ABSTRACT
With the recent COVID-19 pandemic, the importance of vaccine development, distribution, and uptake has come to the forefront of the public eye. Effectively fielding vaccines during an emergency—whether that emergency is a result of an infectious disease or not—requires an understanding of usual vaccine-related processes; the impact of outbreak, complex emergencies, mass gatherings, and other events on patients, communities, and health systems; and ways in which diverse resources can be applied to successfully achieve needed vaccine uptake. In this review, both the emergency setting and briefly vaccine product design are discussed in these contexts in order to provide a concise source of general knowledge from experts in fielding vaccines that can aid in future vaccine ventures and increase general awareness of the process and barriers in various settings.
Introduction
In light of the recent COVID-19 pandemic, the importance of understanding the process and barriers to vaccine development, distribution, and uptake is more important than ever.Citation1 In order for a vaccine to be available to any individual, it must be designed, produced, validated, approved in the jurisdictions where it will be used, manufactured to scale, delivered and staged, and ultimately distributed, accepted, and administered to a population mobilized to receive it. Intention is critical, with each aspect of making and using a vaccine aligned to the use case, including the setting of use. Most vaccines globally are administered in constrained settings through established health services and public health programming. As such, vaccine design, manufacturing, delivery, and uptake rely upon decades of experience by the health infrastructure as well as community patterns of accessing related services. Certain settings, though, challenge assumptions, logistics, and other operational realities on which effective vaccine use in populations depend. Among these are outbreaks, complex emergencies, and mass gatherings. In this article, specific aspects of how such settings impact vaccine-associated logistics are addressed.
There are substantial resource challenges in every setting. Every community is faced with choices regarding how it will apply its wealth and other resources to meet a goal. The richest countries weigh a large breadth of resources against a broad array of challenges that might be successfully met. The poorest countries balance few resources against a broad array of unmet challenges and a narrow list of accomplishable goals. These dynamics do not diminish in an outbreak setting. Much remains to be learned regarding how best to approach vaccine use in any setting. Nonetheless, lessons exist that can help all of us improve the way that we plan for and employ vaccines, regardless of setting. In this review, experts in fielding vaccines provide these lessons learned to aid in future vaccine ventures and increase general awareness of the process and barriers in various settings.
Impact of the setting
Access
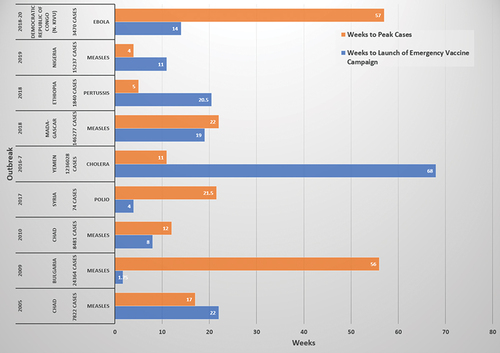
Access is perhaps the most well-recognized aspect of vaccine readiness that differs between well-resourced and resource-limited settings when faced with an emerging or reemerging infectious disease threat. A wealthy country is more likely to have established regulatory and commercial access to a vaccine product, whether the vaccine is novel or existing. It may create the vaccine, be able to both create and produce the vaccine with available biomedical manufacturing infrastructure, or already be positioned to procure the vaccine under existing, agreed overarching, or template contracts. The vaccine or its underlying technology is more likely to already be known by regulatory entities and so achieve emergency use authorization or licensure quickly, depending upon the product, nature of the emergency, and the jurisdiction. In contrast, a country with more limited resources likely will not have an occasional use or novel vaccine in stockpile. Furthermore, the country may not be able to produce the vaccine quickly, even if the formulas for production are made openly available, as a consequence of limited in-place technology coupled with reagent availability. Additionally, while globally more people reside in low-resource settings than high-resource settings, high-resource settings are able to leverage wealth and existing commercial relationships to secure vaccines more effectively, outcompeting broader markets of need. A further complication greatly impacting novel vaccine distribution is export control. Even if wealthy country manufacturing enables a ready supply appropriate for global distribution, sovereign state interests may restrict the ability to allow sufficient quantities of vaccine to enter the global marketplace. All of these barriers to access translate to low-resourced countries waiting weeks to months for vaccine supply after an outbreak is identified ().
Figure 1. Timeline from outbreak onset to peak cases and launch of emergency vaccine campaign in low- to middle-income countries. Select outbreaks from low- to middle-income countries demonstrating the often-lengthy delay in launching an emergency vaccine campaign after outbreak identification.Citation56–64 Case peaks before vaccine campaigns are able to launch or achieve high rates of delivery are common.
Vaccine access initiatives and partnerships
In the non-outbreak setting, strides have been made in vaccine access against pervasive, cosmopolitan threats such as pediatric pneumococcal disease.Citation2 The Global Alliance for Vaccines and Immunizations (GAVI) aims to provide vaccine access to children in some of the lowest-resourced countries in the world.Citation3 While more work needs to be done with an estimated 500,000 children aged <5 y dead from pneumococcal infections in 2015, GAVI and others are making progress advancing use of the pneumococcal vaccine around the world, incorporating financing innovations, as well, to facilitate access.Citation4 In other instances, public-private partnerships have formed to address concerns regarding the ability to facilitate vaccine access to diverse countries. An example of this is the Pox Protein Public-Private Partnership (P5) looking ahead to a potential role for modified Vaccinia Ankara (MVA) in an anti-human immunodeficiency virus (HIV) global vaccine programCitation5 and bringing potential shared aspects of production and distribution into product development discussions.
Analogous initiatives have occurred in the setting of pandemic-threat management as well. There is a renewed discussion internationally that would allow countries to augment their internal regulatory systems with cooperative or international regulatory frameworks. The World Health Organization (WHO) addressed the challenges of disease vigilance and health-related regulatory processes in its 5-y action plan, and it supports systems of assurance and pre-qualification.Citation6 The WHO led Pandemic Influenza Preparedness (PIP)-Framework was established in the aftermath of the 2009–10 influenza A (H1N1) pandemic, where a combination of limited supply and restricted purchasing and distribution of vaccines left low-resource afflicted countries perceiving that they had contributed to knowledge of and products against the pandemic without experiencing timely benefits of vaccine as they underwent subsequent pandemic waves.Citation7 The PIP Framework has succeeded in increasing transparency on how influenza viruses that may be candidate seed strains for vaccine or research are shared between qualified laboratories through its Influenza Virus Traceability Mechanism and templates for sample sharing.Citation8 During the rise of Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), another vaccine access facilitating partnership arose between WHO, GAVI, UNICEF, and the Coalition for Epidemic Preparedness Innovations (CEPI; a global partnership investment group for advancing medical countermeasures against pandemic threats). Referred to as COVAX, this partnership has facilitated access to hundreds of millions of anti-SARS-CoV-2 vaccine doses from various sources to at-risk countries of low resource.Citation9 Parallel, long-lived initiatives for pledged and physical stockpiling exist on a smaller scale against threats associated with outbreaks and epidemics—in particular, yellow fever, cholera, and smallpox.Citation10–12 Yellow fever provides an example of how global supply can be very limited in contrast to need even for well-established vaccines when the marketplace is insufficient to sustain large-scale production and supply, even when the product is considered generic and past most patent protections.Citation13 Well-resourced nations typically manage their own national stockpiles that may feed as pledged doses into global efforts, such as from the U.S. Strategic National Stockpile.Citation14 Bilateral relationships between countries also exist in emergencies, often when the two countries share a common history or current or aspirational commercial relationships. Such sharing has also been employed in what is called soft power, as part of how countries cultivate new or burgeoning political and economic relationships, as has been reported in the context of COVID-19 vaccines between China and Pakistan, India and Bangladesh, and others.Citation15
Purchasing and gifting mechanisms are not the only global approaches to ensuring that vaccines are available to low-resource, at-risk countries. WHO has a pre-qualification process that allows medical countermeasures eligibility for the essential medicines list and other procurement programming by the organization.Citation16 This process’s outputs are used by some regulatory bodies in resource-challenged countries to accelerate regulatory clearance. WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) influences both global programs and country-level vaccine administration policy recommendations by undertaking risk-benefit analyses regarding what individuals in which settings ought to receive a candidate vaccine in and out of emergencies.Citation17 This has direct effects on determinations of vaccine distribution to different countries. The spread of SARS-CoV-2 and its novel coronavirus disease (COVID-19) has led to renewed calls for broader technology transfer to resource-challenged countries so that they can manufacture and distribute their own vaccines and ultimately design their own, as well. The narratives by both advocates and industry have focused on realities of both intellectual property and consequences to how each country structures those systems. Achieving effective distributed production of locally important medical countermeasures globally requires strategies for workforce development, sustainable approaches to manufacturing that address reagents, utilities such as power and clean water, equipment management lifecycles, and other aspects essential to good manufacturing practices.Citation18
Access to routine vaccines
While much of this review focuses on issues of fielding timely vaccine products in response to an emerging or reemerging infectious disease threat, both health and non-health emergencies also significantly impact routine vaccination. Low access and uptake in this context can be striking in crises involving refugees and internally displaced populations. A 2015 systematic review found uptake of critical vaccine interventions such as tetanus in pregnancy as low as 1:10 to 2:5 despite evidence of awareness by at-risk women of the need.Citation19 Why this is the case in emergent large, aggregate settings is sometimes not obvious. To varying degrees, scale of need relevant to both supply and distribution requirements, leveraging impromptu health service systems, and trust by vulnerable persons toward the providers, registration systems, the vaccine, and whether secondary gain by officials is present, all contribute to the complexity of effectively delivering routine and emergent vaccination in these settings. Natural and pandemic-threats disrupt routine vaccination of stable populations, as well. In the United States (U.S.), a recent study of adherence among adolescents to recommendations from the Advisory Committee on Immunization Practices, regularly convened by the Centers for Disease Control and Prevention (CDC), showed a marked decline in routine, scheduled vaccinations lasting at least 3 months into the COVID-19 pandemic.Citation20 This was assessed by cataloging pediatric prescriptions, and the authors attributed it to disruptions in routine healthcare access.
In less well-resourced settings, such access disruptions can lead to palpable increases in preventable deaths. An analysis of routine, scheduled vaccinations in West Africa during the 2014–6 epidemic of Ebola virus disease (EVD) predicted up to 16,000 excess measles deaths associated with decreased healthcare access and, consequently, a 75% reduction in vaccination rates.Citation21 Access issues linked to the decline in mitigation measures, such as vitamin A repletion in malnourished settings to prevent poor outcomes in measles, compounded the risk reflected from these estimates. Distraction from epidemics disrupts surveillance of routinely reported diseases, also, relevant to safe and effective vaccine programming. This occurred with vaccine-derived polio in Guinea under pressure from EVD.Citation22 In both EVD and COVID-19, the application of healthcare precautions and societal measures to limit onward transmission impacted access. Wage loss, as well as shortages in fuel and other aspects making transportation more difficult, exacerbated decreased ability and interest to take time to get vaccinated. Diminished healthcare workforce from illness or flight, patient reticence to engage healthcare settings because of their association with the emergency, and those who are more severely ill, all work together to impact how readily people seek vaccination, and how successful they are in obtaining it when sought. Natural threats such as earthquakes, flooding, rockslides, or community-wide pressures such as drought and famine join these forces with potential mass migration, and physical destruction of roads, healthcare facilities. Stop gap measures as health services adjust to demands and adapt around such challenges have included mobile vaccination teams, rotating vaccine-focused clinics, distributed provision of vaccine services by community health aides, and other extended providers in concert with robust risk communication and social mobilization, incorporating community leadership and diverse stakeholders, such as traditional healers.
Vaccine access during complex emergencies and mass gatherings
Complex emergencies add both dispersed and organized violence intended to disrupt stability, encourage participation and fealty of noncombatants, or act against perceived alliances between combatants and health initiatives. An International Committee of the Red Cross report cites 64 attacks against persons implementing vaccine programming in 2013, affecting internationally deployed and local healthcare workers alike.Citation23 A relationship between violence in eastern Democratic Republic of Congo (DRC) and EVD case rates during their recent epidemic has been established.Citation24 Access to screening, case finding, and vaccination all likely contributed to this effect, through a combination of direct decline in availability of services and the indirect effects of potential retaliation. Despite the existence of a safe and effective Ebola vaccine on site, violence in Beni, DRC, in 2019 halted vaccination and dropped traceable case contacts from 9:10 to less than 1:5.Citation25 Strategies to interrupt violence as a factor in public health emergency response as well as health maintenance programming require persistent multi-stakeholder engagement requiring whole-of-society participation.
Additionally, mass gatherings pose their own unique challenges to vaccine access. Mass gatherings are sometimes anticipated like the Hajj pilgrimage or planned and scheduled events such as the World Cup, but unplanned ones, such as during population migrations from regular droughts in the Sahel or from an escalating conflict, also exist. The principal challenge in these settings is scale, the presence of need accumulated over a much shorter period than in place services evolved to support a much lower density of access. For planned events, organizers can encourage participants to use their access at home to complete vaccination prior to attending them. The ability to travel and even venue ticket purchase can be contingent upon having done so. Saudi Arabia communicates specific requirements for entry to participate with the Hajj and Umrah, for instance.Citation26 For unplanned but anticipated events, particularly those associated with migration, services can target transportation routes so that the burden of access is de-densified. Unfortunately, regular human migration due to drought in the Sahel has long been associated with vaccine-relevant health services gaps and vulnerability.Citation27 Even large human migrations may have very distinct features by location that matter for planning and execution of vaccine campaigns. Bidirectional flow of internally displaced persons between United Nations camps and their points of origin are the norm in South Sudan, while more limited bidirectional flow occurs among Palestinians to and from Jordan and the occupied territories and among Mexicans and other Latin Americans across the U.S.-Mexico border. In contrast, unidirectional flow from sub-Saharan Africa east into the Middle East and north through the Sahara are common.Citation28 Each instance impacts the ability of healthcare workers to attempt more than one encounter with any given migrant.
The international disaster response health services cluster is often relied upon to advise or execute the many ways to provision surge healthcare access. Whether for contingency or event planning or response to a sudden event, preparation for such action requires substantial lead time.
Storage and distribution
Depending upon the threat, vaccine stockpiles may exist in a physical form managed by the WHO or other international actor, in virtual form where vaccines are either present or anticipated in various sourcing national and non-governmental stockpiles, or already distributed to at-risk settings. Aspects of the product itself and human use regulatory requirements that influence this are discussed in a later section. The administration of stockpiles and their delivery is complex. Barriers include the process of procurement; the need to construct sharing agreements and consider aspects of liability; managing requirements imposed by the products’ lifecycle such as production timing, cold chain, and expiration dates; storage needs at every level of distribution; intercontinental, regional, and sub-regional transportation that may include air, sea, and ground transportation; customs requirements and handling; and delays in advising or ensuring receiving at-risk countries’ logistical readiness. Fortunately, global experiences with polio, smallpox, yellow fever, and more recently cholera and pandemic influenza A (H1N1) vaccination campaigns provided robust experience to many stakeholders on both the delivery and receiving end of getting vaccines to communities. While equitable access and distribution continue to be a struggle against every threat, and it can seem like systems get reinvented in each emergency to make storage and distribution happen, the substrate for effective management is present. Adaptive mechanisms for more recent responses to EVD and COVID-19 like COVAX relied upon experienced, in place logistics partners such as GAVI, WHO, UNICEF, World Food Program, and catalyzing influences such as from CEPI.
Another important step in global logistics in at-risk settings happened under the U.S. President’s Emergency Program for AIDS Relief (PEPFAR). Dry, cool, and cold storage requirements for available antiretroviral therapy in the early 2000s coupled with diverse in-country distribution needs resulted in a deliberate program focus on logistical aspects of provisioning therapy. This led to investments in dispersed pharmaceutical-appropriate storage, as well as training and mentored inventory and distribution management relationships. While PEPFAR countries represented a subset of those globally with need, it helped to enculturate the importance of expressly incorporating logistics in public health services planning in at-risk settings beyond exigent emergency response.
Despite these gains in experience and infrastructure, public health logistics innovation remains needed at every level of resource. In the U.S., those in low resource, rural areas are less likely to obtain influenza vaccines unless it is through the established setting of a clinician’s visit as opposed to more innovative approaches such as pharmacies, churches, and workplaces.Citation29 While many U.S. studies focus on whether residents obtain vaccines or not, key components to their ability to get vaccines are having the infrastructure and systems in place to distribute and store them, together with a sufficient number of trained professionals to administer the vaccines. When three major hurricanes devastated Puerto Rico in 2017, more than 2 million USD in vaccines were lost because of power outages or structural damage (Personal Communication, 2021). Since that time, the Puerto Rico Department of Health has sought to build capacity in vaccine administration through purchase and distribution of additional refrigeration equipment and generators, creation of storage facilities and an extensive Vaccine Storage Network (VSN), and recruitment and training of health care providers and pharmacists to increase the availability of vaccines across the islands. With their efforts, the VSN extended access from eight regional centers for vaccine storage to nearly 70, so that residents in even the most rural locations would be served. This work was completed just as the COVID-19 vaccine became available to residents. Consequently, delivery of new vaccine to patients could be accomplished far more quickly than even non-emergency circumstances. This system contributed to Puerto Rico leading the country in vaccine completion rates (78% fully vaccinated compared to 63% nationwide).Citation30
Distributing small-scale logistics centers together with vaccine delivery is an approach used in outbreaks and mass gatherings, as well. Fielding a resource effectively requires applying it where it has value, and so placement must be matched to where meeting need is feasible and ideally sustainable. Solar-powered cold storage in Yemen, for instance, re-enabled routine vaccination programs in several locales after 5 y of conflict had made uncertain power the norm.Citation31
Information management is an often under-appreciated aspect of effective vaccine logistics. Systems for this fulfill three key functions: (1) active management of operational execution; (2) successful planning through demand forecasting, supply-chain gap-analysis, and time-studies; and, (3) quality assurance, adverse reaction reporting, and follow-up tracking. This last element is an increasingly important tool to counter vaccine disinformation. Effectively linking vaccine manufacturer information to a chain-of-custody that connects vaccine origin, destination, and recipient is particularly difficult when existing healthcare system information management infrastructure cannot be relied upon. Additional quality assurance requirements such as temperature records for each process step, tracking who participates in the process and how, material inspection reports, and other information makes achieving a good understanding of vaccine safety and performance in unconstrained settings that much more difficult. Both manufacturers and public health agencies have vested interests in thorough vaccine quality assurances throughout the vaccine supply-chain and processes. Also, rapid and accurate vaccine-related adverse reaction investigations are increasingly critical as each vaccine recipient’s social media claims can quickly become a flashpoint for spreading disinformation and vaccine-hesitancy. Among surveyed persons who declined rVSV-EBOV vaccination in the Democratic Republic of Congo 2018 Ebola virus disease epidemic, three in four expressed insufficient awareness of risks and benefits.Citation32 There are initiatives to field digitized solutions to immunization registries in low- and middle-income countries.Citation33
Measures to overcome logistics challenges in locales with limited health infrastructure sometimes incorporate “greenfield” operational footprints. These are entirely parent organization owned logistics networks and information management systems dropped into an area of need. While this approach may succeed in alleviating outside-of-country stakeholder concerns regarding the feasibility of a vaccine program, there are drawbacks to this approach. High costs and delays in deployment are common. Donor fatigue occurs. Additionally, host countries can view the effort as undermining their sovereignty. In practice, such efforts rarely bring all required assets, equipment, personnel, and support structures to deliver vaccine autonomously. Instead, a significant number of local resources, vendors, and healthcare staff become co-opted into processes. Because the intent is to act separately, it ironically can yield unfortunate consequences for the local healthcare system, which experiences personnel absenteeism, vendor service levels and availability drop, and prices increase above market norms to conform with international organization price-points. Vaccine delivery in the DRC against EVD has pivoted away from “greenfield” vaccine operations, empowering advocates of more local approaches to African COVID-19 vaccination, believing that leveraged rather than co-opted community health infrastructure can extend reach and delivery of vaccines.Citation34
Personnel, supplies, and equipment necessary for implementing vaccine programming are subject to the same security challenges as those who live and work in the communities they seek to service. Complex emergencies may require increasing security measures for personnel, facilities, and transit. This must be balanced against exacerbating perceptions that vaccine efforts represent a particular side to conflict. Effective measures may be hard or soft – they might include increased vigilance, careful scheduling, limiting dispersed stockpiling, or unobtrusive escorts. However, whatever security mechanics are employed, they must be de-escalatory in nature and facilitate, not diminish opportunities for access to the vaccine. Accords facilitating vaccine access can be reached and may be served by broadening the stakeholders in the conversation such as faith and other community leaders whose influence may span conflicting parties.
Uptake issues
Well-considered frameworks on promoting vaccine acceptance and demand exist, applicable to routine and emergency settings alike.Citation35–37 These frameworks acknowledge that a wide mix of factors come together to yield vaccine uptake, namely perceived disease risk, vaccine confidence, social norms, presence of trusted broker reinforcement, an individual sense of right to access care as might be impacted by gender and minority equity issues, practical aspects of access such as direct and indirect costs (e.g., travel time, lost work), availability, and nature of the relationship the individual has with health systems generally and specifically related to the vaccine. In impoverished settings, stark realities of hunger may compete with attempts to demonstrate the need for vaccines.Citation38
Outbreaks, complex emergencies, and mass gatherings are sources of uncertainty at every step of an individual’s decision-making as to whether or not to seek and receive a vaccine. Even after overcoming the hurdles of receiving and storing supplies on-site, launching a campaign does not always equate to high uptake, potentially limiting the vaccine’s impact on curbing cases in these situations (). The very nature of the threat often is not broadly understood—when a threat is new, people lack a frame of reference to appreciate consequences of a lack of vigilance. And, over time, appreciation of risk can become fatigued. A recent U.S. survey revealed persistent COVID-19 vaccine hesitancy among younger persons (less than age 55) and those less educated, while also showing relatively increased hesitancy among some minority groups.Citation39,Citation40
In established communities in outbreaks and complex emergencies, norms regarding vaccination are unseated by the event. In mass gatherings, this is further complicated by a large, new set of communities that have not had time to establish norms relevant to emergency health services. Emergencies and movement away from usual support systems may exacerbate a sense of unworthiness or fear. Complex emergencies have the added challenge of combatant-applied pressure on whether participation in a vaccine campaign is an act of allegiance, which could be punishable. One example of this is Boko Haram’s hindrance of polio vaccination in Nigeria,Citation41 purportedly employing violence against both healthcare workers and recipients. Old wounds from imperialism of the global north into the global south complicates such discussions, providing a ready retreat from willingness to accept pandemic-threat solutions from those who are not trusted by local communities.
Taken together, there is a danger in over attributing individual reticence regarding the nature of the vaccine itself or the threat as a reason for low vaccine uptake. Aspects like those discussed in the previous sections that make it possible to freely consider taking a vaccine must be addressed for a vaccine campaign to succeed. However, open, multi-stakeholder deliberation that acknowledges what is known and not known, and is clear when information has changed, is recommended.Citation42,Citation43
Designing vaccine products that enhance access, storage and distribution, and uptake
When considering what aspects of the setting and use case should be incorporated in design approaches for vaccines employed in emergencies, the obvious ones are the most important. Deployed vaccines must be both safe and effective in meeting aims that are well articulated. However, additional design elements can ease logistical burdens.
Approaches to formulation drive many facets of logistics. While cold chain capabilities are more widely distributed than often realized, the mRNA platform −80°C requirements for COVID-19 vaccination posed several challenges.Citation44 However, availability of doses to resource-limited settings had more to do with alternative vaccine approaches than cold chain capability. Furthermore, strain on the amount of −80°C storage was ameliorated by later stability testing as more experience with related products was gained. However, the environmental conditions most conducive to broad distribution should be considered so that early assessment of stability as well as lyophilization, freeze-drying, and other approaches as relevant to the fielded vaccine technology can be fully explored.Citation45
When useful for magnitude of immunogenicity, immune compartment effects, dose sparing, vaccine acceptability, or other practical aspects of delivery, mechanism of administering the vaccine should be further elaborated early in the development process. Yellow fever vaccine availability through stockpiling has required rationing in recent years. However, in both emergency setting and longitudinal assessment, one-fifth fractional doses administered intradermally have been shown to provide similar neutralizing antibody levels to full dose intramuscular administration.Citation46–48 Oral administration of cholera vaccine has enabled widely distributed, rapid deployment with a diverse workforce as well as high uptake.Citation49
The ability to manufacture vaccine rapidly in diverse settings has been a topic of discourse in the COVID-19 pandemic and other recent high-profile epidemics that preceded it.Citation50 Technologies offer promise of rapid adaptability, though being able to transfer technology requires a broad spectrum of workforce, utilities and technology-associated infrastructure, reagent and other supply, commercial, and regulatory investments. National interests may contribute into these challenges through export controls and competitive purchasing.Citation51,Citation52 Common narratives blaming patent protections belie this constellation of barriers that can be met methodically. If the deeper issues are solved, companies and governments could license manufacture of emergency products. One piece of such a solution is the Medicines Patent Pool, a United Nations-backed activity that seeks increase development and distribution of medicines in low- and middle-income countries (LMIC). Partnering with civil society, industry, patient groups, international organizations, and governments, it facilitates cooperative work through shared intellectual property and licensing.Citation53 Industry partners have used this mechanism in the COVID-19 pandemic.Citation54,Citation55
Product manufacturers tend to consider the regulatory space as an extrinsic factor. However, disclosing development plans, early engagement of regulatory stakeholders, honest, rolling evaluations and communications of results anchored in use case and avoiding corporate or agency success branding, and stepwise explanations for why successive levels of regulatory clearance are being sought could help a transparency agenda and aid risk communication.
Summary
In addition to baseline population health, vaccine access, storage and distribution strategy, and factors impacting vaccine uptake are all key facets of any setting where use of a vaccine is indicated. Outbreaks, complex emergencies, mass gatherings, and other resource-stretching events introduce challenges to how each of these aspects of vaccine programming are executed. Lessons from prior emergencies and opportunities in vaccine, support system, and program design all assist developers and operational implementers alike in attaining results through vaccination. However, efforts must be deliberate, involve broad stakeholders, and start as early as possible in order to ensure the broadest utility when opportunities to intervene on disease transmission may be limited.
Despite the COVID-19 pandemic, women in a small village in South Sudan volunteered to retrieve polio vaccines for the children in their village by traveling over 30 km by foot.Citation65 Credit: @whosouthsudan (with permission).

Disclaimer
Views are those of the authors and do not necessarily represent those of the State of Nebraska or any other agency.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ullah I, Khan KS, Tahir MJ, Ahmed A, Harapan H. Myths and conspiracy theories on vaccines and COVID-19: potential effect on global vaccine refusals. Vacunas. 2021 ;22(2):1–9. doi:10.1016/j.vacun.2021.01.001.
- Feldman C, Anderson R. Recent advances in the epidemiology and prevention of Streptococcus pneumoniae infections. F1000res. 2020:9. doi:10.12688/f1000research.22341.1.
- The Global Alliance for Vaccines and Immunizations. The global alliance for vaccines and immunizations; 2022 Mar 1 [accessed 2022 Mar 1]. https://www.gavi.org/.
- Pneumococcal AMC. The global alliance for vaccines and immunizations; 2022. Updated 2021 May 11 [accessed 2022 Mar 1]. https://www.gavi.org/investing-gavi/innovative-financing/pneumococcal-amc?gclid=Cj0KCQiA09eQBhCxARIsAAYRiymWMniKEBUJ2rWfmqcrwbTDByVB-YXfAS8qniOzokWguRXyBx9wicoaAvzhEALw_wcB .
- Pox protein public-private partnership (P5); 2022 Mar 1. Updated 2017 Nov 17 [accessed 2022 Mar 1]. https://www.niaid.nih.gov/research/pox-protein-public-private-partnership.
- Delivering quality-assured medical products for all 2019-2023: WHO’s five-year plan to help build effective and efficient regulatory systems; 2019:35. https://apps.who.int/iris/handle/10665/332461 .
- Pandemic Influenza Preparedness (PIP) Framework. World health organization; 2022 Mar 1. https://www.who.int/initiatives/pandemic-influenza-preparedness-framework.
- Influenza Virus Traceability Mechanism. 2022 Mar 1. https://extranet.who.int/ivtm2.
- Coalition for Epidemic Preparedness Innovations. 2022 Mar 1. https://cepi.net/covax/.
- Yellow fever vaccine support. The global alliance for vaccines and immunizations; Updated 2019 Dec 5 [accessed 2022 Mar 1]. https://www.gavi.org/types-support/vaccine-support/yellow-fever.
- Cholera vaccine. World health organization; [ accessed 2022 Mar 1]. https://www.who.int/teams/immunization-vaccines-and-biologicals/diseases/cholera.
- Smallpox vaccines. World health organization; [accessed 2022 Mar 1]. https://www.who.int/news-room/feature-stories/detail/smallpox-vaccines.
- Hansen CA, Barrett ADT. The present and future of yellow fever vaccines. Pharmaceuticals (Basel). 2021 Sep 1;14(9). doi:10.3390/ph14090891.
- Strategic National Stockpile. U.S. department of health & human services; Updated 2021 Aug 16 [accessed 2022 Mar 1]. https://chemm.hhs.gov/sns.htm .
- Jennings M. Vaccine diplomacy: how some countries are using COVID to enhance their soft power; 2021 Feb 22 [accessed 2022 Mar 1]. https://theconversation.com/vaccine-diplomacy-how-some-countries-are-using-covid-to-enhance-their-soft-power-155697.
- Welcome to Vaccines Prequalification. World health organization; [accessed 2022 Mar 1. https://extranet.who.int/pqweb/vaccines
- Strategic Advisory Group of Experts on Immunization (SAGE). World health organization; Updated 2021 Dec 2 [accessed 2022 Mar 1]. https://www.who.int/groups/strategic-advisory-group-of-experts-on-immunization.
- Current Good Manufacturing Practice (CGMP). Regulations. U.S food & drug administration; Updated 2020 Sep 21 [accessed 2022 Mar 1]. https://www.fda.gov/drugs/pharmaceutical-quality-resources/current-good-manufacturing-practice-cgmp-regulations.
- Lam E, McCarthy A, Brennan M. Vaccine-Preventable diseases in humanitarian emergencies among refugee and internally-displaced populations. Hum Vaccin Immunother. 2015;11(11):2627–2636. doi:10.1080/21645515.2015.1096457.
- Elam-Evans LD, Yankey D, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020 Aug 21;69(33):1109–1116. doi:10.15585/mmwr.mm6933a1.
- Takahashi S, Metcalf CJ, Ferrari MJ, et al. Reduced vaccination and the risk of measles and other childhood infections post-Ebola. Science. 2015 Mar 13;347(6227):1240–1242. doi:10.1126/science.aaa3438.
- Fernandez-Garcia MD, Majumdar M, Kebe O, et al. Emergence of vaccine-derived polioviruses during ebola virus disease outbreak, Guinea, 2014-2015. Emerg Infect Dis. 2018 ;24(1):65–74. doi:10.3201/eid2401.171174.
- Health care in danger: violent incidents affecting the delivery of health care, January 2012 to December 2013; 2014. https://www.icrc.org/en/doc/assets/files/publications/icrc-002-4196.pdf.
- Kraemer MUG, Pigott DM, Hill SC, et al. Dynamics of conflict during the Ebola outbreak in the democratic republic of the Congo 2018-2019. BMC Med. 2020 Apr 27;18(1):113. doi:10.1186/s12916-020-01574-1.
- Ebola Response Workers Are Killed in Congo. The New York Times. https://www.nytimes.com/2019/11/28/world/africa/ebola-congo.html.
- Hajj and Umrah Health Requirements. The embassy of the kingdom of Saudi Arabia; [accessed 2022 Mar 1]. https://www.saudiembassy.net/hajj-and-umrah-health-requirements.
- Carnell MA, Guyon AB. Nutritional status, migration, mortality, and measles vaccine coverage during the 1983-1985 drought period: Timbuktu, Mali. J Trop Pediatr. 1990 ;36(3):109–113. doi:10.1093/tropej/36.3.109.
- World Migration Report. International Organization for Migration (IOM); 2020 [accessed 2022 Mar 1]. https://worldmigrationreport.iom.int/wmr-2020-interactive/.
- Bennett KJ, Pumkam C, Probst JC. Rural-Urban differences in the location of influenza vaccine administration. Vaccine. 2011 Aug 11;29(35):5970–5977. doi:10.1016/j.vaccine.2011.06.038.
- Vaccinating People in Puerto Rico. Centers for disease control and prevention; Updated 2021 Aug 30 [accessed 2022 Mar 1]. https://www.cdc.gov/vaccines/covid-19/health-departments/features/puerto-rico.html.
- Ali AQ. Enhancing immunization programmes in Yemen. UNICEF; [accessed 2022 Mar 1]. https://www.unicef.org/yemen/stories/enhancing-immunization-programmes-yemen.
- Kasereka MC, Sawatzky J, Hawkes MT. Ebola epidemic in war-torn democratic republic of Congo, 2018: acceptability and patient satisfaction of the recombinant vesicular stomatitis virus - zaire ebolavirus vaccine. Vaccine. 2019 Apr 10;37(16):2174–2178. doi:10.1016/j.vaccine.2019.03.004.
- Electronic immunization registries in low- and middle-income countries; 2021. https://static1.squarespace.com/static/59bc3457ccc5c5890fe7cacd/t/60aee1bfd163646306fb924c/1622073794356/Digital+Square+EIR+Landscape_Final.pdf.
- Rogers AB, Barrie MB, Fallah MP, Kelly JD. Equitable and feasible distribution of SARS-CoV-2 vaccines for all in Africa. Am J Trop Med Hyg. 2021 Jun 28;105(2):278–280. doi:10.4269/ajtmh.21-0264.
- Risk communication and community engagement preparedness and readiness framework: Ebola response in the democratic republic of the Congo in North Kivu. World health organization; [accessed 2022 Mar 1]. https://www.who.int/publications/i/item/9789241514828.
- Acceptance and demand. World health organization; [accessed 2022 Mar 1. https://www.who.int/teams/immunization-vaccines-and-biologicals/essential-programme-on-immunization/demand.
- Kuppalli K, Brett-Major DM, Smith TC. COVID-19 vaccine acceptance: we need to start now. Open Forum Infect Dis. 2021;8(2):ofaa658. doi:10.1093/ofid/ofaa658.
- Drexler M. The troubled history of vaccines and conflict zones. National public radio. https://www.npr.org/sections/goatsandsoda/2021/08/29/1031007332/the-troubled-history-of-vaccines-and-conflict-zones.
- Siegler AJ, Luisi N, Hall EW, et al. Trajectory of COVID-19 vaccine hesitancy over time and association of initial vaccine hesitancy with subsequent vaccination. JAMA Netw Open. 2021 Sep 1;4(9):e2126882. doi:10.1001/jamanetworkopen.2021.26882.
- Monte LM. Household pulse survey shows many don’t trust COVID vaccine, worry about side effects. United States census bureau; 2021 Dec 28 [accessed 2022 Mar 1]. https://www.census.gov/library/stories/2021/12/who-are-the-adults-not-vaccinated-against-covid.html.
- Haruna Umar KL. Nigeria’s Boko Haram extremists hamper polio eradication. AP News; 2018 Apr 16. https://apnews.com/article/3f27aea190ae418483d0eb281eeb97d8.
- Omer SB, Benjamin RM, Brewer NT, et al. Promoting COVID-19 vaccine acceptance: recommendations from the lancet commission on vaccine refusal, acceptance, and demand in the USA. Lancet. 2021 Dec 11;398(10317):2186–2192. doi:10.1016/S0140-6736(21)02507-1.
- Wynia ALTCVLMLKKDCFAFSMDB-MM. Uncertainty, scarcity and transparency: public health ethics and risk communication in a pandemic; 2022.
- Uddin MN, Roni MA. Challenges of storage and stability of mRNA-based COVID-19 vaccines. Vaccines (Basel). 2021 Sep 17;9(9). doi:10.3390/vaccines9091033.
- Hansen LJJ, Daoussi R, Vervaet C, Remon JP, De Beer TRM. Freeze-Drying of live virus vaccines: a review. Vaccine. 2015 Oct 13;33(42):5507–5519. doi:10.1016/j.vaccine.2015.08.085.
- Roukens AHE, van Halem K, de Visser AW, Visser LG. Long-term protection after fractional-dose yellow fever vaccination: follow-up study of a randomized, controlled, noninferiority trial. Ann Intern Med. 2018 Dec 4;169(11):761–765. doi:10.7326/M18-1529.
- Roukens AH, Vossen AC, Bredenbeek PJ, van Dissel JT, Visser LG. Intradermally administered yellow fever vaccine at reduced dose induces a protective immune response: a randomized controlled non-inferiority trial. PLoS One. 2008 Apr 23;3(4):e1993. doi:10.1371/journal.pone.0001993.
- Casey RM, Harris JB, Ahuka-Mundeke S, et al. Immunogenicity of fractional-dose vaccine during a yellow fever outbreak - final report. N Engl J Med. 2019 Aug 1;381(5):444–454. doi:10.1056/NEJMoa1710430.
- Pezzoli L; Oral Cholera Vaccine Working Group of the Global Task Force on Cholera C. Global oral cholera vaccine use, 2013-2018. Vaccine. 2020 Feb 29;38(Suppl 1):A132–A140. doi:10.1016/j.vaccine.2019.08.086.
- Maxmen A. The fight to manufacture COVID vaccines in lower-income countries. Nature. 2021;597(7877):455–457. doi:10.1038/d41586-021-02383-z.
- U.S. Export Control. International trade administration; [accessed 2022 Mar 1]. https://www.trade.gov/us-export-controls .
- Andrew PD. A nation battling flu, and short vaccine supplies; 2009 Oct 25. https://www.nytimes.com/2009/10/26/health/26flu.html.
- MPP welcomes WHO announcement of the first COVID-19 mRNA vaccine technology transfer hub to be established in South Africa. Medicines patent pool; 2021 Jun 22. https://medicinespatentpool.org/news-publications-post/who-covid-19-mrna-vaccine-tech-transfer-hub-sa.
- Pfizer and The Medicines Patent Pool (MPP). Sign licensing agreement for COVID-19 oral antiviral treatment candidate to expand access in low- and middle-income countries; 2021 Nov 16. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-medicines-patent-pool-mpp-sign-licensing.
- Aboulenein A. Merck signs pact to broaden generic manufacturing of COVID-19 pill. Reuters; 2021 Oct 27. https://www.reuters.com/business/healthcare-pharmaceuticals/merck-signs-pact-broaden-generic-manufacturing-covid-19-pill-2021-10-27/.
- Guerrier G, Guerra J, Fermon F, Talkibing WB, Sekkenes J, Grais RF. Outbreak response immunisation: the experience of Chad during recurrent measles epidemics in 2005 and 2010. Int Health. 2011 ;3(4):226–230. doi:10.1016/j.inhe.2011.06.003.
- Marinova L, Kojouharova M, Mihneva Z. An ongoing measles outbreak in Bulgaria, 2009. Euro Surveill. Jul 2 2009;14(26).
- Muscat M, Marinova L, Mankertz A, et al. The measles outbreak in Bulgaria, 2009-2011: an epidemiological assessment and lessons learnt. Euro Surveill. 2016;21(9):30152. doi:10.2807/1560-7917.ES.2016.21.9.30152.
- Mbaeyi C, Moran T, Wadood Z, et al. Stopping a polio outbreak in the midst of war: lessons from Syria. Vaccine. 2021 Jun 23;39(28):3717–3723. doi:10.1016/j.vaccine.2021.05.045.
- Federspiel F, Ali M. The cholera outbreak in Yemen: lessons learned and way forward. BMC Public Health. 2018 Dec 4;18(1):1338. doi:10.1186/s12889-018-6227-6.
- Sodjinou VD, Douba A, Nimpa MM, Masembe YV, Randria M, Ndiaye CF. Madagascar 2018-2019 measles outbreak response: main strategic areas. Pan Afr Med J. 2020;37:20. doi: 10.11604/pamj.2020.37.20.24530.
- Mitiku AD, Argaw MD, Desta BF, et al. Pertussis outbreak in southern Ethiopia: challenges of detection, management, and response. BMC Public Health. 2020 Aug 11;20(1):1223. doi:10.1186/s12889-020-09303-2.
- Jean Baptiste AE, Wagai J, Luce R, et al. Measles outbreak in complex emergency: estimating vaccine effectiveness and evaluation of the vaccination campaign in Borno State, Nigeria. BMC Public Health. 2019;21(1):437. doi:10.1186/s12889-021-10436-1.
- Ebola Virus Disease Democratic Republic of the Congo. External situation report 98; 2020. https://apps.who.int/iris/bitstream/handle/10665/332654/SITREP_EVD_DRC_20200623-eng.pdf.
- How local communities made polio vaccination possible despite the fear of COVID-19. https://www.afro.who.int/news/how-local-communities-made-polio-vaccination-possible-despite-fear-covid-19.
