distance and were unable to obtain the vaccination service, they may be forced to default their children’s immunization.
Caregivers whose partners were involved in child vaccination were 1.820 times more likely to vaccinate their child than poor involvement. The finding was in line with a study done in Wogera.Citation5 According to research, the presence of a male partner with a positive attitude toward health and vaccination contributed to immunization coverage.Citation5 In most cases, women have taken on childcare responsibilities, including visits to immunization sites. If the child develops any immunization-related side effects, the husband would chastise the woman for vaccinating the child. If the husband was involved in child immunization, he would be more understanding and accepting of the side effects as well as the importance of vaccination.Citation50 We live in a society where men have an impact on the general activities and well-being of their families and loved ones. In most cases, women must obtain male consent before requesting child immunization.
Strength and limitations of the study
Strengths
This is a valuable community based study to compare the full immunization coverage of children aged 12–23 months in urban and rural settings and may have important policy implications for the further improvement of immunization services. The study addresses the effect of socio demographic characteristics, accessibility and availability of service, male partner involvement, health professionals’ availability on immunization sites, social organizations like the health development army and fear of COVID 19 infection at a health institution on the full immunization of children. In Ethiopia, the majority of the population lives in rural areas and our study included many participants from a rural areas.
Limitations
Since the study was also done in a single portion of the country, it may not reflect the whole picture of vaccination coverage in the whole country. Questions about the immunization history of children in the absence of vaccination cards require appropriate recall of events. This might introduce recall bias in the data obtained. This might over or under estimate the coverage level of the service. However to reduce recall bias different strategies were informed by the interviewer are the sites of vaccination given (oral, injection and scar) and at what age the child should receive specific antigen, these strategies are used to assess immunization coverage. In addition, reports of vaccination may have been exaggerated because of its social desirability. The cross-sectional design confers some difficulty in demonstrating temporality between full vaccination status and some independent variables such as the mother’s educational level or knowledge of the benefits of immunization. This limits the extent to which a cause-effect relationship, or lack of it, could be assigned to the associations explored.
Conclusions and recommendations
Vaccination coverage was higher in urban than in rural areas, but it is still far below the WHO recommended target. Residence, wealth index, ANC follow-up, and fear of COVID 19 infection at a health institution were found to be significantly associated with overall full vaccination. Knowledge and place of delivery were found to be significantly associated with full vaccination in urban areas. Distance and male partner involvement were found to be significantly associated with full vaccination in rural areas. As a result, interventions will be made to improve coverage, particularly by utilizing the identified factors such as improving ANC service and promoting institutional delivery, promoting male involvement, and health education and communication, which are critical for alleviating poor knowledge about child immunization.
Authors’ contribution
GA, conceived the research idea and developed the proposal, participated in data collection and analysis, and drafted the paper. MM & AS enriched the concept and proposal write-up participated in data analysis and drafting of the manuscript, and critically reviewed the manuscript. All the authors read and approved the final manuscript.
Abbreviations
AOR Adjusted Odds Ratio
ANC Antenatal Care
BCG Bacille Calmette-Guérin
COVID 19 Corona Virus Diseases 2019
COR Crude Odds Ratio
CI Confidence
EDHS Ethiopian Demographic and Health Survey
EPI Expanded Program of Immunization
GVAP Global Vaccine Action Plan
HSDP-IV Health Sector Development Plan IV
IPV Injectable Polio Vaccine
OPV Oral Polio Vaccine
PCV Pneumococcal Conjugate Vaccine
SD Standard Deviation
SPSS Statistical Package for Social Science
SSA Sub Saharan Africa
TT Tetanus Toxoid
WHO World Health Organization
Ethical approval and consent from the participant
The Institutional Review Board (IRB) of Wolaita Sodo University approved all experimental protocols. All methods were carried out in accordance with relevant guidelines and regulations. Before the interview, informed written consent was obtained from all mothers or caregivers of children aged 12–23 months. Participants were informed that they could skip any questions that they didn’t want to answer completely or partially and that they could stop at any time. By not recording identifying information, the confidentiality of the individual information was ensured.
Acknowledgment
I want to thank my colleagues for giving suggestions. I am very grateful to the Wolaita Sodo University College of Health Science and Medicine for giving me the ethical clearance. We are also indebted to thank the respective universities and study participants for their cooperation during data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- UN Inter-agency Group for Child Mortality Estimation. Levels & trends in child mortality; 2021. https://reliefweb.int/sites/reliefweb.int/files/resources/Levels-and-trends-in-child-mortality-IGME-English_2021.pdf
- Who U. World Bank. State of the world’s vaccines and immunization, Geneva. World Health Organization; 2009:1–12.
- WHO. The immunization coverage fact sheet. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage
- WHO Immunization Agenda 2030. A global strategy to leave no one behind. https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030
- Kassahun MB, Biks GA, Teferra AS. Level of immunization coverage and associated factors among children aged 12–23 months in Lay Armachiho District, North Gondar Zone, Northwest Ethiopia: a community-based cross-sectional study. BMC Res Notes. 2015;8(1):239. doi:10.1186/s13104-015-1192-y.
- Ethiopia National Expanded Programme on Immunization. Comprehensive multi-year plan 2016 - 2020, Federal Ministry of Health (FMoH). Ethiopia: Addis Ababa; 2016.
- Negussie A, Kassahun W, Assegid S, Hagan AK. Factors associated with incomplete childhood immunization in Arbegona district, southern Ethiopia: a case-control study. BMC Public Health. 2016;16(1):27. doi:10.1186/s12889-015-2678-1.
- Gelagay AA, Geremew AB, Teklu A, Mekonnen ZA, Gera R, Ba-Nguz A, and Tilahun B. Full immunization coverage and its determinants among children aged 12-23 months in Wogera district, Northwest Ethiopia. Ethiopian J Health Dev. 2021 Nov 25;35(3):21–23.
- Central Statistical Agency (CSA) of Ethiopia. Ethiopian demographic and health survey: key indicators report. Ethiopia: Addis Ababa; 2016.
- Central Statistical Agency(CSA) of Ethiopia. Ethiopian demographic and health survey. Ethiopia: Addis Ababa ; 2011.
- World Health Organization(WHO). Global vaccine action plan: regional vaccine action plans progress reports. Tech Rep. 2016;5–10.
- Dadi AF. Full immunization coverage and associated factors among children aged 12-23 months in a hard-to-reach areas of Ethiopia; 2019.
- Ethiopian Public Health Institute (EPHI). Central Statistical Agency (CSA). Federal Ministry of Health (FMoH). Mini demographic and health survey; 2019. https://microdata.worldbank.org/index.php/catalog/3946/related-materials
- Yadita ZS, Ayehubizu LM, Olorunsaiye CZ. Full immunization coverage and associated factors among children aged 12–23 months in Somali Region, Eastern Ethiopia. PloS One. Dec 7, 2021;16(12):e0260258. doi:10.1371/journal.pone.0260258.
- Dechasa Ela W, Dechasa W. An assessment of child immunization coverage and its determinants in Sinana District. Southeast Ethiopia BMC Pediatr. 2015;15(31). doi:10.1186/s12887-015-0345-4.
- Dilie Tga A. Vaccination coverage and associated factors among children aged 12–23 months in Debre Markos Town, Amhara Regional State. Ethiopia Adv Public Health. 2017;4–5.
- Mohamud AN, Feleke A, Worku W, Kifle M, Sharma HR. Immunization coverage of 12–23 months old children and associated factors in Jigjiga District, Somali National Regional State, Ethiopia. BMC Public Health. 2014;14(1):1–9. doi:10.1186/1471-2458-14-865.
- Ayal Debie BT. Assessment of full vaccination coverage and associated factors among children aged 12-23 months in Mecha district, North West Ethiopia: a cross-sectional study. Sci J Public Health. 2014;2:344–346.
- Asrat Meleko MG, and Birhanu F. Assessment of child immunization coverage and associated factors with full vaccination among children aged 12–23 months at Mizan Aman Town, Bench Maji Zone, Southwest Ethiopia. Int J Pediatr. 2017;11.
- Alemayehu Gonie Mekonnen ADBaETA. Immunization coverage of 12–23 months old children and its associated factors in Minjar-Shenkora district, Ethiopia: a community-based study. BMC Pediatr. 2019;19(198):1–8.
- Hailu S, Astatkie A, Johansson KA, Lindtjørn B, Angelillo IF. Low immunization coverage in Wonago district, southern Ethiopia: a community-based cross-sectional study. PloS One. 2019;14(7):e0220144. doi:10.1371/journal.pone.0220144.
- Lake MW, Boulanger L, Wasswa P, Merbratu M, Fenta A. Factors for low routine immunization performance: a community-based cross-sectional study in Dessie town, south Wollo zone, Ethiopia, 2014. Adv Appl Sci. 2016;1:7–17.
- Gelaye SS, Snr MK, Baraki AG. Rural vaccination coverage among children aged 12–23 months was higher than the urban counterparts: a comparative cross-sectional study in Pawi District, Ethiopia. Pediatr Health Med Ther. 2021;12:119. doi:10.2147/PHMT.S299064.
- Tolera D. Assessment of magnitude and factors associated with full immunization coverage in children aged 12–23 months in Addis Ketema sub-city, Addis Ababa, Ethiopia. Addis Ababa: Institutional Repository; 2014.
- Okwaraji YB, Mulholland K, Schellenberg J, Andarge G, Admassu M, Edmond KM. The association between travel time to health facilities and childhood vaccine coverage in rural Ethiopia. A community-based cross-sectional study. BMC Public Health. 2012;12(1):476. doi:10.1186/1471-2458-12-476.
- Fite RO, Hailu LD. Immunization coverage of 12 to 23 months old children in Ethiopia; 2019.
- Animaw W, Taye W, Merdekios B, Tilahun M, Ayele G. Expanded program of immunization coverage and associated factors among children age 12–23 months in Arba Minch town and Zuria District, southern Ethiopia, 2013. BMC Public Health. 2014;14(1):464. doi:10.1186/1471-2458-14-464.
- Ebrahim Y, Salgedo WB. Childhood immunization coverage in Tehulederie district, northeast of Ethiopia: a community based cross-sectional study. Int J Curr Res. 2015;7:20234–20240.
- Jimma MS, GebreEyesus FA, Chanie ES, Delelegn MW. Full vaccination coverage and associated factors among 12-to-23-month children at Assosa Town, Western Ethiopia, 2020. Pediatr Health Med Ther. 2021;12:279. doi:10.2147/PHMT.S306475.
- Tesfaye TD, Temesgen WA, Kasa AS. Vaccination coverage and associated factors among children aged 12–23 months in Northwest Ethiopia. Hum Vaccines Immunother. 2018;14(10):2348–2354. doi:10.1080/21645515.2018.1502528.
- Etana B, Deressa W. Factors associated with complete immunization coverage in children aged 12–23 months in ambo Woreda, Central Ethiopia. BMC Public Health. 2012;12(1):566. doi:10.1186/1471-2458-12-566.
- Ayano B. Factors affecting fully immunization status of children aged 12–23 months in Hosanna Town, South Ethiopia. J Pregnancy Child Health. 2015;2:185.
- Mohammed H, Atomsa A. Assessment of child immunization coverage and associated factors in Oromia regional state, eastern Ethiopia. Sci Technol Arts Res J. 2013;2(1):36–41. doi:10.4314/star.v2i1.98842.
- Kidane T, Tekie M. Factors influencing child immunization coverage in a rural district of Ethiopia, 2000. Ethiop J Health Dev. 2003;17:105–110.
- Beyene E, Worku A, Bisrat F, Fantahun M. Factors associated with immunization coverage among children age 12-23 months: the case of zone 3, Afar regional state, Ethiopia. Ethiop Med J. 2013;51 Suppl 1:41–50.
- Asresie MB. Determinants of full immunization among children aged 12-23 months in Ethiopia. Further analysis of the 2016 Ethiopian demographic health survey.
- Ketema DB, Assemie MA, Alamneh AA, Alene M, Chane KY, Alamneh YM, Birhanu MY, Alebel A. Full vaccination coverage among children aged 12–23 months in Ethiopia: a systematic review and meta-analysis. BMC Public Health. 2020;20(1):1. doi:10.1186/s12889-020-08940-x.
- Sibeudu FT, Uzochukwu BS, and Onwujekwe OE. Rural-Urban comparison of routine immunization utilization and its determinants in communities in Anambra States, Nigeria. SAGE Open Med. 2019;7:1–8.
- Adeleye OA, Mokogwu N. Determinants of full vaccination status in a rural community with accessible vaccination services in South-South Nigeria. J Community Med Primary Health Care. 2015;27:12–19.
- Ijarotimi IT, Fatiregun AA, Adebiyi OA, Ilesanmi OS, Ajumobi O. Urban-Rural differences in immunization status and associated demographic factors among children 12-59 months in a southwestern state, Nigeria. PloS One. 2018 Nov 5;13(11):e0206086. doi:10.1371/journal.pone.0206086.
- Fenta SM, Biresaw HB, Fentaw KD, Gebremichael SG. Determinants of full childhood immunization among children aged 12–23 months in sub-Saharan Africa: a multilevel analysis using demographic and health survey data. Trop Med Health. 2021 ;49(1):1–2. doi:10.1186/s41182-021-00319-x.
- Sanou A, Simboro S, Kouyaté B, Dugas M, Graham J, Bibeau G. Assessment of factors associated with complete immunization coverage in children aged 12-23 months: a cross-sectional study in Nouna district, Burkina Faso. BMC Int Health Hum Rights. 2009;9(1):1–5. doi:10.1186/1472-698X-9-S1-S10.
- Masood T, Mehraj J, Guriro S, Shaikh MA. Factors affecting full immunization coverage among children aged 12-23 months in urban and rural areas of Sindh. Indian J Sci Technol. 2020 Mar 20;13(12):1283–1292. doi:10.17485/IJST/v13i12.149859.
- Bazie GW, Adimassie MT. Modern health services utilization and associated factors in North-East Ethiopia. PloS One. 2017 Sep 26;12(9):e0185381. doi:10.1371/journal.pone.0185381.
- Guo B, Xie X, Wu Q, Zhang X, Cheng H, Tao S, and Quan H. Inequality in the health services utilization in rural and urban china: a horizontal inequality analysis. Medicine. 2020;99(2):1–7.
- Sisay S, Endalew G, Hadgu G. Assessment of mothers/caregivers healthcare-seeking behavior for childhood illness in rural Ensaro District, north Shoa zone, Amhara region, Ethiopia 2014. Glob J Life Sci Biol Res. 2015;1:15.
- Naeem M, Khan MZ, Adil M, Abbas SH, Khan MU, Khan A, Naz SM. Inequity in childhood immunization between urban and rural areas of Peshawar. J Ayub Med Coll Abbottabad. 2011 Sep 1;23(3):134–137.
- Bramer CA, Kimmins LM, Swanson R, Kuo J, Vranesich P, Jacques‐carroll LA, Shen AK. Decline in child vaccination coverage during the COVID-19 pandemic—Michigan care improvement registry, May 2016-May 2020. Am J Transplant. 2020;20(7):1930. doi:10.1111/ajt.16112.
- Aregawi HG, Gebrehiwot TG, Abebe YG, Meles KG, Wuneh AD, Ortiz JR. Determinants of defaulting from completion of child immunization in Laelay Adiabo District, Tigray region, northern Ethiopia: a case-control study. PloS One. 2017;12(9):e0185533. doi:10.1371/journal.pone.0185533.
- Sturm LA, Mays RM, Zimet GD. Parental beliefs and decision making about child and adolescent immunization: from polio to sexually transmitted infections. J Dev Behav Pediatr. 2005;26(6):441–452. doi:10.1097/00004703-200512000-00009.