 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
COVID 19 vaccination has recently been launched globally to halt the pandemic. But COVID 19 vaccines have some adverse effects that raise concerns in the global community. This study aimed to evaluate and compare the adverse effects of Janssen and Oxford-AstraZeneca vaccinated adults. A community-based cross-sectional study was conducted from March 15 to 30, 2022 among 421 (211 Janssen and 210 Astra Zeneca vaccinated) adults recruited by a convenience sampling technique in Debre Tabor Town, Northwest Ethiopia. Data were collected via face-to-face interviews and by reviewing the immunization card. Chi-square test, independent t-test, and Mann-Whitney test were used to compare the adverse symptoms and related parameters between the two vaccines. A linear regression model was also used to identify predictors of the number of post-vaccination symptoms. The majority (75.8%) of participants reported at least one side effect after vaccination. Adverse symptoms had a significantly greater occurrence (p < .05) among recipients of the AstraZeneca vaccine (84.8%) than receivers of the Janssen vaccine (66.8%). The main adverse symptoms were injection site pain, fever, fatigue, arthralgia, and myalgia in both vaccines. Significant variations (p < .05) between the receipts of the two vaccines were shown in injection site pain, fever, and arthralgia. The total number of symptoms was significantly higher (p < .05) in participants with female sex, younger age, BMI <25 kg/m2, no prior COVID 19, and those who had received AstraZeneca vaccine. Thus, the authors advise that they should receive vaccines with no hesitation, while continuous tracking of vaccine safety is kept in place.
Introduction
Recently, a worldwide vaccination campaign against COVID 19 was initiated in an effort to halt the pandemic.Citation1 Billions of doses of COVID-19 vaccines have been administered so far to billions of people worldwide.Citation2 In Ethiopia, COVID 19 vaccination program was commenced on 13 March 2021, with priority being given to health professionals and the elderly. Then COVID 19 vaccination targeting all people aged 12 years and above began on 16 November 2021.Citation3 To date, 21.3 million (18.5%) of the Ethiopian population have been vaccinated with WHO approved COVID-19 vaccines, namely Oxford-AstraZeneca, Janssen, and Pfizer-BioNTech vaccines. The AstraZeneca (AZD-1222) and Janssen (Johnson & Johnson or Ad26. COV2.S) vaccines are adenovirus-based vector vaccines with the efficacy of 81% and 66%, respectively.Citation4 Whereas the Pfizer-BioNTech (BNT162b2) vaccine is an mRNA-based vaccine with an efficacy of 95%.Citation5
Although vaccines successfully lower the severity of COVID 19, they are not without adverse symptoms. As a matter of fact, no vaccine or medicine is free from any side effects or complications, and similarly, the COVID 19 vaccine can cause side effects.Citation6–8 Post-vaccination adverse effects are not rare and can be observed after all COVID 19 vaccines.Citation9,Citation10 However, not all vaccinated individuals have these adverse effects, with roughly 50–90% of vaccinated people experiencing any adverse symptoms.Citation5,Citation11,Citation12 The adverse effects can range from local symptoms such as pain, redness, itch, warmth, and swelling at the injection site, to transient systemic symptoms like headache, nausea, tiredness, arthralgia, myalgia, chills and shivers, diarrhea, and fever throughout the rest of the body.Citation13,Citation14 Preliminary reports showed that the COVID 19 vaccine side effects are mostly mild to moderate and acute lasting for a few days.Citation15 Nonetheless, serious side effects such as anaphylaxis to a vaccine component (such as polyethylene glycol) and thrombosis and thrombocytopenia syndrome due to the dropping of platelet counts have been reported in exceedingly rare cases after vaccination with AstraZeneca, Janssen, Pfizer, and Moderna vaccines.Citation16–21 Leukopenia like neutropenia, which may increase the risk of severe infections, has also been reported in some instances after COVID 19 vaccination.Citation22 In addition, COVID 19 vaccines are linked with hepatotoxicity as evidenced by a report showing drug-induced liver damage and abnormal liver function test from vaccine recipients. Liver biomarkers including alkaline phosphatase, total bilirubin, direct bilirubin, and aspartate transaminase are all remarkably elevated among COVID 19 vaccinated individuals.Citation23
The potential side effects of the vaccines have raised great concerns among the global communities and have become obstacles against COVID 19 vaccination.Citation24–26 Thus, at this stage of vaccination deployment, surveillance of the broader population is essential. There are some reports on the adverse effects of the COVID 19 vaccines in several countries around the globe.Citation5,Citation11,Citation24,Citation27,Citation28 But no population-level data on adverse effects after COVID-19 vaccination was available in Ethiopia, and it was unclear which vaccines were associated with which adverse effects. Hence, the purpose of this study was to evaluate the adverse effects of the two most common vaccines in Ethiopia, Janssen and AstraZeneca vaccines, and to compare the adverse effects of the two vaccine types. The findings from this study might provide important information on vaccine safety and adverse events to the public, and reduce misunderstandings and conspiracy beliefs about the COVID-19 vaccines. Consequently, it could lower vaccine hesitancy, improve their confidence in vaccine safety, and thereby accelerate the COVID 19 vaccinations.
Methods and materials
Study design, period, and setting
A community-based retrospective cross-sectional study was conducted from March 15 to 30, 2022 in Debre Tabor Town, Northwest Ethiopia. Debre Tabor is the administrative town of the South Gondar Zone, which is located 103 km away from Bahir Dar and 667 km Northwest of Addis Ababa. The town has six kebeles (the smallest administrative unit in Ethiopia), with more than 200 thousand people currently residing in the town. Due to financial constraints and lack of funding, the study was only conducted in Debre Tabor Town.
Population
The source population included all adults (aged 18 or older) who had received the COVID-19 vaccine from Janssen or AstraZeneca in Debre Tabor Town. While the study population involved all eligible persons who were 18 years of age or older and had received a Janssen or AstraZeneca vaccination during the study period in the chosen kebeles of Debre Tabor.
Eligibility criteria
Due to a vaccine shortage in Ethiopia, the AstraZeneca and Janssen vaccines are primarily given to adults aged 18 or above, while the Pfizer-BioNTech vaccine is mainly reserved for those aged between 12 and 18 years. Thus, our study included only volunteer adult participants (aged 18 years or older) who received Janssen or Astra Zeneca vaccines at least one week before the data collection period in Debre Tabor Town. Participants with serious illnesses or those under the age of 18 were, however, excluded from the study. Furthermore, participants who received vaccines other than Janssen and Astra Zeneca vaccines, as well as those who were unaware of the type of vaccine they received or did not have an immunization certificate, were excluded from the study.
Study variables
Post-vaccination adverse symptoms were taken as a dependent variable, while socio-demographic factors (age, sex, marital status, education, and occupation) and clinical related variables (smoking, chronic medical illness, allergy history, BMI, prior COVID 19, vaccine type, and vaccine dose) were considered independent variables.
Sample size determination and sampling procedures
The sample size was calculated using the single population proportions formula by considering Zα/2 at a 95% confidence level = 1.96 and margin of error (d) = 0.05, with the assumption the proportion of 50% post-vaccination side effects (P) due to lack of prior similar study in Ethiopia. The sample size (n) was calculated 384. After adding a 10% non-response rate, the final sample size became 421. Then three kebeles out of the six kebeles were selected by lottery method and a proportional sample size was allocated to each kebele. Finally, a convenience sampling technique was employed to select 421 (211 Janssen vaccinated and 210 AstraZeneca vaccinated) study participants during the data collection period.
Data collection instruments and procedures
Data were collected using a pretested structured questionnaire that was developed by the authors after a thorough review of previous related literature.Citation29–31 The final draft of the questionnaire comprised three parts: The first one covers the socio-demographic characteristics, the second part embodies clinical-related data, and the last category focused on post-vaccination side effects related data. The severity of self-reported adverse effects of COVID 19 vaccines was categorized into mild, moderate, and severe symptoms. The adverse effects were classified as ‘mild;’ if the participants experienced symptoms that did not require a symptom reliever, not interrupted their daily activities, and did not seek medical attention. Moderate symptoms were adverse effects that may or may not require a symptom reliever and did not seek medical attention but interrupted their daily activities. Self-reported adverse symptoms are considered ‘severe’ if they sought medical attention or visit a health institution regardless of the need for a symptom reliever and interruption of daily activities.Citation31 Data were collected from adult participants who get vaccinated at least one week before the data collection period. Recruitment of eligible adults was done at the household level by visiting all the houses of each selected kebele of the town until the required sample size was achieved. While the Janssen vaccine is a one-dose COVID 19 vaccine, the AstraZeneca vaccine is a two-dose schedule as first dose and second dose. If the participants had taken more than two doses of the AstraZeneca vaccine, the more recent (the second) dose of the vaccine was taken to investigate the adverse effects. The data collection was done by four trained data collectors under the supervision of two supervisors via face-to-face interviews and by reviewing their immunization certificates to check the type of vaccine they took.
Data processing and analysis
After the data were checked for completeness and internal consistency, data entry, cleaning, and statistical analysis were done using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). Frequencies and percentages were used to express categorical variables. Whereas mean and standard deviation (SD) were used to present normally distributed continuous data, the median and interquartile range (IQR) were used to describe non-normally distributed continuous variables. Comparisons between categorical variables were made using the Chi-square test or Fisher’s exact test as appropriate. To compare the differences in numerical outcome variables, independent sample t-tests or Mann-Whitney tests were used, depending on whether they fit the assumption of normality or not. A multiple linear regression model was used to assess the predictors of the total number of post-COVID 19 vaccination adverse effects. The results from linear regression analysis were expressed using the adjusted beta (β) as regression coefficients at 95% Confidence Intervals (CI). Those variables with p-values of less than .05 were assumed to be statistically significant.
Data quality control
The questionnaire was prepared in English and translated to the local Amharic, and then retranslated back to the English version to ensure consistency. The questionnaire was reviewed by a panel of experts for construct and content validity. Pretesting was also done among 5% of the study population to ensure the validity of the tool. Then appropriate amendments, such as wording, logical sequence, inconsistencies, and errors in the skip pattern before the commencement of the actual data collection were carried out. Extensive training was given to the data collectors and supervisors on the objectives of the study, the content of the measuring tool, confidentiality, and informed consent. The data were also collected under supervision and the questionnaires were checked for completeness on the daily basis to ensure the data quality.
Results
Socio-demographic characteristics
A total of 421 study participants (211 Janssen vaccinated and 210 AstraZeneca vaccinated) were involved in this study. Age ranged between 18 and 69 years, with a mean (SD) of 39.1
7.6 years and the majority were under 50 years (77%). More than half of them were male (54.2%), while a great proportion (70.1%) were married. Roughly 44.4% of respondents had completed primary education and half of them were merchants (50.6%) ().
Table 1. Socio demographic characteristics of the study participants (n = 421).
Clinical related characteristics
Among all participants, few had a history of cigarette smoking (2.1%), chronic medical illness (3.8%), and allergy (4.0%). The mean body mass index (BMI) of participants was 22.4 9.1, with about 73.6% of them having less than 25 kg/m2 BMI and 26.4% having 25 kg/m2 or greater BMI. Only 9.3% of participants reported a previous history of COVID 19 infection (). Out of all participants, 211 (50.1) and 210 (49.9%) of them were the receivers of the Johnson & Johnson (Janssen) vaccine and Oxford-AstraZeneca vaccine, respectively. Most of them, 402 (95.5%), received only a single dose of COVID 19 vaccine at the time of the interview (Data not shown).
Table 2. Clinical related characteristics of the study participants (n = 421).
Post-COVID 19 vaccination symptoms
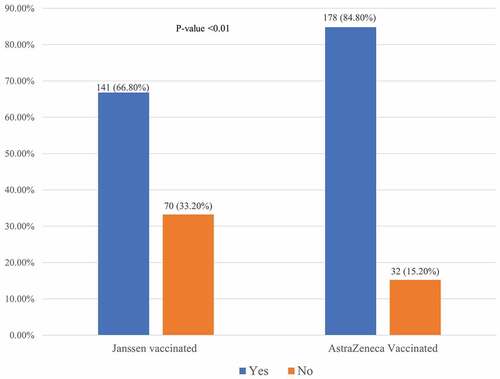
The study participants were interviewed on vaccine-related symptoms after the injection of the COVID 19 vaccine. Nearly three-quarters (75.8%) of the study participants reported one or more post-vaccination side effects. About 66.8% and 84.8% of Janssen vaccinated and AstraZeneca vaccinated respondents, respectively, experienced at least one side effect following vaccination. There was a statistically significant difference (p-value <.01) in adverse effects reported among Janssen vaccinated and AstraZeneca vaccinated participants ().
Figure 1. The occurrence of adverse effects after vaccination with Janssen and Astra Zeneca vaccines. Significantly higher occurrence of side effects (p-value <.01) was seen in the AstraZeneca vaccine than Janssen vaccine.
A total of 16 side effects following vaccination were analyzed in this study, with their number ranging between 1 and 13 per participant. The most frequently reported symptoms in both Janssen and AstraZeneca vaccine receipts were injection site pain (39.8% vs 51.9%) followed by fatigue (34.6%) for receivers of the Janssen vaccine and fever (37.1%) for receipts of the AstraZeneca vaccine. Nonetheless, significant differences (p-value <.05) have been observed between the two vaccines in the proportions of injection site pain, fever, and arthralgia. However, none of the participants reported any instances of severe allergic reactions or blood clotting (). The mean total number of side effects reported after vaccination with either of the vaccines was calculated, and a statistically significant difference has been seen in the mean +SD number of side effects between Janssen and AstraZeneca vaccinated respondents (4.2 3.1 vs 6.7
4.6, p-value <.05). However, the differences between the two vaccines in terms of the mean onset and median duration of post-vaccination side effects were insignificant. Most participants experienced mild 198 (62.1%) and moderate symptoms 110 (34.5%), while only 11 (3.5%) of them reported severe side effects after vaccination. Out of all participants, only a few of them required hospitalization (0.9%) and treatment (6.6%) after receiving both vaccines, with no significant difference between the two vaccines ().
Table 3. Post vaccination adverse symptoms among recipients of the Janssen and AstraZeneca vaccines.
Table 4. Comparison of post-vaccination adverse symptoms and related parameters between Janssen and AstraZeneca vaccinated participants.
Predictors of the number of post-vaccination side effects
Multiple independent variables were entered simultaneously into a multiple linear regression model to determine whether they were predictors of the mean total number of post-vaccination symptoms. Accordingly, the mean total number of adverse symptoms was significantly higher among younger participants compared to their older counterparts (β: 0.21; 95% CI:0.15, 7.71; p = .009) and females compared to males (β: 0.19; 95% CI: 0.11, 5.52; p = .023). In contrast, the mean total score of vaccine side effects was significantly lower among participants with a BMI of less than 25 kg/m2 (β: −0.16; 95% CI: −0.09, −0.89; p = .041). Similarly, participants having prior COVID 19 had a significantly lower number of post-vaccination symptoms compared to their counterparts (β: −0.27; 95% CI: −0.14, −1.90; p = .012). A significantly higher mean total score of post-vaccination symptoms was observed after receiving the AstraZeneca vaccine in comparison to the Janssen vaccine (β: 0.31; 95% CI: 0.19, 2.69; p = .001) ().
Table 5. Predictors of the mean total number of post-vaccination symptoms of the study participant.
Discussion
COVID 19 vaccines have been developed in response to the ongoing surge in COVID 19 cases, and it was emerged as the most important weapons to fight against this disease and to stop the global pandemic.Citation6 Despite the fact that the COVID-19 vaccines effectively lower the disease severity, they are associated with adverse effects or complications.Citation6–8 In this study, about 75.8% of individuals experienced at least one post-vaccination side effect, with AstraZeneca vaccine symptoms having a significantly higher occurrence (84.8%) than that of the Janssen vaccine (66.8%). This is in line with other studies, showing that most participants (up to 90%) reported one or more adverse effects.Citation13–32–Citation34 The most commonly reported post-vaccination symptoms in both Janssen and AstraZeneca vaccine recipients were injection site pain, fever, fatigue, arthralgia, and myalgia. But injection site pain, fever, and arthralgia were observed to differ significantly between the two vaccines, with 51.9% vs39.8%; 41.6% vs 33.7%, and 40.0% vs 24.6% for AstraZeneca and Janssen vaccines, respectively. These findings are consistent with prior studies.Citation27–Citation29–34–Citation38 Adverse effects such as fever, fatigue, arthralgia, and myalgia following COVID 19 vaccination generally occur due to excessive body immune responses to the vaccine that lead to inflammatory reactions (reactogenicity).Citation39
Our study also indicated that the mean onset of symptoms was nearly 11 hours after the Janssen or AstraZeneca vaccination. The median duration of symptoms was 3 days after receiving the Janssen vaccine and 2 days after the AstraZeneca vaccination. This is congruent with a number of studies, stating that the onset of the symptoms was mostly before 24 hours and the side effects wore off in a few days after vaccination.Citation15–27,Citation28–32–Citation40–42 The findings of this study also indicated that most side effects were mild (62.1%) and moderate (34.5%) following vaccination. But there were low rates of severe side effects (3.5%) and only a few reported requiring hospitalizations (0.9%) or treatment (6.6%) after receiving either the Janssen or AstraZeneca vaccines, with no significant variation between the two vaccines. This data is in line with other studies that found most post-vaccination symptoms were mild to moderate and usually resolved within a few days after vaccination without the need for hospitalization or treatment.Citation11,Citation27,Citation28,Citation32,Citation34,Citation36,Citation41,Citation43
Our analysis showed that female sex, younger age, BMI of less than 25 kg/m2, no prior history of COVID 19, and receivers of AstraZeneca vaccines were significantly correlated with a higher mean total number of adverse effects after COVID 19 vaccination. Females had a significantly higher number of adverse effects than males. This is in agreement with several other studies, revealing that the female gender has a significantly higher risk for adverse effects than the male counterparts.Citation13,Citation30,Citation32,Citation34,Citation35,Citation38,Citation44,Citation45 The possible explanation may be that females tend to elicit more antibody led immune responses to vaccination than males and are thus more likely to develop more number of side effects.Citation28,Citation46–48 Nevertheless, our results contradict another report showing that the incidence of post-vaccination side effects is significantly more prevalent in males than in females.Citation38 Remarkably, the mean total number of post-vaccination side effects among younger participants was significantly higher compared to older participants. This is in correspondence with other studies reporting that younger adults are more likely to suffer from post-vaccination side effects than older ones.Citation15–34–Citation36–38–Citation44 This could be because younger people induce stronger immune responses than the elders, making them more likely to experience a greater number of side effects. Our result, however, opposes the study finding by Omeish et al.Citation32 that showed that increasing age is associated with higher odds of reporting adverse effects.
More intriguingly, our study found that participants with a higher BMI (25 kg/m2 or above) had a significantly lower mean number of vaccine-related symptoms than those with a BMI of less than 25 kg/m2. This finding is in line with several previous studies.Citation13,Citation46,Citation49,Citation50 A study done in Spain revealed that obese individuals had lower antibody titers and experienced a lower percentage of COVID-19 vaccine-associated adverse effects when compared to non-overweight/obese individuals.Citation50 Another study also demonstrated that central obesity is linked with lower antibody titers in response to the COVID-19 mRNA vaccine.Citation49 Similarly, a study among Italian health workers indicated that a higher BMI is significantly associated with lower antibody titers in response to the COVID-19 vaccine.Citation46 Since post-vaccination symptoms suggest the level of body’s immune responses (or antibody titers), the number of adverse events in those overweight and obese persons will be reduced. However, this is contrary to the preexisting notion that obese individuals are associated with low-grade chronic inflammation that potentiates a higher risk of disease severity and mortality related to COVID19.Citation51 As there is an increased inflammation linked with obesity, one may expect that vaccine-induced immune response may amplify the existing chronic inflammation in obese people and may increase the number of post-COVID 19 vaccination adverse effects among those with higher BMI. But this does not occur in obese/overweight individuals as shown in our study and other related prior studies. This could be explained by the weaker ability to mount humoral immune responses to COVID-19 vaccines among people who are heavier (overweight or obese) due to the associated metabolic derangement and immune dysfunction (including impaired cellular immunity).Citation13,Citation46,Citation52,Citation53 This could also be because of a poor seroconversion rate in obese individuals.Citation54 Additionally, it might be because BMI is less accurate in measuring actual body fat deposition and, consequently, inflammation in that individual.Citation55 This calls for researchers to carry out further explanatory research.
The current study also indicated that those who had no prior COVID 19 infections had a significantly greater mean total number of adverse effects than those with a history of COVID 19 infection. This is in parallel with previous studies that demonstrated more frequent reports of adverse reactions by infection-naive respondents than those with prior COVID-19 infection before vaccination.Citation39,Citation56 This could be attributed to the lower proportion of participants who had previous COVID 19 infection in our study sample, and most of them had received a single vaccine dose. Consequently, they were primed with the first vaccine dose and induced a robust immune response that in turn considerably increase the number of post-vaccination adverse symptoms (reactogenicity) than individuals who had previously been infected with COVID 19.Citation57 However, inconsistent results were reported by other studies, which indicated that participants with prior COVID 19 infections showed more post-vaccination adverse symptoms.Citation13,Citation32,Citation34,Citation44,Citation58,Citation59 This conflicting finding may be due to the differences in sociodemographic, genetic, and the number of COVID 19 infected people. The inconclusive result on the correlation between prior COVID-19 and post-vaccination adverse symptoms was also reported by Baden et al.Citation12 Thus, further studies need to be done to fully determine the relationship between these two variables and reach a conclusion. All COVID-19 vaccines have the potential to cause adverse effects, however, the number of side effects varies depending on the type of vaccine.Citation29 The current study pointed out that the mean number of adverse symptoms was significantly higher among participants who received the AstraZeneca vaccine than those who were vaccinated with the Janssen vaccine. It is in concordance with the other studies, which stated that the AstraZeneca vaccine recipients were more prone to side effects and reported a significantly higher number of symptoms than other vaccines.Citation13,Citation15,Citation32,Citation34,Citation39,Citation44,Citation60
Overall, our study revealed that a sizable percentage of people who had received the COVID 19 vaccine had at least one adverse symptom, which is consistent with multiple previous related studies. The adverse effects were typically mild or moderate symptoms that go away on their own or are otherwise manageable. This suggests that the benefit of the vaccine outweighs its risks, and therefore it is important to deter people from believing nonscientific rumors and conspiracies. In addition, recent reports have demonstrated that COVID 19 vaccines are associated with reduced efficacy as observed by the gradual waning of antibody titers after vaccination.Citation61 The efficiency of the vaccine in preventing COVID 19 infections is more notably reduced in new SARS-COV-2 variants, such as Omicron variants.Citation61,Citation62 Despite the reduced effectiveness of the COVID 19 vaccines, the vaccine-induced immune response still confers a substantial level of protection that is crucial in preventing COVID 19 from progressing into a severe type and leading to mortality. More recently, booster dose vaccination after primary immunization has been introduced globally to improve vaccine effectiveness against SARS-CoV-2 infection, including novel variants.Citation62
Limitation of the study
Even though our study sheds light on the side effects of the Janssen and AstraZeneca COVID-19 vaccines, it has some limitations. Firstly, information bias might occur as the study used self-reported data. Secondly, respondents might be liable for recall bias as the interview was conducted some days after the COVID-19 vaccination. Thirdly, because our study focused on the adverse effects of the recent (second dose) of the AstraZeneca vaccine for those who had received two doses, it did not compare the adverse symptoms between the first and second doses of AstraZeneca vaccines.
Conclusion
The majority of participants experienced one or more post-vaccination side effects, with a significantly higher occurrence in the AstraZeneca vaccine than in the Janssen vaccine. The most common adverse symptoms in both vaccine recipients were injection site pain, fever, fatigue, arthralgia, and myalgia, with significant variation between the receipts of the two vaccines in symptoms, such as injection site pain, fever, and arthralgia. A significantly higher number of adverse effects were observed among participants with female sex, younger age, BMI of less than 25 kg/m2, no prior history of COVID 19, and AstraZeneca vaccination. This study provides critical evidence to the general public regarding COVID-19 vaccine-related symptoms and complications, which may help to dispel misunderstandings and conspiracy beliefs about vaccination. This may reduce vaccine hesitancy, boost vaccine uptake confidence, and increase vaccine trust among the recipients. Thus, the authors advise that participants should receive vaccines with no hesitation, while continuous tracking of vaccine safety is kept in place.
Abbreviations
| BMI | = | Body Mass Index |
| CI | = | Confidence Interval |
| COVID 19 | = | coronavirus disease of 2019 |
| IQR | = | interquartile range |
| SARS-CoV-2 | = | Severe Acute Respiratory Syndrome Coronavirus-2 |
| SD | = | standard deviation |
| WHO | = | World Health Organization |
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Ethical approval and consent to participate
An ethical approval letter from the Ethical Review Committee of Debre Tabor University and a written collaboration letter from Debre Tabor Town Health Department were obtained to carry out this study. The study objective was explained and written consent was obtained from each selected study participant. Participants were also informed that participation is voluntary that they can withdraw from the study at any time and that their decision to continue or not in the study will not influence their provision of healthcare services. Confidentiality of information provided by study subjects was also kept by making the data collection procedure anonymous.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
We are thankful to Debre Tabor University, Debre Tabor Town Health Department, data collectors, supervisors, and study participants for their collaboration and involvement in this research work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduction Targeted Ther. 2020;5(1):1–9. doi:10.1038/s41392-020-00352-y.
- Holder J Tracking coronavirus vaccinations around the world. The New York Times. 2021;30.
- Organization WH. Ethiopia launches a COVID-19 vaccination campaign targeting the 12 years and above population. 2021 November 25.
- Doroftei B, Ciobica A, Ilie O-D, Maftei R, Ilea C. Mini-Review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics. 2021;11(4):579. doi:10.3390/diagnostics11040579.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577.
- Bhatta M, Nandi S, Dutta S, and Saha MK. Coronavirus (SARS-CoV-2): A systematic review for potential vaccines. Hum Vaccines Immunother . 2021;18(1):1865774.
- Chapin-Bardales J, Gee J, Myers T. Reactogenicity following receipt of mRNA-based COVID-19 vaccines. Jama. 2021;325(21):2201–2202. doi:10.1001/jama.2021.5374.
- Kimmel SR. Vaccine adverse events: Separating myth from reality. Am Fam Physician. 2002;66:2113.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi:10.1038/s41586-020-2639-4.
- Walsh EE, Frenck RW, Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi:10.1056/NEJMoa2027906.
- Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi:10.1056/NEJMoa2101544.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, and Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med . 2020;384:403–416.
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: A prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi:10.1016/S1473-3099(21)00224-3.
- Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, Singh K, Yadav D, Sharma P, Misra S, et al. Adverse events reported from COVID-19 vaccine trials: a systematic review. Indian J Clin Biochem. 2021;36(4):427–439. doi:10.1007/s12291-021-00968-z.
- Abu-Hammad O, Alduraidi H, Abu-Hammad S, Alnazzawi A, Babkair H, Abu-Hammad A, Nourwali I, Qasem F, Dar-Odeh N. Side effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines. 2021;9(6):577. doi:10.3390/vaccines9060577.
- Hunter PR. Thrombosis after covid-19 vaccination. Bmj. 2021:n958. doi:10.1136/bmj.n958.
- Lee EJ, Cines DB, Gernsheimer T, Kessler C, Michel M, Tarantino MD, Semple JW, Arnold DM, Godeau B, Lambert MP, et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV -2 vaccination. Am J Hematol. 2021;96(5):534–537. doi:10.1002/ajh.26132.
- Zhongming Z, Linong L, Xiaona Y, Wangqiang Z, Wei L. ‘It’Sa very special picture.’why vaccine safety experts put the brakes on AstraZeneca’s COVID-19 vaccine. 2021.
- Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdox1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi:10.1056/NEJMoa2104840.
- Tobaiqy M, Elkout H, MacLure K. Analysis of thrombotic adverse reactions of COVID-19 AstraZeneca vaccine reported to EudraVigilance database. Vaccines. 2021;9(4):393. doi:10.3390/vaccines9040393.
- Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID-19 vaccine AstraZeneca” exposure. J Clin Med. 2021;10(8):1599. doi:10.3390/jcm10081599.
- Sing CW, Tang CTL, Chui CSL, Fan M, Lai FTT, Li X, Wan EYF, Wong CKH, Chan EWY, Hung IFN, et al. COVID -19 vaccines and risks of hematological abnormalities: Nested case–control and self-controlled case series study. Am J Hematol. 2022;97(4):470–480. doi:10.1002/ajh.26478.
- Mann R, Sekhon S, and Sekhon S. Drug-Induced liver injury after COVID-19 vaccine. Cureus. 2021;13(7):e16491.
- Sampath V, Rabinowitz G, Shah M, Jain S, Diamant Z, Jesenak M, Rabin R, Vieths S, Agache I, Akdis M, et al. Vaccines and allergic reactions: The past, the current COVID‐19 pandemic, and future perspectives. Allergy. 2021;76(6):1640–1660. doi:10.1111/all.14840.
- Kirzinger A, Kearney A, Hamel L, Brodie M. Kff/the Washington Post frontline health care workers survey. The Washington Post/KFF Survey Project. 2021.
- Meo S, Bukhari I, Akram J, Meo A, Klonoff DC. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur Rev Med Pharmacol Sci. 2021;25(3):1663–1669. doi:10.26355/eurrev_202102_24877.
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, et al. Safety and immunogenicity of ChAdox1 nCov-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396(10267):1979–1993. doi:10.1016/S0140-6736(20)32466-1.
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Safety and efficacy of the ChAdox1 nCov-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi:10.1016/S0140-6736(20)32661-1.
- Hatmal MM, Al-Hatamleh MA, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM, Mohamud R. Side effects and perceptions following COVID-19 vaccination in Jordan: A randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Vaccines. 2021;9(6):556. doi:10.3390/vaccines9060556.
- Alhazmi A, Alamer E, Daws D, Hakami M, Darraj M, Abdelwahab S, Maghfuri A, Algaissi A. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines. 2021;9(6):674. doi:10.3390/vaccines9060674.
- Camacho Moll ME, Salinas Martínez AM, Tovar Cisneros B, García Onofre JI, Navarrete Floriano G, Bermúdez de León M. Extension and severity of self-reported side effects of seven COVID-19 vaccines in Mexican population. Front Public Health. 2022;10:834744. doi:10.3389/fpubh.2022.834744.
- Omeish H, Najadat A, Al-Azzam S, Tarabin N, Abu Hameed A, Al-Gallab N, Abbas H, Rababah L, Rabadi M, Karasneh R, et al. Reported COVID-19 vaccines side effects among Jordanian population: A cross sectional study. Hum Vaccines Immunother. 2022;18(1):1981086. doi:10.1080/21645515.2021.1981086.
- Canas LS, Österdahl MF, Deng J, Hu C, Selvachandran S, Polidori L, May A, Molteni E, Murray B, Chen L, et al. Disentangling post-vaccination symptoms from early COVID-19. EClinicalMedicine. 2021;42:101212. doi:10.1016/j.eclinm.2021.101212.
- Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Diabetes Metab Syndr: Clin Res Rev. 2021;15(5):102207. doi:10.1016/j.dsx.2021.102207.
- Riad A, Jouzová A, Üstün B, Lagová E, Hruban L, Janků P, Pokorná A, Klugarová J, Koščík M, Klugar M, et al. COVID-19 vaccine acceptance of Pregnant and Lactating Women (PLW) in Czechia: an analytical cross-sectional study. Int J Environ Res Public Health. 2021;18(24):13373. doi:10.3390/ijerph182413373.
- Zhu F-C, Li Y-H, Guan X-H, Hou L-H, Wang W-J, Li J-X, Wu S-P, Wang B-S, Wang Z, Wang L, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395(10240):1845–1854. doi:10.1016/S0140-6736(20)31208-3.
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: Interim analysis of 2 randomized clinical trials. Jama. 2020;324(10):951–960. doi:10.1001/jama.2020.15543.
- Adam M, Gameraddin M, Alelyani M, Alshahrani MY, Gareeballah A, Ahmad I, Azzawi A, Komit B, Musa A. Evaluation of post-vaccination symptoms of two common COVID-19 vaccines used in Abha, Aseer Region, Kingdom of Saudi Arabia. patient Preference Adherence. 2021;15:1963. doi:10.2147/PPA.S330689.
- Mahallawi WH, Mumena WA. Reactogenicity and Immunogenicity of the Pfizer and AstraZeneca COVID-19 Vaccines. Front Immunol. 2021;12:5169. doi:10.3389/fimmu.2021.794642.
- Organization WH. Interim recommendations for use of the ChAdox1-S [recombinant] vaccine against COVID-19 (AstraZeneca COVID-19 vaccine AZD1222, SII Covishield, SK Bioscience). 2021 [Accessed 2021 May 21].
- Mohammed RA, Garout RM, Wahid S, Ayub F, ZinAlddin LMF, and Sultan I. A survey on the side effects of Pfizer/BioNTech COVID-19 vaccine among vaccinated adults in Saudi Arabia. Cureus. 2021;13(11):e19222.
- Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, et al. Safety and immunogenicity of the ChAdox1 nCov-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi:10.1016/S0140-6736(20)31604-4.
- Shay DK, Gee J, Su JR, Myers TR, Marquez P, Liu R, Zhang B, Licata C, Clark TA, Shimabukuro TT. Safety monitoring of the Janssen (Johnson & Johnson) COVID-19 Vaccine — United States, March–April 2021. MMWR Morb Mortal Wkly Rep. 2021;70(18):680–684. doi:10.15585/mmwr.mm7018e2.
- Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, Pletcher MJ, Marcus GM. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Network Open. 2021;4(12): e2140364-e. doi:10.1001/jamanetworkopen.2021.40364.
- Alghamdi A, Ibrahim A, Almutairi R, Joseph M, Alghamdi G, and Alhamza A. A cross-sectional survey of side effects after COVID-19 vaccination in Saudi Arabia: Male versus female outcomes. J Adv Pharm Educ Res. 2021 Apr-Jun;11(2):51–56.
- Pellini R, Venuti A, Pimpinelli F, Abril E, Blandino G, Campo F, Conti L, De Virgilio A, De Marco F, Di Domenico EG, et al. Obesity may hamper SARS-CoV-2 vaccine immunogenicity. MedRxiv. 2021.
- Flanagan KL, Fink AL, Plebanski M, Klein SL. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33(1):577–599. doi:10.1146/annurev-cellbio-100616-060718.
- Voigt EA, Ovsyannikova IG, Kennedy RB, Grill DE, Goergen KM, Schaid DJ, Poland GA. Sex differences in older adults’ immune responses to seasonal influenza vaccination. Front Immunol. 2019;10:180. doi:10.3389/fimmu.2019.00180.
- Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, Caputi A, Rossetti R, Spoltore ME, Filippi V, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID‐19 mRNA vaccine. Diabetes Metab Res Rev. 2022;38(1):e3465. doi:10.1002/dmrr.3465.
- Iguacel I, Maldonado AL, Ruiz-Cabello AL, Casaus M, Moreno LA, Martínez-Jarreta B. Association between COVID-19 vaccine side effects and body mass index in Spain. Vaccines. 2021;9(11):1321. doi:10.3390/vaccines9111321.
- Mohammad S, Aziz R, Al Mahri S, Malik SS, Haji E, Khan AH, Khatlani TS, Bouchama A. Obesity and COVID-19: What makes obese host so vulnerable? Immun Ageing. 2021;18(1):1–10. doi:10.1186/s12979-020-00212-x.
- Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7(1):66–75. doi:10.3945/an.115.010207.
- McLarnon A. Influenza immunity impaired in obesity. Nat Rev Endocrinol. 2012;8(1):3. doi:10.1038/nrendo.2011.199.
- Painter SD, Ovsyannikova IG, Poland GA. The weight of obesity on the human immune response to vaccination. Vaccine. 2015;33(36):4422–4429. doi:10.1016/j.vaccine.2015.06.101.
- Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, Santos RD, Arsenault B, Cuevas A, Hu FB, et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat Rev Endocrinol. 2020;16(3):177–189. doi:10.1038/s41574-019-0310-7.
- Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, Frias EC, Stewart JL, Van Eyk JE, Braun JG, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27(6):981–984. doi:10.1038/s41591-021-01325-6.
- Bauernfeind S, Salzberger B, Hitzenbichler F, Scigala K, Einhauser S, Wagner R, Gessner A, Koestler J, Peterhoff D. Association between reactogenicity and immunogenicity after vaccination with BNT162b2. Vaccines. 2021;9(10):1089. doi:10.3390/vaccines9101089.
- Powell AA, Power L, Westrop S, McOwat K, Campbell H, Simmons R, Ramsay ME, Brown K, Ladhani SN, Amirthalingam G, et al. Real-World data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March−June 2021, England. Eurosurveillance. 2021;26(28):2100634. doi:10.2807/1560-7917.ES.2021.26.28.2100634.
- Mathioudakis AG, Ghrew M, Ustianowski A, Ahmad S, Borrow R, Papavasileiou LP, Petrakis D, Bakerly ND. Self-Reported real-world safety and reactogenicity of COVID-19 vaccines: A vaccine recipient survey. Life. 2021;11(3):249. doi:10.3390/life11030249.
- Andrzejczak-Grządko S, Czudy Z, Donderska M. Side effects after COVID-19 vaccinations among residents of Poland. Eur Rev Med Pharmacol Sci. 2021;25(12):4418–4421. doi:10.26355/eurrev_202106_26153.
- Coppeta L, Ferrari C, Somma G, Mazza A, D’Ancona U, Marcuccilli F, Grelli S, Aurilio MT, Pietroiusti A, Magrini A, et al. Reduced titers of circulating anti-SARS-CoV-2 antibodies and risk of COVID-19 infection in healthcare workers during the Nine Months after immunization with the BNT162b2 mRNA vaccine. Vaccines. 2022;10(2):141. doi:10.3390/vaccines10020141.
- Abebe EC, Dejenie TA, Ayele TM, Admasu FT, Muche ZT, Adela GA. Mutational pattern, impacts and potential preventive strategies of omicron SARS-CoV-2 variant infection. Infect Drug Resist. 2022;15:1871. doi:10.2147/IDR.S360103.
