ABSTRACT
Efficacy and safety data on quadrivalent influenza vaccines (QIVs) for immunization of Indian children are scarce. This phase 3, registration study evaluated the immunogenicity, safety, and tolerability of a QIV in Indian children aged 6–35 months (Group 1) and 3–17 y (Group 2). Subjects received one or two doses (0.5 mL each) of the study vaccine based on their priming status. Immunogenicity (post-vaccination geometric mean fold increase in hemagglutination inhibition [HI] titers and proportion of patients with seroprotection and seroconversion against the four influenza strains), unsolicited adverse events (AEs), and tolerability were analyzed. Among 118 subjects enrolled in each group, the geometric mean(standard deviation) fold increase in HI titers against A(H3N2), A(H1N1), B(Victoria), and B(Yamagata) strains were 31.7(5.33), 10.5(6.06), 4.1(5.70), and 8.6(5.34) in Group 1 and 14.0(4.37), 9.2(4.26), 14.3(6.73), and 14.4(5.41) in Group 2, respectively. Seroprotection was achieved by 91.2%, 83.3%, 41.2%, and 68.4% subjects in Group 1 and 100%, 95.8%, 73.7%, and 89.8% subjects in Group 2, respectively. Seroconversion was achieved by 87.7%, 66.7%, 41.2%, and 64.9% subjects in Group 1 and 89.0%, 78.8%, 69.5%, and 75.4% subjects in Group 2, respectively. Vaccination site pain and fever were the most common local and systemic reactions, respectively. Systemic reactions were more frequent in Group 1 (16.9% vs 7.6%). Most subjects (>90%) did not experience inconvenience within 7 d of vaccination; <10% in both groups reported unsolicited AEs. Thus, the QIV had a positive benefit/risk profile in Indian children/adolescents aged 6 months to 17 y.
CTRI Registry No: CTRI/2018/05/014191
Registry Name: Clinical Trials Registry – India
Date of Trial Registration: May 29, 2018
Study Dates: August 03, 2018 (first subject first visit) to January 31, 2019 (last subject last visit)
Drugs Controller General of India [DCGI] permission letter number: CT-03/2018
Introduction
Influenza or ‘the common flu’ is an acute respiratory infectious disease caused by influenza virus.Citation1 Out of four types of influenza viruses (A to D), influenza A and B are the most common and widely circulating influenza viruses that cause flu outbreaks worldwide.Citation2 As per World Health Organization (WHO), influenza remains a substantial contributor in global burden of diseases, causing 3–5 million cases of severe respiratory tract infections and 3–6 million respiratory deaths per year globally.Citation1,Citation3
Influenza is one of the major causes of high morbidity and mortality in high-risk categories, including elderly people ≥65 y, children 6 months to 5 y of age (children younger than 2 y are at the highest risk), pregnant women (at any stage of pregnancy) and women up to 2 weeks after the end of pregnancy, and people of any age with chronic medical conditions.Citation4 Children have a higher annual global incidence of influenza in comparison to adults (20–30% versus 5–10%)Citation1,Citation5 due to their lower previous exposure to circulating influenza viruses, which causes a significant increase in school absenteeism, hospitalizations, and fatal cases.Citation6 Once infected, children shed a higher titer of virus for a longer period of time (2 weeks),Citation7–9 causing them to be the main source of transmission of influenza virus and secondary illness to close contacts and communities, which causes a high socioeconomic and clinical burden on the society. In 2017, Purakayastha et al.Citation10 estimated 16,000,000 cases of influenza in under-fives in India in 2016, including 11,000,000 influenza associated outpatient visits, 1,500,000 respiratory hospitalizations, and 28,000 deaths. According to virological data obtained from the influenza surveillance network led by National Institute of Virology, India, respiratory excess death estimates were 51.1 deaths/100,000 population for subjects aged ≥65 y, 9.8 deaths/100 ,000 for subjects aged <5 y, and 1.1 deaths/100 000 for those aged 5–64 y. For children <5 y, the prevalent circulating virus subtype strain was found to be associated with the highest mortality rate.Citation11 Hence, child vaccination is expected to reduce the overall impact of influenza within the household and the wider community, lower the risk of laboratory-confirmed influenza-related pediatric deaths, and serve as a cost-beneficial way to prevent influenza infection.Citation12 Both CDC and WHO recommend vaccination of household contacts and caregivers of infants so as to reduce the risk of transmission of influenza infection to the infant, who may further serve as a gateway to infection for a wider community.Citation1,Citation13
All children aged 6 months to 8 y who are being vaccinated for the first time should receive 2 vaccine doses separated by ≥4 weeks to generate sufficient protective immune response. This would increase the vaccine effectiveness against the infection and provide residual protection for the subsequent season.Citation14 Once the child is primed, a single dose of vaccine is sufficient to elicit protective antibodies.Citation15
The American Academy of Pediatrics (AAP) 2019–2020 recommendations for prevention and control of influenza in children suggested that all pediatric influenza vaccines to be quadrivalent influenza vaccine (QIV).Citation16 As compared to trivalent inactivated influenza vaccine (TIV) vaccines with only one B-strain, QIV vaccines contain two influenza B strains from both Victoria and Yamagata lineages, which increase the likelihood of better protection against influenza B diseases. Various studies have shown QIV to have a higher immunogenicity with comparable reactogenicity and safety profile to TIV.Citation17–20 The QIV was also found to be cost-effective (in terms of direct and indirect costs) in infants and children (6 months to 9 y) and elderly subjects (≥80 y).Citation21 It was associated with 69% fewer medical visits, 75% fewer hospitalizations, 77% fewer absences from school, and 61% fewer parental absences from work in children with moderate-to-severe influenza.Citation22 Influvac® Tetra is an inactivated subunit QIV that has previously shown adequate immune response and a favorable safety profile in healthy Indian adults (aged 18–60 y) and elderly subjects (>61 aged y).Citation23
The present study was a registration trial to evaluate the immunogenicity via pre- and post-vaccination antibody titers and proportion of subjects achieving seroprotection and seroconversion, reactogenicity (tolerability), and safety of Influvac® Tetra in infants/toddlers (6–35 months) and children/adolescents (3–17 y) from India.
Materials and methods
Study design
This was a Phase III, open-label baseline-controlled study in children and adolescents aged 6 months to 17 y to assess the immunogenicity, reactogenicity, and safety of QIV in India. The study was conducted between August 2018 and January 2019 across nine sites in India (one site each in Punjab, West Bengal, Tamil Nadu, and Uttar Pradesh, two sites in Gujarat, and three sites in Karnataka). The study protocol and informed consent/assent forms were reviewed and approved by all relevant institutional review boards (Appendix A) and DCGI, and the study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, International Council for Harmonization guideline E11 on Clinical Investigation of Medicinal Products in the Pediatric Population, and Schedule Y requirements of the Drugs and Cosmetic Rules of India, 1945. Written informed assent from subjects or written informed consent from subjects’ parents/legally acceptable representatives (LARs) were obtained prior to participation. Source data were retained by individual centers. Data were captured in electronic case report forms and submitted to a central server maintained by the study monitor IQVIA and audit trailed.
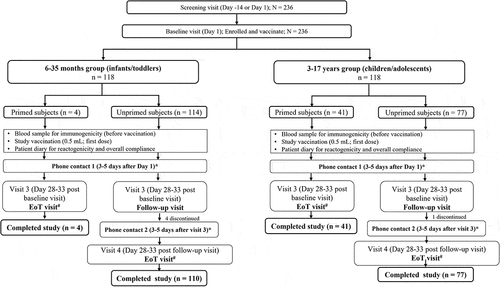
Subjects were categorized into primed and unprimed based on their prior vaccination status. Children and adolescents aged ≥9 y or children aged <9 y who had received ≥2 doses of a seasonal influenza vaccine at least 1 month apart were considered as primed subjects. Children aged <9 y who had previously not received ≥2 doses of a seasonal influenza vaccine were considered as unprimed subjects. Both primed and unprimed subjects received one 0.5 mL (15 µg per strain) dose of study vaccine on D 1. After 28–33 d, blood samples were collected from the primed subjects to assess immunogenicity, and the unprimed subjects received the second 0.5 mL dose vaccine. After 28–33 d of receiving the second dose of the vaccine, blood samples were collected from the unprimed subjects to assess immunogenicity ().
Figure 1. Study schematics.
Vaccine was administered intramuscularly in the deltoid muscle of the upper arm or in the anterolateral region of the thigh in infants and toddlers, if preferred because of limited muscle mass. Vaccination was performed in the opposite arm from which the blood sample was taken for immunogenicity assessment. All children were observed at the study site for at least 30 min after administration of the vaccine to monitor for any immediate adverse reactions.
During the conduct of the study, any medication(s) that could influence the immune system, systemic use of corticosteroids or any planned vaccinations were prohibited. However, use of topical applications (e.g., creams, ocular drops, and inhalation and intranasal sprays), within the dosage of the package insert and without any sign of systemic exposure, was allowed.
Study Population
Subjects (males and females) aged ≥6 months-17 y with stable health condition or underlying illness (if their signs and symptoms were in control) or on a stable dose of prescribed medication for at least 3 months were eligible to participate in the study after receiving the assent/consent from them/their parent(s) or LARs. Subjects who were 6–24 months of age on D 1 were included if they were born at full term of pregnancy (≥37 weeks) and had birth weight ≥2.5 kg.
Subjects were excluded from the study if they had a history of hypersensitivity to influenza vaccine or its components, Guillain–Barŕe syndrome, other progressive neurological disease or seizures, immunosuppressive or immunodeficient condition based on medical history and physical examination, or any other disorders as per investigator discretion; ongoing aspirin therapy; prior receipt of any seasonal or pandemic influenza vaccine or laboratory-confirmed influenza infection in the 6 months preceding the study vaccination; receipt of any vaccine including routine childhood vaccines within 30 d or planned vaccination during the study within 30 d after any study vaccine administration, immunosuppressive medications/immunoglobulins or any blood products within 3 months, or cytotoxic drugs, anticancer chemotherapy, or radiation therapy within 36 months; fever and/or acute disease or infection on the day of study vaccination; a solid organ or bone marrow/stem cell transplant recipient; known drug or alcohol abuse; planned surgery requiring a general anesthetic; positive urine pregnancy test in case of adolescents of childbearing potential, or were the family members of the sponsor or contract research organization. Subjects were stratified by age groups 6–35 months and 3–17 y at enrollment.
Vaccine
Inactivated subunit QIV Influvac® Tetra, Abbott Biologicals B.V., Netherlands,Citation24 contains 15 µg of hemagglutinin and neuraminidase of each of the four viral strains (A/Michigan/45/2015 (H1N1) pdm09-like virus, A/Singapore/INFIMH-16-0019/2016 (H3N2)-like virus, B/Phuket/3073/2013-like virus, and B/Brisbane/60/2008-like virus), as recommended by the WHO for the 2018 season, in a total volume of 0.5 mL dose.
Outcomes
Immunogenicity
The primary objective of the study was to describe the immunogenicity of QIV in infants/toddlers aged 6–35 months (6–35 months group) and children/adolescents aged 3–17 y (3–17 y group). The primary variables were pre- and post-vaccination hemagglutination inhibition (HI) antibody geometric mean titer (GMT) against the four vaccine strains, geometric mean fold increase (GMFI) in HI titers, and the proportion of seroconverted and seroprotected subjects post-vaccination. Antibody titers were assessed using HI assay and were performed by VisMederi in Siena, Italy. For transfer (import/export) of human biological samples for commercial purposes, notification issued by the Directorate General of Foreign Trade, Ministry of Commerce & Industry, Government of India, 2016, was followed. After sera were separated from the blood samples, they were stored at −20°C until titration. The test was done in duplicates for each sample; pre- and post-vaccination sera were titrated simultaneously. The titer assigned to each sample was calculated as the geometric mean of two independent measurements.
The seroconversion rate for each age group was defined as the percent of subjects with either a pre-vaccination titer <10 and a post-vaccination titer ≥40 or a pre-vaccination titer ≥10 and at least a fourfold post-vaccination increase. The seroprotection rate for each group was defined as the percent of subjects with an HI titer ≥40 post-vaccination. Seroconversion and seroprotection rates and GMFI were summarized according to the derived serology criteria that were used by the Committee for Medicinal Products for Human Use (CHMP) in defining a satisfactory immune response to influenza vaccination in the context of the requirements for annual influenza strain updates.Citation24 Since no criteria have been established for children, in this study, criteria for adults have been used instead. These criteria included seroconversion rate >40%, seroprotection rate >70%, and GMFI >2.5.Citation25
Reactogenicity and Safety
The secondary objective of the study was to describe the reactogenicity and safety of QIV in 635 months and 3–17-y groups. The secondary variables were reactogenicity and overall inconvenience during first 7 d after vaccination, and unsolicited adverse events (AEs). Reactogenicity assessment included solicited vaccination site reactions (local reactions) and solicited general adverse events (systemic reactions) within first 7 d of vaccination. All solicited reactions were considered as vaccination related. The local reactions included redness, swelling, induration (hardening), vaccination site pain, and ecchymosis (bruising). The systemic reactions included fever, irritability/fussiness, drowsiness, sweating, diarrhea/vomiting, and loss of appetite (for subjects 6 months to 5 y of age) or fever, headache, fatigue, gastrointestinal symptoms (nausea, vomiting, diarrhea, and/or abdominal pain), myalgia, arthralgia, malaise, sweating, and shivering (for subjects 6–17 y of age). Fever was defined as body temperature ≥38.0°C (measured by oral or rectal method, as applicable). Both local and systemic reactions, and the overall inconvenience within 7 d of vaccination were recorded in the subject diary and rated on a 4-point scale (Grade 0 = none; Grade 1 = mild; Grade 2 = moderate; and Grade 3 = severe). Subject diaries were completed or modified by the subjects or their LARs, as appropriate. Because children aged 6 months to 5 y were expected to have limitations in their verbal expression of local and systemic reactions, different grading criteria were used for this age group for assessment of the local reaction of vaccination site pain and the systemic reactions of irritability/fussiness and loss of appetite. Vaccination site pain was graded as none (grade 0), minor reaction to touch (grade 1), cries/protests on touch (grade 2) or cries when arm is moved/spontaneously painful (grade 3) for subjects aged 6 months to 5 y and as none (grade 0), painful on touch (grade 1), painful when arm is moved (grade 2), or pain that prevented normal daily activity (grade 3) for subjects aged ≥6 y. In addition, for the 6 months to 5 y age group, irritability/fussiness was recorded as behavior as usual (grade 0), crying more than usual/did not interfere with normal daily activity (grade 1), crying more than usual/interfered with normal daily activity (grade 2), or crying that could not be comforted/prevented normal daily activity (grade 3). Further, loss of appetite was recorded as appetite as usual (grade 0), eating less than usual/no effect on normal daily activity (grade 1), eating less than usual/interfered with normal daily activity (grade 2), or not eating at all (grade 3).
Unsolicited AEs and serious AEs (SAEs) were recorded from the time of signing the informed consent/assent form until 28–33 d after last study vaccination. If the onset of solicited events was after 7 d of vaccination, these events were considered as unsolicited AEs. Unsolicited AEs were coded using Medical Dictionary for Regulatory Activities Version 21.0.
An independent Data Safety Monitoring Board (DSMB) consisting of independent experts was established to assess the safety data of children in 6–35 months group after enrolling onethird of the target sample size.
Statistical Analyses
The sample size of 118 vaccinated subjects per age group, i.e., a total of 236 subjects secured an overall statistical power of 0.9 that all three CHMP criteria would be met for all four strains.Citation25 The sample size allowed for a dropout rate of ±5%.
The statistical analysis was performed using the SAS® system version 9.4 (SAS Institute, Cary, NC). Immunogenicity and safety parameters were assessed in all subjects who received the study vaccine and had a valid post-vaccination serology result. The primary immunogenicity variables were summarized by the number of observations (n), geometric mean, geometric standard deviation (SD), and corresponding 95% confidence interval (CI) for the geometric mean. For qualitative endpoints, per category, the number and proportion of subjects with non-missing data (n, %) and corresponding 95% CI for the proportion using the Clopper–Pearson method were presented.
Results
Study participants
A total of 236 subjects (118 subjects in each age group) were enrolled and vaccinated at 9 study sites in India. The DSMB did not signal any safety concerns in one-third of the total sample enrolled for the 6–35 months group; hence, enrollment was continued for the remaining two-thirds of subjects in this group. All primed subjects (n = 45) received a single dose of study vaccination, and all but 1 of the unprimed subjects (n = 191 all <9 y: 77 aged 3–17 y and 114 aged 6–35 months) received 2 doses of study vaccine as planned. Out of 236 subjects, 4 (1.7%) subjects in the 6–35 months group and 1 (0.8%) subject in the 3–17 y group discontinued the study due to withdrawal of consent (2×) or loss to follow-up (3×) All 236 subjects were included in the analysis of the safety and tolerability data. The four subjects in the 6–35 months group who discontinued the study were excluded from the immunogenicity analysis because for them no post-vaccination HI titers were available.
All subjects were Asians, and 54.7% were males. The mean age (SD) was 19.7 (8.27) months for subjects aged 6–35 months and 96.3 (42.46) for subjects aged 3–17 y. In all, 58.5% subjects in the 6–35 months age group and 50.8% subjects in the 3–17 y group were males. Only 4 (3.4%) subjects in the 6–35 months group and 2 (1.7%) subjects in the 3–17 y group had been previously vaccinated against seasonal influenza. A total of 15 subjects (6–35 months group: 11 subjects and 3–17 y group: 4 subjects) required concomitant medications during the study where more than 1% of the subjects used paracetamol (3%) and chlorphenamine/paracetamol/phenylephrine combined preparation and azithromycin (1.3% each).
Immunogenicity
Pre- and post-vaccination geometric mean HI antibody titers and GMFI in HI titers
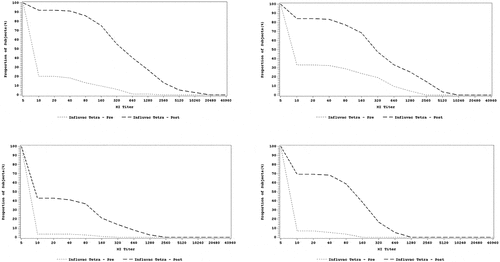
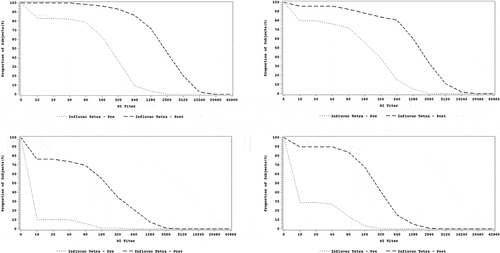
The GMT and GMFI in HI titers are presented in . In both age groups, the post-vaccination GMT titers were higher than the pre-vaccination GMTs for all four strains. Both the pre- and post-vaccination GMT were higher for A strains compared to B strains in both age groups.
Table 1. Geometric mean HI titer and geometric mean fold increase in HI titer.
The GMFI in HI titers was >2.5 for all four strains in both age groups; it varied between 4.1 and 31.7 in the 6–35 months group and between 9.2 and 14.4 in the 3–17 y group.
Seroconversion and seroprotection rates
The seroconversion and seroprotection rates are presented in . The seroconversion rate was >40% for A and B strains in both age groups, varying from 41.2% to 87.7% in the 6–35 months group and from 69.5% to 89% in the 3–17 y group. Seroconversion rates were slightly higher for A strains than B strains in both age groups. The seroprotection rate was >70% for A strains in the 6–35 months group and for A and B strains in the 3–17 y group. However, the seroprotection rate was <70% for B strains in the 6–35 months group.
Table 2. Seroconversion and seroprotection rates.
The reverse cumulative distribution (RCD) curves for HI assay for each strain across both age groups are provided in . The post-vaccination titers ranged higher than the pre-vaccination titers for all the 4 strains in both age groups. Pre-vaccination and post-vaccination titers ranged higher for A strains as compared with B strains in both age groups.
Reactogenicity
Local reactions
Local reactions were observed in 16 (13.6%) subjects in the 6–35 months group and 20 (16.9%) subjects in the 3–17 y group. The most common local reaction was vaccination site pain across both age groups (6–35 months group: 15 [12.7%]; 3–17 y group: 20 [16.9%]). All the events of vaccination site pain were mild or moderate in severity (mild [6–35 months group: 14 subjects; 3–17 y group: 19 subjects] and moderate [6–35 months: 1 subject; 3–17 y group: 1subject]) and lasted for 3–4 d. One (0.9%) additional subject in the 6–35 months group reported mild swelling, which lasted for 3 d.
Systemic reactions
Systemic reactions were observed in 20 (16.9%) subjects in the 6–35 months group and 9 (7.6%) subjects in the 3–17 y group. The most common systemic reaction was fever across both groups (6–35 months group: 9 [7.8%]; 3–17 y group: 7 [5.9%]). All the events of fever were mild (38–38.4°C) or moderate (38.5-39°C) except 1 severe event (>39°C) in the 3–17 y group. Other systemic reactions of mild or moderate severity were irritability/fussiness (6 [5.2%]), drowsiness, diarrhea/vomiting, and loss of appetite (4 [3.4%] each) in the 6–35 months group, and headache and fatigue/tiredness (2 [2.5%] each), and malaise (1 [1.2%]) in the 3–17 y group. All the systemic reactions were resolved within 5 d of vaccination except 2 reactions (fever and loss of appetite) in the 6–35 months group, which lasted for ≥7 d.
Overall inconvenience
Majority of the subjects in both groups did not experience any inconvenience within 7 d of vaccination (6–35 months group: 106 [91.4%]; 3–17 y group: 117 [99.2%] subjects). Only 11 subjects reported inconvenience; 10 subjects in the 6–35 months group reported mild inconvenience and 1 subject in the 3–17 y group reported moderate inconvenience. None of the subjects in either of the groups reported severe inconvenience after study vaccination. The inconvenience after first and second post-vaccination within 7 d was explored in unprimed subjects. Majority (100 [90.9%]) of the unprimed subjects did not consider one vaccination to be more inconvenient than the other. Only 8 (7.3%) subjects had higher inconvenience after first vaccination, and 2 (1.8%) subjects had higher inconvenience after second vaccination.
Unsolicited adverse events
Less than 10% of the subjects reported unsolicited AEs; 10 (8.5%) subjects in the 6–35 months group reported 22 AEs, and 4 (3.4%) subjects in the 3–17 y group reported 7 AEs. The most common AE was pyrexia (5.9%) in the 6–35 months group and nasopharyngitis (2.5%) in the 3–17 y group ().
Table 3. Incidence of unsolicited adverse events.
All the events were mild or moderate in severity. A total of 12 related AEs was reported in the study (6–35 months group: 7 related events [4 events of pyrexia, 2 events of cough, and 1 event of malaise] in 5 subjects; 3–17 y group: 5 related events [3 events of nasopharyngitis and 2 events of cough] in 3 subjects).
No deaths or discontinuations due to AEs were reported in the study. One unrelated SAE of generalized tonic-clonic seizure was reported in the 3–17 y group. This subject had a known history of seizure with two previous episodes over a period of 2.5 y prior to study entry.
Discussion
This Phase III, open-label, baseline-controlled study showed 0.5 mL dose of inactivated subunit QIVs to be safe and immunogenic with low reactogenicity in children and adolescents (6 months to 17 y of age) in an Indian population.
The primary objective of the study was to describe the immunogenicity of QIV in both age groups with respect to HI GMTs, GMFI, and seroconversion and seroprotection rates. Additionally, reverse cumulative distribution plots were used to determine distribution of antibody titers in each of the age groups. The HI assay is the most widely used, established, and reproducible serological techniques to demonstrate immunogenicity against influenza virusCitation23 and therefore has been used as the primary outcome measure in this study. The present study showed higher post-vaccination HI titers in comparison to pre-vaccination HI titers for all 4 strains in both age groups, demonstrating a clear immunogenic response to the vaccine. For both age groups, the pre- and post-vaccination GMTs, seroconversion and seroprotection rates were higher for A strains than B strains, which may be due to a relatively higher circulation of influenza A as compared to influenza B over the over the past decade in India resulting in higher preexisting immunity derived from previous natural infection of influenza A.Citation26
In this study, the HI data showed strong serological responses for each of the influenza strains in both age groups. Historically, an HI titer of ≥40 in adult subjects was the serological correlate for 50% reduction or more in the risk of influenza infections or disease.Citation27,Citation28 The former CHMP criteria for the derived serological parameters GMFI, seroconversion, and seroprotection (GMFI >2.5, seroconversion rate >40%, and seroprotection rate >70%) were defined by the CHMP of European Medicines Agency for adults/elderly in the context of the requirement to demonstrate adequate immunogenicity of influenza vaccines (i.e., meeting at least 1 out of 3 individual criteria for each strain in each age group) following an updated strain composition in annual update clinical studies. However, no criteria have been established for children and, therefore, in this study, the criteria for adults have been used.
The study vaccine was found to be safe and well-tolerated in both age groups. Majority of the AEs were mild or moderate and unrelated to the study vaccine. Only one SAE (generalized tonic-clonic seizure) was reported in the study, which was considered as not related to study vaccine. The study vaccine also showed a relatively low level of reactogenicity (<20% of subjects in both age groups) within 7 d of vaccination as compared to comparable studies.Citation20,Citation29 All reactions reported were generally short-lasting and mild or moderate in severity in both age groups for the majority of the subjects.
Our study has few strengths and limitations. The strengths being that this is the first study that evaluated the immunogenicity, reactogenicity, and safety of a full 0.5 mL dose of inactivated subunit QIV in Indian children aged below 3 y of age, which constitutes a higher-risk category for the complications of influenza. Second, this was a pan-India study conducted across different geographical regions. Third, multiple serological assessments (HI titer, GMFI, and seroconversion and seroprotection rates) were considered to assess the immunogenicity of the study vaccine. The limitations of the study are the lack of a comparator arm in the study as no other QIV was approved in this age group (6 months to 17 y) in India at the time when the study was designed; the duration of antibody response to 4 influenza strains was not evaluated beyond 28 d after final vaccination; the enrollment was restricted to healthy children and adolescents aged below 18 y, and other high-risk categories like immunocompromised subjects or residents of nursing home and other long-term care facilities were not enrolled in the study, which further restricted the generalizability of the results across the entire population. Finally, the sample size was based on the primary objective, i.e., on meeting the CHMP criteria for the induced immune response. This means that the claims that can be made based on the results of the study, are also limited to the immunogenicity of the vaccine. Thus, the sample size may not have been sufficient for reactogenicity, safety, and tolerability.Citation30 Future studies can be strengthened by including tolerability, reactogenicity and safety in the primary objectives, so that important safety findings can also qualify as evidence.
In conclusion, the current study demonstrated that QIV elicited an adequate immune response against influenza A and B strains with an acceptable safety and reactogenicity profile in Indian children and adolescents aged 6 months to 17 y. The favorable benefit–risk ratio supports the use of QIV for seasonal vaccination of infants, toddlers, children, and adolescents in India.
Author’s contributions
All authors met the International Council of Medical Journal Editors’ criteria for authorship and all those who met those criteria are listed as authors. All authors participated in the design, implementation, analysis, and/or interpretation of the study. All the authors were involved in drafting the manuscript or revising it critically for important intellectual content and provided final approval of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Acknowledgments
The authors would like to thank the participating study volunteers, and the research staff at the centers involved in the study. The authors also thank the medical writing team of Data Sciences, Safety, and Regulatory, IQVIA (India) for providing their support in developing this manuscript.
Disclosure statement
Serge van de Witte is an employee of Abbott Healthcare Products B.V. Sneha Nair and Ashfaque Shaikh are employees of Abbott India Ltd. All other authors were investigators in the study and received grant support from Abbott to conduct this study. No other conflict of interest is declared for the work presented in this article.
Data availability statement
Data that support the findings of this study will be made available upon reasonable request.
Additional information
Funding
References
- World Health Organization (WHO). Influenza (seasonal)-ask the experts: Q&A. WHO; 2018 Nov 6 Archived from the original on 2019 Oct 25 [accessed 2020 Jun 16]. https://www.who.int/news-room/fact-sheets/detail/influenza-seasonal.
- Content source: Centers for Disease Control and Prevention, National Center for ×Immunization and Respiratory Diseases. Types of influenza viruses. U.S. Department of Health & Human Services; 2019 Nov 18 [accessed 2020 Jun 16]. https://www.cdc.gov/flu/about/viruses/types.htm.
- Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, Cohen C, Gran JM, Schanzer D, Cowling BJ, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1–9. PMID: 29248255. doi:10.1016/S0140-6736(17)33293-2.
- Immunization Action Coalition (IAC). Influenza: questions and answers information about the disease and vaccines. Saint Paul, Minnesota: IAC; 2020 [accessed 2020 Feb 6]; https://www.immunize.org/catg.d/p4208.pdf.
- World Health Organization (WHO). Biologicals: influenza. WHO; 2019 Jun 19 [accessed 2020 Jun 12]. http://www.who.int/biologicals/vaccines/influenza/en/.
- Viboud C, Boelle PY, Cauchemez S, Lavenu A, Valleron AJ, Flahault A, Carrat F. Risk factors of influenza transmission in households. Br J Gen Pract. 2004;54(506):684–689. PMID: 15353055
- Glass LM, Glass RJ. Social contact networks for the spread of pandemic influenza in children and teenagers. BMC Publ Health. 2008 PMID: 18275603;8(1):61. doi:10.1186/1471-2458-8-61.
- Schanzer D, Vachon J, Pelletier L. Age-specific differences in influenza a epidemic curves: do children drive the spread of influenza epidemics? Am J Epidemiol. 2011;174(1):109–117. PMID: 15353055. doi:10.1093/aje/kwr037.
- von Kries R, Weiss S, Falkenhorst G, Wirth S, Kaiser P, Huppertz HI, Tenenbaum T, Schroten H, Streng A, Liese J, et al. Post-Pandemic seroprevalence of pandemic influenza a (H1N1) 2009 infection (swine flu) among children <18 years in Germany. PLoS One. 2011;6(9):e23955. PMID: 21915270. doi:10.1371/journal.pone.0023955.
- Purakayastha DR, Vishnubhatla S, Rai SK, Broor S, Krishnan A. Estimation of burden of influenza among under-five children in India: a meta-analysis. J Trop Pediatr. 2018;64(5):441–453. PMID: 29112737. doi:10.1093/tropej/fmx087.
- Narayan VV, Iuliano AD, Roguski K, Bhardwaj R, Chadha M, Saha S, Haldar P, Kumar R, Sreenivas V, Kant S, et al. Burden of influenza-associated respiratory and circulatory mortality in India, 2010-2013. J Glob Health. 2020;10(1):010402. PMID: 32373326. doi:10.7189/jogh.10.010402.
- Flannery B, Reynolds SB, Blanton L, Santibanez TA, O’Halloran A, Lu PJ, Chen J, Foppa IM, Gargiullo P, Bresee J, et al. Influenza vaccine effectiveness against pediatric deaths: 2010–2014. Pediatrics. 2017;139(5):e20164244. PMID: 28557757. doi:10.1542/peds.2016-4244.
- Centers for Disease Control and Prevention (CDC). Study of flu-related deaths in children shows healthy children at risk. U.S.CDC. 2018 Feb 12 [accessed 2020 Jun 12]. https://www.cdc.gov/flu/spotlights/2017-2018/flu-death-children.htm.
- Thompson MG, Clippard J, Petrie JG, Jackson ML, McLean HQ, Gaglani M, Reis EC, Flannery B, Monto AS, Jackson L, et al. Influenza vaccine effectiveness for fully and partially vaccinated children 6 months to 8 years old during 2011–2012 and 2012–2013. Pediatr Infect Dis J. 2016;35(3):299–308. PMID: 26658375. doi:10.1097/INF.0000000000001006.
- SAGE Working Group. Background paper on influenza vaccines and immunization. 2012. https://www.who.int/immunization/sage/meetings/2012/april/1_Background_Paper_Mar26_v13_cleaned.pdf.
- Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2019–2020. Pediatrics 2019;144:e20192478. PMID: 31477606. doi:10.1542/peds.2019-2478.
- Domachowske JB, Pankow-Culot H, Bautista M, Feng Y, Claeys C, Peeters M, Bl I, Jain V. A randomized trial of candidate inactivated quadrivalent influenza vaccine versus trivalent influenza vaccines in children aged 3-17 years. J Infect Dis. 2013;207:1878–1887. PMID: 23470848. doi:10.1093/infdis/jit091.
- Greenberg DP, Robertson CA, Landolfi VA, Bhaumik A, Senders SD, Decker MD. Safety and immunogenicity of an inactivated quadrivalent influenza vaccine in children 6 months through 8 years of age. Pediatr Infect Dis J. 2014;33:630–636. PMID: 24445833. doi:10.1097/INF.0000000000000254.
- Rodriguez Weber MA, Claeys C, Aranza Doniz C, Feng Y, Innis BL, Jain VK, Peeters M. Immunogenicity and safety of inactivated quadrivalent and trivalent influenza vaccines in children 18–47 months of age. Pediatr Infect Dis J. 2014;33(12):1262–1269. PMID: 25386965. doi:10.1097/INF.0000000000000463.
- Langley JM, Martinez AC, Chatterjee A, Halperin SA, McNeil S, Reisinger KC, Aggarwal N, Huang LM, Peng CT, Garcia-Sicilia J, et al. Immunogenicity and safety of an inactivated quadrivalent influenza vaccine candidate: a phase III randomized controlled trial in children. J Infect Dis. 2013;208(4):544–553. PMID: 23847058. doi:10.1093/infdis/jit263.
- You JH, Ming WK, Chan PK. Cost-Effectiveness of quadrivalent influenza vaccine in Hong Kong – a decision analysis. Hum Vaccin Immunother. 2015;11(3):564–571. PMID: 25714506. doi:10.1080/21645515.2015.1011016.
- Jain VK, Rivera L, Zaman K, Espos JR, Sirivichayakul C, Quiambao BP, Rivera-Medina DM, Kerdpanich P, Ceyhan M, Dinleyici EC, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med. 2013;369(26):2481–2491. PMID: 24328444. doi:10.1056/NEJMoa1215817.
- Basu I, Agarwal M, Shah V, Shukla V, Naik S, Supe PD, Srivastava MK, Giriraja KV, Pinjar P, Mishra PK, et al. Immunogenicity and safety of two quadrivalent influenza vaccines in healthy adult and elderly participants in India - a phase III, active-controlled, randomized clinical study. Hum Vaccin Immunother. 2021;1–10. PMID: 33957854. doi:10.1080/21645515.2021.1885278.
- Wijnans L, Voordouw B. A review of the changes to the licensing of influenza vaccines in Europe. Influenza Other Respir Viruses. 2016;10:2–8. doi:10.1111/irv.12351.
- Trombetta CM, Perini D, Mather S, Temperton N, Montomoli E. Overview of serological techniques for influenza vaccine evaluation: past, present and future. Vaccines (Basel). 2014;2(4):707–734. PMID: 26344888. doi:10.3390/vaccines2040707.
- World Health Organization. FluNet - Global Influenza Surveillance and Response System (GISRS). [accessed 2020 Jun 24]. http://www.who.int/influenza/gisrs_laboratory/flunet/en/.
- de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-Inhibiting antibody to influenza virus. Dev Biol (Basel). 2003;115:63–73. PMID: 15088777.
- Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond). 1972;70(4):767–777. PMID: 4509641. doi:10.1017/s0022172400022610.
- Vesikari T, Nauta J, Lapini G, Montomoli E, van de Witte S. Immunogenicity and safety of quadrivalent versus trivalent inactivated subunit influenza vaccine in children and adolescents: a phase III randomized study. Int J Infect Dis. 2020;92:29–37. PMID: 31838217. doi:10.1016/j.ijid.2019.12.010.
- Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Da Silva FT. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4(1):39. PMID: 31583123. doi:10.1038/s41541-019-0132-6.