ABSTRACT
The aim of this study was to establish whether a universal pneumococcal vaccination for older adults in Norway is likely to be cost-effective from the perspective of the health care provider. A decision tree model developed by the Public Health Agency of Sweden was adopted to the Norwegian setting. Two cohorts, consisting of 65-year-olds and 75-year-olds grouped into vaccinated and unvaccinated, were followed over a 5-year time horizon. In the base case, the 23-valent polysaccharide vaccine (PPV23) was used while the 13-valent pneumococcal conjugate vaccine (PCV13) was included in scenario analyses only. The costs and health benefits (measured in quality adjusted life years (QALY) gained) were compared in the two cohorts between the vaccinated and unvaccinated groups. The impact of indirect effects of the vaccine, such as herd immunity and serotype replacement, were not investigated. The relative importance of change in price was assessed by performing one-way sensitivity analyses. Under base-case assumptions, the programme for the 75-year-old cohort is expected to be cost-effective from the health care perspective at the current pharmacy purchasing price and at 75% vaccination coverage as it falls below the lower end of the cost-effectiveness threshold range (NOK 9467/EUR 964). In comparison, for the 65-year-old cohort the cost per QALY gained is approximately NOK 780 206 (EUR 79 451) under the base-case assumptions which falls within the acceptable ranges in a Norwegian context for both the 65- and 75-year-old cohorts. There is no exact cost-effectiveness threshold in Norway. However, introducing a vaccination programme against pneumococcal disease for 65-year-olds and 75-year-olds in Norway is likely to fall within the acceptable cost-effectiveness threshold range.
Introduction
Pneumococcal infections are caused by Streptococcus pneumoniae, commonly referred to as pneumococcus.Citation1 S pneumoniae is the most common cause of community-acquired pneumonia (CAP), bacterial meningitis, bacteremia, and otitis media.Citation2 Additionally, it is an important cause of sinusitis, septic arthritis, osteomyelitis, peritonitis, and endocarditis.Citation3
Following the introduction of the conjugate pneumococcal vaccine (PCV) in the Norwegian childhood immunization programme, we observed substantial reductions in severe invasive pneumococcal disease (IPD) and pneumonia caused by vaccine serotypes in children targeted for vaccination, as well as in unvaccinated adults through indirect herd protection. However, the burden of pneumococcal disease remains considerable in Norway, especially among the older adults and clinical risk groupsCitation4 due to increases in IPD incidence caused by replacement with non-vaccine serotypes. In Norway, after 2016, a declining incidence of IPD caused by both PCV13 (13-valent pneumococcal conjugate vaccine) and non-PCV13 IPD was observed, though the proportion of non-PCV13 serotypes is increasing compared to PCV13 serotypes.Citation4
In Norway, the 23-valent polysaccharide vaccine (PPV23) is not implemented in a universal pneumococcal vaccination programme for older adults. However, all persons 65 years and older, as well as medical risk groups are recommended pneumococcal vaccination.Citation5 PPV23 is currently the recommended vaccine for medical risk groups and the older adults in Norway. Only a few, selected high-risk groups are recommended PCV13 in series with PPV23.Citation6 The current uptake of pneumococcal vaccination in Norway is assumed to be suboptimal in adultsat approximately 15%.Citation4 Currently vaccination outside the childhood immunization programme is only financed for selected medical high-risk groups.Citation7 In Denmark, a pneumococcal vaccination programme has been administered free of charge for persons aged 65 years and older, as well as for high-risk groups since 2020.Citation8 In Sweden, people with certain underlying diseases, as well as those who are 75 years-of-age and older, will be offered vaccination against pneumococcal infections within a free-of-charge national vaccination programme from the autumn of 2022.Citation9
The continued dissemination of non-vaccine serotypes, as well as the low uptake of pneumococcal vaccination warrants the need to inform health policy makers on the effect and cost-effectiveness of introducing 23-valent polysaccharide vaccine (PPV23) in a universal vaccination programme for older adults in Norway. This study addresses the issue of whether universal vaccination for older adults with PPV23 would be a cost-effective policy from the health care provider perspective. The analysis takes into consideration the uncertainties related to the variation in the vaccine price and coverage but does not consider the potential for indirect protection (herd immunity) among unvaccinated individuals as well as serotype replacement effects in the whole population.
Methods
Decision tree model
A deterministic cohort decision-tree model developed at the Public Health Agency of Sweden was adapted to the Norwegian setting. The model is described in detail elsewhere.Citation10 A modified schematic from the Swedish analysis using the same model is presented in . The base case analysis measures the impact of vaccinating with PPV23 for a hypothetical 65-year-old and 75-year-old cohort in Norway compared to no vaccination. The 13-valent conjugate vaccine (PCV13) was only included in a scenario analysis. The model was constructed to follow a hypothetical cohort of 65-year-olds and 75-year-olds over a five-year time horizon. A cohort size of 55,614 individuals was used for the 65-year-old cohort while a cohort size of 31,925 individuals was used for the 75-year-old cohort. These cohort sizes were based on population data from Statistics Norway from 2015. The cycle length was set to one year. Individuals enter the model in a susceptible state and then progress depending on their risk of disease, vaccination coverage and vaccination effectiveness.
Figure 1. Simplified schematic overview of the decision tree model.
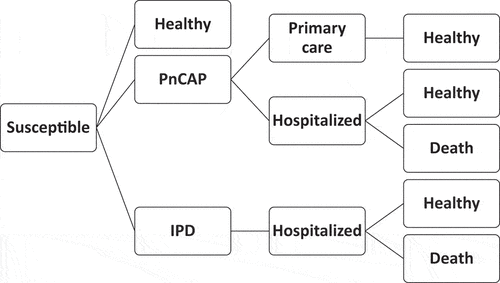
The model was developed in Excel (2016) software. A collaboration working to assess the impact of pneumococcal vaccination on older adults was previously established among the Nordic countries. The work included a systematic literature review,Citation11 and it was agreed to strive to re-use models and estimates for health economic evaluations where appropriate. In line with the objective of the Nordic collaboration, the Swedish model was chosen.
The number of quality-adjusted life-year (QALY) gained from the vaccination programme is used as the primary outcome measure for the programme. This is compared to its net cost, which is the additional cost of vaccination minus the expected savings from the programme in terms of reduced use of health care resources.
Pneumococcal infection can lead to a number of outcomes such as invasive disease, pneumonia, ear infections, sinusitis, bronchitis, arthritis, conjunctivitis, and peritonitis. However, the model only considers invasive pneumococcal disease (IPD) and noninvasive pneumococcal community-acquired pneumonia (PnCAP) as randomized and non-randomized observational studies regarding vaccine efficacy are available. All IPD cases were assumed to require hospital admission (100%) while PnCAP cases were assumed to require either hospital admission (25%) or general practitioners (GP) consultation (75%). These assumptions were made in collaboration with clinical experts.
Future benefits and costs were discounted according to the Norwegian guidelines for single technology assessment (STA) at 4% per annum.Citation12 In the base-case analysis, we assumed vaccination coverage of 75% among both the 65- and 75-year-olds cohorts as this is the target vaccination coverage.
The impact of herd protection, (i.e., changes in disease incidence among unvaccinated individuals), was not considered in this work. Cross-protection (i.e., the protection conferred by a serotype of pneumococci that prevents infection by a closely related serotype of pneumococci) or serotype replacement (i.e., the resistance to sub-types of serotypes if the frequency of a sub-type of serotype declines due to high levels of immunity allowing other serotypes to replace it) were not considered in the analysis.Citation13 Serotype distribution that may be expected to occur after the introduction of pneumococcal vaccination was, however, considered in the base-case analysis. The impact of changes in vaccine price, vaccination coverage, and inpatient PnCAP incidence were further investigated in one-way sensitivity analyses.
Epidemiological data
No comprehensive community acquired pneumonia (CAP) outpatient data was available from Norwegian sources.Citation14,Citation15 We assumed that the Swedish register data for outpatient CAP would be similar enough to the Norwegian setting to justify the use of the Swedish dataset.Citation15 As such, for the base-case analysis the age-specific incidence rates of CAP outpatient for the 65- and 75- year-old cohorts were derived by extracting the data from Swedish register sourcesCitation16 and used in line with the methodology in the Swedish model. In the Swedish study CAP was defined as a first-listed discharge diagnosis of pneumonia, or first-listed diagnosis of meningitis, septicemia, or empyema in addition to a pneumonia diagnosis. Analyses were restricted to patients without previous hospital care during the last 30 days to restrict episodes to CAP. The share of CAP which is caused by Streptococcus pneumoniae was estimated as 9% for outpatient CAP based on Leven et al. 2018.Citation17 The model only included the share of CAP that was estimated to be due to pneumococcal infection, i.e. noninvasive pneumococcal community acquired pneumonia (PnCAP).
CAP inpatient estimates were based on hospital discharge data from the Norwegian Patient Register (NPR).Citation18 CAP was defined as a first-listed discharge diagnosis of pneumonia according to the listed ICD10-codes. Episodes per person were defined as one or several admissions for CAP within the same 30 days. Further details and description of the data compilation can be found elsewhereCitation18 In line with the Swedish study, we assumed that 30% of the cases would be caused by pneumococci.Citation19 Norwegian CAP data including only noninvasive episodes from 2015 was used to estimate the incidence of PnCAP. For the 65-year-old cohort the incidence (per 100.000) was estimated as 815 × 0.3 = 244 and for the 75-year-old cohort the incidence (per 100.000) was estimated as 1770 × 0.3 = 531.
The age-specific incidence of IPD cases per 100.000 population from the Norwegian Surveillance System for Communicable Diseases (MSIS) is based on average estimates over a five-year period from 2015 to 2019.Citation20 There is variation in the estimates over time and, as such, we think that using average estimates over a five-year period is more representative than using estimates from a single year. MSIS is the most reliable data source on IPD in Norway, as detailed information on laboratory detection of S. pneumoniae from sterile area by isolation, nucleic acid, or antigen test (not urine) is available. The epidemiological parameters used are presented in .
Table 1. Annual incidence of invasive pneumococcal disease (IPD), noninvasive pneumococcal community acquired pneumonia (PnCAP) parameters for a hypothetical cohort of 65 year- and 75-year-olds over a five-year time horizon (year since vaccination).
Vaccine effectiveness (VE)
The vaccine effectiveness (VE) estimates against pneumococcal disease used in the model for the 65- and 75- year-old cohorts are shown in . These estimates were based on a combination of data sources which are described in more detail in the sub-sections below. PPV23 was used in the base-case analysis, while PCV13 was only included in a scenario analysis.
Table 2. Vaccine effectiveness (VE) on invasive pneumococcal disease (IPD) and noninvasive pneumococcal community-acquired pneumonia (PnCAP) applied in the Norwegian setting*, per year since vaccination, vaccine type; 23-valent polysaccharide vaccine (PPV23) and 13-valent pneumococcal conjugate vaccine (PCV13), and age group.
Table 3. Overview of model input parameters.
Table 4. Estimated number of Norwegian cases of (invasive pneumococcal disease (IPD) and noninvasive pneumococcal community-acquired pneumonia (PnCAP) in the epidemiological model, with and without vaccination with 23-valent polysaccharide vaccine (PVV23), depending on age and year after vaccination, given the input parameters in , and 75% vaccination coverage.
Serotype distribution
Norwegian national data on IPD from 2017 indicates that 127 (23%) of the isolates belonged to serotypes included in PCV13, and 390 (70%) of the isolates belonged to serotypes included in PPV23. In the age group ≥65 years, 226 (68%) of the cases were caused by serotypes included in PPV23, and 74 (22%) of the cases were caused by serotypes included in PCV13.Citation21 We assumed the same serotype distribution for pneumococcal pneumonia.
PPV23
No comprehensive data from Norway was available for the vaccine effectiveness for the 65-year-old cohort. Thus, we applied the same data as in the study by Wolff et al. Estimates of the vaccine effectiveness against IPD for PPV23 following the first year after vaccination were extracted from a review by Kraicer-Melamed et al.Citation22 and a study by Kim et al.Citation23 To obtain the vaccine effectiveness against IPD for Norway, the adjusted PPV23 serotype-specific vaccine effectiveness in the 75-year-old cohort was estimated to be 69.9%. This was then multiplied by the share of IPD that is vaccine type-specific for Norway which is 70%.Citation21 English data from a study by Djennad et al.Citation24 was used to estimate vaccine effectiveness against IPD in the years 2–5 following vaccination. Similar calculations were applied to estimate the vaccine effectiveness for PPV23 against PnCAP in the 65-year-old cohort. For the first year following vaccination, the estimated vaccine effectiveness of 38.9% was extracted from a Japanese study by Suzuki et al.Citation19 The 38.9% estimate was multiplied by 70% to obtain the estimate of 28%. To obtain the vaccine effectiveness against CAP for the years 2–5 following vaccination similar calculations were conducted ().
No comprehensive data from Norway was available for the vaccine effectiveness for the 75-year-old cohort. Thus, we applied the same data from Djennad et al.Citation24 to estimate the vaccine effectiveness against IPD in the years 1 to 5 following vaccination. Suzuki et al.Citation19 suggests that the adjusted PPV23 serotype-specific vaccine effectiveness against PnCAP is 28.2%. This estimate was multiplied by the share of PnCAP that is assumed to be vaccine type-specific in Norway to arrive at an estimate of vaccine effectiveness of PPV23 against PnCAP (20%).
PCV13
In line with the Swedish model, the protective effect against IPD and PnCAP for PCV13, for both the 65-year-old cohort and the 75-year-old cohort, was calculated in the same way as described above and data was collected from the CAPITA trial.Citation25,Citation26 Vaccine effectiveness against pneumococcal disease was based on intention to treat results of the CAPITA trials and for IPD was adjusted to reflect the serotype distribution in Norway.
Health outcomes
In compliance with the Norwegian guidelines for STA of pharmaceuticals, health-related quality of life (HRQoL) data was based on the standardized generic instrument EQ-5D.Citation12 The outcome measure QALY gained simultaneously captures gains from reduced morbidity (quality gains) and reduced mortality (quantity gains) and integrates these into a single measure. Reductions in health-related quality of life due to pneumococcal disease (calculated on a scale of 0–1 where 1 is equivalent to perfect health and 0 equates death) were derived from the literature and based on utility values from the Netherlands.Citation27 For IPD a utility value of 0,694 was used. For inpatient PnCAP a utility value of 0,694 was used while a utility value of 0,761 was used for outpatient PnCAP. No published age or gender-specific EQ-5D data from the general Norwegian population is available. The QALY weight among the healthy population was based on a utility value from a Swedish study (0,765).Citation28 Severe adverse events are rare and as such we did not consider these in the analysis.Citation29,Citation30 Therefore, no disutility values were applied in the model.
Cost estimates
Past cost-effectiveness analyses of vaccination programmes in NorwayCitation31–33 have used the maximum pharmacy retail price as listed by the Norwegian Medicines Agency.Citation34 The publicly listed maximum pharmacy retail price includes value-added tax (VAT) of 25% but in the Norwegian context it is recommended that the price used excludes VAT.Citation35 However, in this analysis we use the pharmacy purchasing price to estimate the cost of the PPV23 and PCV13 vaccines as this is more representative of current practice (as laid out in an internal communication from the Norwegian Institute of Public Health) (see ). The PCV13 vaccine dose was also set to the pharmacy purchasing price but only used in a scenario analysis. Vaccines implemented in national immunization programmes (NIP) in Norway are, however, acquired through tenders. The realistic vaccine price is, therefore, typically lower than the pharmacy purchasing price when included in a NIP. Based on experience with previous tenders for vaccines included in the Norwegian NIP, the rebate varies between 25–75%.Citation36 In compliance with the Norwegian guidelines for STA of pharmaceuticals,Citation12 the cost of administering a vaccine is calculated as the renumeration for fee-for-service per the GP Fees List Collective Agreement 2021–2022 (takst2ad)Citation37 multiplied by two (168 × 2 = 336 NOK/EUR 34). In line with the Norwegian guidelines for STA of pharmaceuticals,Citation12 an additional cost of 150 NOK (EUR 15) for a single subcutaneous injection is used per vaccine delivery. Thus, the total cost of one vaccination delivery per person includes the vaccine cost, the cost of a single subcutaneous injection and the renumeration of GP fee-for-service multiplied by two. A one-time cost of EUR 127,291 covering implementation and information costs for the first year of the programme was included. This estimate is based on the costing of other vaccination programmes (as laid out in an internal communication from the Norwegian Institute of Public Health).
The average case cost of a hospital admission for either IPD or PnCAP was derived from the Diagnosis Related Group (DRG) which was established based on the International Classification of Diseases (ICD) 10 diagnostic codes.Citation38 Each DRG code has a relative weight, which determines the reimbursement for that DRG. Each DRG weight represents the average case cost for cases in that DRG relative to the average case cost for all DRGs. The relative weight was multiplied by the unit cost for 2022 (NOK 47 742/EUR 4862).
In the Norwegian guideline for the management of more severe infections in the primary sector a Penicillin or Amoxicillin dosage of 1,3 g 4 times a day for 7 to 10 days is recommended.Citation39 The cost of antibiotic treatment is based on the official retail prices in Norway as given by the Norwegian Medicines Agency and calculated in the same way as above for the vaccine price. In the Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance from Norway report from 2018,Citation40 it is estimated that, in terms of the number of prescriptions per 1000 inhabitants, penicillin accounts for 25% while amoxicillin accounts for 75%. These estimates were used in the model. All cases which did not require hospitalization were assumed to require treatment with antibiotics.
Costs were measured in 2022 Norwegian kroner (NOK) and converted to € EUR using the average annual 2022 exchange rate (EUR1 = NOK9.820).Citation41
Sensitivity and scenario analyses
The most likely parameter values and assumptions were used in the base-case. However, several one-way sensitivity analyses were also performed looking at the effect of changing one parameter at a time. The rebate rates were varied in sensitivity analyses to detect the impact on the vaccine pharmacy purchasing price for PPV23 if the price was reduced by 25%, 50% and 75%, respectively. The share of PnCAP (inpatient) that is caused by S. pneumoniae was varied from 30% in the base case to 20% in a sensitivity analysis. The vaccination coverage was also varied from 75% to 50% in a sensitivity analysis. A scenario analysis in which the PCV13 vaccine price was used instead of the PPV23 vaccine price was performed, in line with the Swedish model. In addition, the impact on the vaccine pharmacy retail price for PCV13 if the price was reduced by 25%, 50% and 75%, respectively, was tested. The share of PnCAP (inpatient) that is caused by S. pneumoniae varied from 30% in the base case to 20% for the PCV13 vaccine.
Results
Base case
PPV23 vaccination would lead to a total decrease in the number of IPD cases by 30% and a decrease of 19% for the CAP cases among the 65-year-old cohort. Among the 75-year-old cohort the IPD cases would be reduced by 30% while the PnCAP cases would be reduced by 15% (See ).
At the current pharmacy purchasing price for PPV23, vaccinating 65-year-olds would result in a total difference in costs of NOK 12 412 345 million (EUR 1 263 986) over the five-year time horizon and a gain of 15,91 QALY. This results in a cost per QALY gained of approximately NOK 780 206 (EUR 79 451) which falls within the current threshold ranges in Norway which starts at NOK 275 000 (EUR 28 004). The corresponding figures for vaccinating 75-year-olds are a total difference in costs of NOK 146 579 (EUR 14 927) and a gain of 15,48 QALY (See ). This results in a cost per QALY gained of approximately NOK 9467 (EUR 964) for the 75-year-old cohort which falls below the lower end of the current threshold ranges in Norway.
Table 5. Base case results (NOK/EUR) – at 75% vaccination coverage.
Sensitivity analyses
The impact of changing the share of PnCAP inpatient that is caused by S. pneumonia was varied from 30% in the base case to 20% in a sensitivity analysis. This did not have an impact on the incremental cost-effectiveness ratio (ICER) for either the 65-year-old cohort or the 75-year-old cohort. The potential effects of variation in vaccine price for PPV23 from the current price to 25%, 50% or 75% rebate lowered the ICER for the 65-year-old cohort (see ). Changing the vaccination coverage from 75% to 50% for the 65-year-old cohort had impact on the ICER (NOK 780 206 (EUR 79 451) in the base-case versus NOK 1 736 657/EUR 176 849). Varying the vaccine price by 25%, 50% and 75% rebate for the 75-year-old cohort changed the result to the dominant strategy (See ). Varying the vaccination coverage rate from 75% to 50% for the 75-year-old cohort did impact the ICER NOK 9467/EUR 964 in the base case versus NOK 596 365/ EUR 60 730.
Table 6. Sensitivity analyses results (NOK/EUR) – varying the price of PVV23 with 25%, 50%, 75% rebate – at 75% vaccination coverage.
Scenario analysis
Using the PCV13 vaccine price and changing the VE data would result in a higher ICER in the base case with a cost per QALY gained of NOK 1 504 034/EUR 153 160 for the 65-year-old cohort and a cost per QALY gained of NOK 436 412/EUR 44 441 for the 75-year-old cohort. However, a rebate of 50% or 75% would result in a dominant strategy for the 75-year-old cohort, while the strategy would fall within the acceptable ranges for the 65-year-old cohort if a rebate of 75% was used (See ). The impact of changing the share of PnCAP inpatient that is caused by S. pneumonia was varied from 30% in the base case to 20% in a sensitivity analysis. This did not have an impact on the ICER. However, changing the vaccination coverage from 75% to 50% would impact the ICER for the 65-year-old cohort (cost per QALY gained NOK 2 800 795/EUR 285 213) while for the 75-year-old cohort varying the vaccination coverage from 75% to 50% would results in a cost per QALY gained of NOK 1 220 815 (EUR 124 319).
Table 7. Sensitivity analyses results (NOK/EUR) – varying the price of PCV13 with 25%, 50%, 75% rebate – at 75% vaccination coverage and changing VE data.
Discussion
This paper considers the possible health effects and costs associated with a universal vaccination programme with the 23-valent pneumococcal polysaccharide vaccine in older adults in Norway. It establishes baseline information on its effect and cost-effectiveness from the health care perspective.
In Norway, there is no exact cost-effectiveness threshold, and thus the maximum amount a decision-maker is willing to pay for a unit of health outcome is uncertain.Citation42 However, estimates of the costs that are displaced at the lower end of the scale start at NOK 275 000 (EUR 28 004) per QALY while approaching an upper limit in the upper severity class which is three times this amount i.e., NOK 825 000 (EUR 84 011) per QALY. Vaccinating the 65-year-old cohort with one dose of PPV23 is likely to be cost-effective compared to not vaccinating the cohort as the ICER falls within the recommended ranges. Vaccinating the 75-year-old cohort is also likely to be cost-effective as the ICER falls below the lower end of the of the recommended ranges. The sensitivity analyses confirmed the results of the base case vaccinating with one dose of PVV23, implying that varying the input parameters of the model would not alter the conclusions, except for varying the vaccination coverage which appear to have an impact on the ICER in both the 65-year-old and 75-year-old cohorts. Vaccinating the 65-year-old cohort with one dose of PCV13 is unlikely to be cost-effective as the cost per QALY falls above (NOK 1 504 034/EUR 153 160) the upper limit on the scale i.e., NOK 825 000 (EUR 84 011) per QALY.
We aimed to use Norwegian data where possible but quality-of life impact, PnCAP outpatient data, vaccine effectiveness and serotype distribution for pneumococcal pneumonia were not available. We acknowledge that using data from other countries with different epidemiology and healthcare systems may have impacted the results in either direction, leading to either under or over-estimation. Since not all data were available from Norway, we had to base our model on other sources. Sweden and Norway are relatively similar countries with subsidized health care services for the population and with a similar burden of pneumococcal disease.
Norway does not have their own local weights and value sets for translating various health states into a quality-of-life score. Therefore, utility values from the Netherlands and Sweden were applied. We acknowledge that transferring utilities from one country to another without an adjustment presents a limitation in the study. However, we would not expect there to be huge fluctuation among weights between Norway and Sweden or the Netherlands.
The health-care perspective was used. Absenteeism from work was ignored in part because of a lack of data on the wider indirect societal costs of pneumococcal disease in Norway. International guidelines favor using a societal perspective in cost-effectiveness analyses. Nonetheless, following the Norwegian government’s guidance using a health-care perspective makes the analysis pertinent and relevant to the Norwegian context.Citation43 Consequently, we did not consider labor production in the analysis. We do, however, acknowledge using the health care perspective only as a limitation as studies from other countries indicate that including a variety of societal outcomes in the analysis may be essential.Citation44 Using the health care perspective may imply that we have, in this instance, underestimated the benefits of vaccination.
We are aware that the indirect effects phenomena of herd immunity and serotype replacement are not readily captured via static models.Citation45 We acknowledge that the decision tree does not accurately represent the nature of the disease and the implied limitation of the work presented here. Nevertheless, most recent cost-effectiveness analyses of either the PPV23 or PCV13 vaccine have used a static approach.Citation46 Herd immunity from childhood vaccination has substantially reduced the burden of pneumococcal disease in Norway, particularly of pneumonia among older adults. However, serotype replacement has partly offset these benefits. Introduction of newer vaccines with broader serotype protection in the childhood immunization programme could reduce the disease burden in adults further, and thus reduce the cost saving from vaccination of adults. We do not expect PPV23 to reduce nasopharyngeal pneumococcal colonization, and we do not expect indirect effects from vaccination of adults with PPV23. We did not account for either herd immunity effects or serotype replacement. This would require a dynamic approach. However, since the uncertainty of quantifying the long-term impact of indirect effects increases when indirect effects are not implicitly included in the model,Citation46 we ignored indirect effects in this analysis.
The cost-effectiveness of vaccination (vs. no vaccination) is typically more favorable when indirect effects are included than when indirect effects are not included. However, as unvaccinated people are protected indirectly by those who are vaccinated this does not necessarily mean that the cost-effectiveness of interventions to increase vaccination coverage or interventions to expand vaccination to different population groups is more favorable when indirect effects are included than when indirect effects are not included. In this study, including the ongoing (and increasing) indirect effects of childhood vaccination would likely make the cost-effectiveness results less favorable for vaccinating 65- and 75-year-olds.
Cross-protection between serotypes 6A and 6B and between 19A and 19F may be relevant but no absolute cross-protection has been observed (and some vaccines will contain both 6A and 6B). Thus cross-protection is deemed of limited clinical significance and disregarded in the analysis.Citation47
A short time horizon of 5-years was used instead of the generally preferred lifetime time horizon. International guidance recommends a time horizon which is long enough to capture the full spectrum of costs and benefits which in many cases will be the lifetime of the cohorts modeled.Citation48 A recent report by the Joint Committee of Vaccination & Immunization (JCVI) also emphasized the use of a lifetime time horizon for vaccines.Citation49 The 5-year time horizon may be too short to capture the impact for PCV13 which has longer lasting protective effects. This may mean that the economic value of PCV13 is underestimated. However, uncertainty about how the epidemiology of Streptococcus pneumonia develops over time with universal vaccination favors a short time horizon. Because of changes in the vaccines used in the child vaccination programme, it is likely that serotype replacement will continue to evolve in the future. In addition, new and expanded conjugate vaccines (15 and 20 serotypes) have recently entered the market. The Norwegian Institute of Public Health has started developing recommendations on how these vaccines could replace or supplement the use of PPV23 for older adults and adults with medical risk conditions. This also supports the use of a relatively short time-horizon in our analysis of PPV23.
We have described the limitations of the study above. In the main, this study is limited by the extent to which geographic transferability of data from study populations in other country settings to a target population in Norway is deemed appropriate. The resulting findings should be interpreted carefully in view of this caveat.
Conclusion
A universal PPV23 vaccination programme against pneumococcal disease for the 75-year-old cohort is likely cost-effective in the Norwegian setting. A universal PPV23 vaccination programme against pneumococcal disease for the 65-year-old cohort is likely to be cost-effective in the Norwegian setting, assuming a threshold scale between NOK 275 000 (EUR 28,004) per QALY and NOK 825 000 (EUR 84,011) per QALY.
Author’s contributions
The model development was carried out by EW. LSN adapted the model and performed the economic calculations. Data collection and analysis were conducted by TML, BAW, JB and LSN. LSN drafted the manuscript. All authors contributed to the interpretation of the data analyses, revising and approval of the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Brooks LRK, and Mias GI . Streptococcus pneumoniae’s virulence and host immunity: aging, diagnostics, and prevention. Front Immunol. 2018 Jun 22;9:1366. doi:10.3389/fimmu.2018.01366. PMID: 29988379; PMCID: PMC6023974.
- File TM Jr. Streptococcus pneumoniae and community-acquired pneumonia: a cause for concern. Am J Med. 2004;117(Suppl 3A):39S–9. doi:10.1016/j.amjmed.2004.07.007.
- Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Ag. 2008;32(3):199–206. doi:10.1016/j.ijantimicag.2008.01.021.
- Winje BA, Vestrheim DF, White RA, Steens A. The risk of invasive Pneumococcal disease differs between risk groups in Norway following widespread use of the 13-valent pneumococcal vaccine in children. Microorganisms. 2021;9(8):1774. doi:10.3390/microorganisms9081774.
- Pneumokokkvaksine - veileder for helsepersonell [Internet]; [accessed 2022 Mar 27]. https://www.fhi.no/nettpub/vaksinasjonsveilederen-for-helsepersonell/vaksiner-mot-de-enkelte-sykdommene/pneumokokkvaksinasjon—veileder-fo/.
- Folkehelseinstituttet. Vaksinasjonsveilederen for Helsepsersonell (Vaskinasjonsboka) [Internet]. Oslo: Folkehelseinstituttet; 2015 [accessed 2022 Mar 1]. https://www.fhi.no/nettpub/vaksinasjonsveilederen-for-helsepersonell/.
- The “blue prescription” regulation [Internet]; [accessed 2022 March 27]. https://lovdata.no/dokument/SF/forskrift/2007-06-28-814.
- Executive order on free pneumococcal vaccination for certain groups of persons. BEK nr 395 af 07/04/2020 [Internet]. The Danish Ministry of Health; [accessed 2022 Mar 1]. https://www.retsinformation.dk/eli/lta/2020/395.
- Hälsoekonomisk utvärdering av pneumokockvaccination som ett särskilt vaccinationsprogram för personer 75 år och äldre. Public Health Agency of Sweden [Internet]; [accessed 2022 Mar 1]. https://www.folkhalsomyndigheten.se/publicerat-material/publikationsarkiv/h/halsoekonomisk-utvardering-av-pneumokockvaccination-som-ett-sarskilt-vaccinationsprogram-for-personer-75-ar-och-aldre/.
- Wolff E, Storsaeter J, Ortqvist A, Naucler P, Larsson S, Lepp T, Roth A. Cost-effectiveness of pneumococcal vaccination for elderly in Sweden. Vaccine. 2020;38(32):4988–4995. doi:10.1016/j.vaccine.2020.05.072.
- Efficacy and effectiveness of pneumococcal vaccination in adults – a second update of the literature. [Effekt av pneumokokkvaksine hos eldre] Report 2019 [Internet]. Oslo: Norwegian Institute of Public Health; 2022. https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2022/efficacy-and-effectiveness-of-pneumococcal-vaccination-in-adults.pdf.
- Norwegian Medicines Agency. Guidelines for the submission of documentation for single technology assessment (STA) of pharmaceuticals [Internet]. Oslo (Norway): Norwegian Medicines Agency; 2018 [accessed 2022 March 1]. https://legemiddelverket.no/Documents/English/Public%20funding%20and%20pricing/Documentation%20for%20STA/Guidelines%2018.10.2021.pdf.
- Nymark L, and Vassall A. A comprehensive framework for considering additional unintended consequences in economic evaluation. Cost Effect Resour Alloc. 2020;18:27. https://doi.org/10.1186/s12962-020-00218-8.
- Holter JC, Muller F, Bjorang O, Samdal HH, Marthinsen JB, Jenum PA, Ueland T, Frøland SS, Aukrust P, Husebye E, et al. Etiology of community-acquired pneumonia and diagnostic yields of microbiological methods: a 3-year prospective study in Norway. BMC Infect Dis. 2015;15(1):64. doi:10.1186/s12879-015-0803-5.
- Roysted W, Simonsen O, Jenkins A, Sarjomaa M, Svendsen MV, Ragnhildstveit E, Tveten Y, Kanestrøm A, Waage H, Ringstad J, et al. Aetiology and risk factors of community-acquired pneumonia in hospitalized patients in Norway. Clin Respir J. 2016;10(6):756–764. doi:10.1111/crj.12283.
- Naucler P, Henriques-Normark B, Hedlund J, Galanis I, Granath F, Ortqvist A. The changing epidemiology of community-acquired pneumonia: nationwide register-based study in Sweden. J Intern Med. 2019;286(6):689–701. doi:10.1111/joim.12956.
- Ieven M, Coenen S, Loens K, Lammens C, Coenjaerts F, Vanderstraeten A, Henriques-Normark B, Crook D, Huygen K, Butler CC, et al. Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect. 2018;24(11):1158–1163. doi:10.1016/j.cmi.2018.02.004.
- Lyngstad TM, Kristoffersen AB, Winje BA, Steens A. Estimation of the incidence of hospitalisation for non-invasive pneumococcal pneumonia in the Norwegian population aged 50 years and older. Epidemiol Infect. 2022;150. doi:10.1017/S0950268822000607.
- Suzuki M, Dhoubhadel BG, Ishifuji T, Yasunami M, Yaegashi M, Asoh N, Ishida M, Hamaguchi S, Aoshima M, Ariyoshi K, et al. Serotype-Specific effectiveness of 23-valent pneumococcal polysaccharide vaccine against Pneumococcal pneumonia in adults aged 65 years or older: a multicentre, prospective, test-negative design study. Lancet Infect Dis. 2017;17(3):313–321. doi:10.1016/S1473-3099(17)30049-X.
- Norwegian Instiute of Public Health. Norwegian surveillance system for communicable diseases [Internet]. Oslo (Norway): Norwegian Instiute of Public Health [accessed 2022 Mar 1]. http://www.msis.no/.
- Årsrapport for 2017. Invasive infeksjoner [Internet]. Oslo (Norway): Norwegian Instiute of Public Health; 2018. https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2018/arsrapport-invasive-infeksjoner-2017.pdf.
- Kraicer-Melamed H, O’-Donnell S, Quach C. The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: a systematic review and meta-analysis. Vaccine. 2016;34(13):1540–1550. doi:10.1016/j.vaccine.2016.02.024.
- Kim JH, Chun BC, Song JY, Kim HY, Bae IG, Kim DM, Choi YH, Jun YH, Choi WS, Kang SH, et al. Direct effectiveness of pneumococcal polysaccharide vaccine against invasive pneumococcal disease and non-bacteremic pneumococcal pneumonia in elderly population in the era of pneumococcal conjugate vaccine: a case-control study. Vaccine. 2019;37(21):2797–2804. doi:10.1016/j.vaccine.2019.04.017.
- Djennad A, Ramsay ME, Pebody R, Fry NK, Sheppard C, Ladhani SN, Andrews NJ. Effectiveness of 23-valent polysaccharide Pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClin Med. 2018;6:42–50. doi:10.1016/j.eclinm.2018.12.007.
- Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AMM, Sanders EAM, Verheij TJM, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372(12):1114–1125. doi:10.1056/NEJMoa1408544.
- van Werkhoven CH, Huijts SM, Bolkenbaas M, Grobbee DE, Bonten MJ. The impact of age on the efficacy of 13-valent Pneumococcal conjugate vaccine in elderly. Clin Infect Dis. 2015;61(12):1835–1838. doi:10.1093/cid/civ686.
- Mangen MJ, Rozenbaum MH, Huijts SM, van Werkhoven CH, Postma DF, Atwood M, van Deursen AMM, van der Ende A, Grobbee DE, Sanders EAM, et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur Respir J. 2015;46(5):1407–1416. doi:10.1183/13993003.00325-2015.
- Burström K, and Rehnberg C. Hälsorelaterad livskvalitet i Stockholms län 2002- 2006; Rapport 2006:1. Enheten för Socialmedisin och Hälsoekonomi. Centrum för Folkhälsa. FORUM för kunnskap og gemensam utvikling. Stockholm, Sweden: Stockholm läns landsting, 2006.
- Blommaert A, Hanquet G, Willem L, Theeten H, Thiry N, Bilcke J, Verhaegen J, Beutels P.Use of pneumococcal vaccines in the elderly: an economic evaluation – Synthesis. Health Technology Assessment (HTA) Brussels: Belgian Health Care Knowledge Centre (KCE). 2016. KCE Reports 274Cs. D/2016/.Please see the link below on page 45: https://kce.fgov.be/sites/default/files/2021-11/KCE_274C_Short-Report_Pneumococcal.pdf, https://kce.fgov.be/sites/default/files/2021-11/KCE_274C_Short-Report_Pneumococcal.pdf.
- Remschmidt C, Harder T, Wichmann O, Bogdan C, Falkenhorst G. Effectiveness, immunogenicity and safety of 23-valent pneumococcal polysaccharide vaccine revaccinations in the elderly: a systematic review. BMC Infect Dis. 2016;16(1):711. doi:10.1186/s12879-016-2040-y.
- Jiménez E, Torkilseng EB, Klemp M Cost-Effectiveness of HPV vaccination of boys aged 12 in a Norwegian setting. Report from Kunnskapssenteret no. 2−2015. Oslo: Norwegian Knowledge Centre for the Health Services; 2015.
- Jiménez E, Wisløff T, Klemp M Cost-Effectiveness of a HPV vaccination catch-up program for females aged 26 years or younger in a Norwegian setting. Report from Kunnskapssenteret no. 5−2012. Oslo: Norwegian Knowledge Centre for the Health Services; 2012.
- Berg AS, Furuseth E, Greve-Isdahl M, Grosvold IW, Næss LS, Nøkleby H, Stålkrantz J, Tunheim G, Wisløff T, Wolden B, et al. Kikhostevaksine til gravide – aktuelt for Norge? [Maternal Immunization against Pertussis. Evaluation for Norway] Rapport 2019. Oslo: Folkehelseinstituttet.
- Norwegian Medicines Agency. Oslo (Norway) [Internet]; [accessed 2022 March 1]. https://legemiddelverket.no/english.
- Norwegian Medicines Agency. Oslo (Norway) [Internet]; [accessed 2022 Mar 27]. https://legemiddelverket.no/offentlig-finansiering/maksimalpris#oversikt-over-maksimalpriser.
- Watle SV, Naess LM, Tunheim G, Caugant DA, Wisloff T. Cost-Effectiveness of meningococcal vaccination of Norwegian teenagers with a quadrivalent ACWY conjugate vaccine. Hum Vaccin Immunother. 2021;17(8):2777–2787. doi:10.1080/21645515.2021.1880209.
- Norwegian Medical Association. General practitioner fees list. Collective Agreement; 2021- 2022 [Internet]; [ accessed 2022 Mar 1]. https://normaltariffen.legeforeningen.no/asset/pdf/Fastlegetariffen-2021-2022.pdf.
- Norwegian: (ISF_regulations_2022_DRGlist somatic). Norwegian Directorate of Health [Internet]; 2022 [ accessed 2022 Mar 1]. https://www.helsedirektoratet.no/tema/finansiering/innsatsstyrt-finansiering-og-drg-systemet/innsatsstyrt-finansiering-isf/ISF-regelverk%202022.pdf/_/attachment/inline/f7f190ab-33fc-4e07-9d36-fd5fc9e4e17e:9d97d72b2acf5c719d19cde034b6174007b84923/ISF-regelverk%202022.pdf.
- Antibiotikabruk i primærhelsetjenesten. Oslo (Norway) [Internet]; [accessed 2022 Mar 27]. https://www.antibiotikaiallmennpraksis.no/index.php?action=showtopic&topic=f6000288c623ddf6aa4c.
- NORM/NORM-VET. Usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. Tromsø/Oslo. 2019 ISSN:1502-2307 (print)/1890-9965 (electronic); 2018.
- Exchange rates. Oslo (Norway); 2022 [ accessed 2022 Mar 10]. https://www.norges-bank.no/en/topics/Statistics/exchange_rates/Internet.
- Norwegian Ministry of Health and Care Services. Principles for priority setting in health care - Summary of a white paper on priority setting in the Norwegian health care sector [Internet]. Oslo (Norway): Norwegian Government; 2016. https://www.regjeringen.no/contentassets/439a420e01914a18b21f351143ccc6af/en-gb/pdfs/stm201520160034000engpdfs.pdf.
- Norheim O, Allgott B, Gjul G, Kjellevold A, Moen A, Sjøli S. Norwegian [Transparent and fair – priorities in health services]. Oslo (Norway): Norwegian Government; 2014. https://www.regjeringen.no/no/dokumenter/NOU-2014-12/id2076730/?ch=1.
- Nymark LS, Miller A, Vassall A. Inclusion of additional unintended consequences in economic evaluation: a systematic review of immunization and tuberculosis cost-effectiveness analyses. Pharmacoecon Open. 2021;5(4):587–603. doi:10.1007/s41669-021-00269-4.
- Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, Brisson M. Dynamic transmission modeling: a report of the ISPOR-SMDM modeling good research practices task force-5. Value Health. 2012;15(6):828–834. doi:10.1016/j.jval.2012.06.011.
- Nymark LS, Sharma T, Miller A, Enemark U, Griffiths UK. Inclusion of the value of herd immunity in economic evaluations of vaccines. A systematic review of methods used. Vaccine. 2017;35(49):6828–6841. doi:10.1016/j.vaccine.2017.10.024.
- Madhi SA, Goldblatt D. The duopoly of ten-valent and 13-valent pneumococcal conjugate vaccines: do they differ? Lancet Infect Dis. 2019;19(5):453–454. doi:10.1016/S1473-3099(18)30785-0.
- Weinstein MC, O’-Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices—modeling studies. Value Health. 2003;6(1):9–17. doi:10.1046/j.1524-4733.2003.00234.x.
- Immunisation and High Consequence Infectious Diseases Team, Global and Public Health Group. Consultation Ron the cost-effectiveness methodology for vaccination programmes and procurement (CEMIPP) report [Internet]; [accessed 2022 Mar 27]. https://www.gov.uk/government/consultations/cost-effectiveness-methodology-for-vaccination-programmes.
