ABSTRACT
The quadrivalent human papillomavirus (qHPV; HPV6/11/16/18) and 9-valent HPV (9vHPV; HPV6/11/16/18/31/33/45/52/58) vaccines have demonstrated efficacy, immunogenicity, and safety in international clinical trials. We report outcomes from three completed clinical trials in India: a single-arm study (V501–029 [NCT00380367]) in Indian girls (aged 9–15 years; N = 110) evaluating qHPV vaccine immunogenicity and safety; a subgroup analysis (n = 225) of Indian girls/boys (9–15 years) and women (16–26 years) from a global study (V503–002 [NCT00943722]) evaluating 9vHPV vaccine immunogenicity and safety; and a qHPV vaccine post-marketing safety surveillance study (V501–125) in Indian females (aged 9–45 years; N = 188) vaccinated during routine care. In V501–029 and V503–002, HPV vaccines were administered as 3 doses (Day 1, Month 2, Month 6). Serum HPV antibodies were evaluated by competitive Luminex immunoassays at Day 1 and Month 7 (both studies) and Months 12, 24, and 36 (V503–002 only). Adverse events (AEs) were collected by Vaccination Report Card. In V501–125, participants were actively surveilled for serious AEs (SAEs) within 30 days post-qHPV vaccination. In per-protocol analyses, qHPV and 9vHPV vaccines induced robust anti-HPV6/11/16/18 (V501–029) and HPV6/11/16/18/31/33/45/52/58 (V503–002) responses, respectively; ≥97% of participants seroconverted at Month 7 for each vaccine HPV type in both studies, and antibody responses persisted through 36 months in V503–002. The most common AEs were injection-site-associated. Most AEs were mild/moderate; no deaths, vaccine-related SAEs, or discontinuations due to AEs were reported. In V501–125, no SAE was reported. Overall, the qHPV and 9vHPV vaccines elicited robust antibody responses and were generally well tolerated in Indian participants.
Introduction
Human papillomavirus (HPV) causes nearly all cervical cancers and a substantial proportion of vulvar, vaginal, anal, penile, oropharyngeal, and other head and neck cancers as well as genital warts and recurrent respiratory papillomatosis.Citation1,Citation2 HPV-related disease poses a substantial public health burden in India, including nearly all of the >124,000 new cases of cervical cancer and a substantial portion of the approximately 5,500 anal, 9,000 vulvar/vaginal, 10,700 penile, and 20,600 oropharyngeal cancers that occur annually in India (based on 2020 estimates).Citation3 Of all countries, India has the highest estimated number of cervical cancer deaths each year (i.e., 60,000 deaths annually [164 deaths daily on average] in 2018).Citation4
Bivalent, quadrivalent (qHPV), and 9-valent (9vHPV) prophylactic HPV vaccines are widely recommended globally following initial licensures in 2007, 2006, and 2014, respectively.Citation5 The bivalent and qHPV vaccines protect against oncogenic HPV types 16 and 18, which account for approximately 70% of HPV-related cancers, while the 9vHPV vaccine protects against five additional oncogenic HPV types (HPV31, 33, 45, 52, and 58), which together with HPV16/18 account for approximately 90% of HPV-related cancers.Citation6 The qHPV and 9vHPV vaccines also protect against HPV6 and 11 which cause most (i.e., approximately 90%) cases of genital warts.Citation7
Multiregional clinical outcome studies supported the licensure of the qHPV and 9vHPV vaccines, thereby enhancing the generalizability of the study results. Analyses conducted in subgroups of study participants defined by region of residence demonstrated high efficacy and robust immunogenicity across the regions where the studies were conducted, such as Europe, Latin America, North America, and the Western Pacific.Citation8–14 However, some regions, such as Sub-Saharan Africa and South Asia, did not substantially contribute to the trials that supported licensure. Generating data in these regions using the same methodology as in the global clinical trials is necessary to support the generalizability of the global trial results to those regions. Results of an immunogenicity and safety study of the qHPV vaccine conducted in Sub-Saharan Africa have been reported.Citation15
Here, we report qHPV vaccine immunogenicity and safety results from a single-arm, open-label immunogenicity and safety trial in Indian girls (9–15 years of age) conducted to support registration of the vaccine in India and post-marketing safety data from a qHPV vaccine safety surveillance study in Indian females (9–45 years of age). We also report 9vHPV vaccine immunogenicity and safety from the subgroup of Indian girls and boys (9–15 years of age) and young women (16–26 years of age) who participated in an international immunogenicity and safety clinical trial that supported registration of the 9vHPV vaccine in India.
Methods
Study designs and populations
The qHPV and 9vHPV vaccine clinical trials conducted in India are summarized in .
Table 1. Overview of qHPV and 9vHPV vaccine clinical trials in India.
Study V501–029 (NCT00380367) was an open-label, single-arm study that assessed immunogenicity and safety of the qHPV vaccine in girls 9–15 years of age at seven centers located in three cities in India (Bangalore, Mumbai, and Pune).
Study V503–002 (NCT00943722) evaluated 9vHPV vaccine immunogenicity and safety in girls and boys 9–15 years of age compared with young women 16–26 years of age across sites in 17 countries, including India. Participants from India were enrolled at seven sites located in seven cities (Hyderabad, Indore, Kolkata, Ludhiana, Mumbai, New Delhi, and Pune). The overall study design, participants, and results have been described previously.Citation16,Citation17
Participants 9–15 years of age in V501–029 and V503–002 were required to be generally healthy and sexually naïve at enrollment and throughout the vaccination period (through Month 7). Participants 16–26 years of age in V503–002 were required to be generally healthy and have no history of abnormal cervical cytology or biopsy result and no more than four lifetime sexual partners. Reasons for exclusion from these two studies included pregnancy, known allergy to any vaccine component, thrombocytopenia, immunosuppression/previous immunosuppressive therapy, or previous receipt of an HPV vaccine.
Study V501–125 was a post-marketing safety surveillance study to assess the safety of qHPV vaccine administered under conditions of routine clinical care in females 9–45 years of age. The study was designed to collect data on a minimum of 500 HPV vaccine doses. Females who were naïve to the HPV vaccine and who opted for vaccination with qHPV vaccine during a routine visit at eight study centers in seven cities in India (Bangalore, Hubbali, Hyderabad, Kolkata, Madurai, Mumbai, and Pune) could participate.
Each study was performed in accordance with the principles of Good Clinical PracticeCitation18 and was approved by the appropriate institutional review boards/ethics committees and regulatory agencies. All participants (or their legal guardians) provided written informed consent prior to participation in accordance with local laws and regulations.
Vaccine administration
In Study V501–029, all participants received three doses of qHPV vaccine, administered intramuscularly at Day 1, Month 2, and Month 6. Each 0.5 mL dose of qHPV vaccine contained approximately 20 µg of HPV6, 40 µg of HPV11, 40 µg of HPV16, and 20 µg of HPV18 L1 virus-like particles as well as aluminum adjuvant (225 µg; amorphous aluminum hydroxyphosphate sulfate).
In Study V501–125, participants received ≥1 dose of commercial qHPV vaccine in a routine clinical setting, with a recommended dosing schedule of Day 1, Month 2, and Month 6.
In Study V503–002, all participants received three doses of 9vHPV vaccine (at Day 1, Month 2, and Month 6). As described previously,Citation16,Citation17 each dose of 9vHPV vaccine contained 30 µg of HPV6, 40 µg of HPV11, 60 µg of HPV16, 40 µg of HPV18, 20 µg of HPV31, 20 µg of HPV33, 20 µg of HPV45, 20 µg of HPV52, and 20 µg of HPV58 L1 virus-like particles, and 500 µg of amorphous aluminum hydroxyphosphate sulfate.
Immunogenicity follow-up
In both Study V501–029 and Study V503–002, serum samples were collected from all participants at Day 1 and Month 7, and from a subset of girls and all boys (Study V503–002 only) at Months 12, 24, and 36 for immunogenicity analysis. In addition, participants aged 16–26 years from Study V503–002 underwent polymerase-chain reaction (PCR) testing of cervical and external genital swabs on Day 1 and Month 7 for detection of HPV DNA; results were used as part of the criteria to define the immunogenicity analysis population, as described.Citation16
In Study V501–029, the HPV-4 competitive Luminex Immunoassay (cLIA) was used to detect antibodies to HPV6, 11, 16, and 18 virus-like particles.Citation19–21 Antibody concentrations were determined in milli-Merck units per mL (mMU/mL). Participants were considered seropositive for a given HPV type if the cLIA concentration for that type was at or above the cutoff value (HPV6: 32 mMU/mL; HPV11: 20 mMU/mL; HPV16: 20 mMU/mL; HPV18: 20 mMU/mL).
In Study V503–002, the HPV-9 cLIA was used to detect antibodies to HPV6, 11, 16, 18, 31, 33, 45, 52, and 58.Citation22 A participant was defined as seropositive for a given HPV type if the anti-HPV cLIA concentration for that type was at or above the following cut off values: HPV6: 30 mMU/mL; HPV11: 16 mMU/mL; HPV16: 20 mMU/mL; HPV18: 24 mMU/mL; HPV31: 10 mMU/mL; HPV33: 8 mMU/mL; HPV45: 8 mMU/mL; HPV52: 8 mMU/mL; and HPV58: 8 mMU/mL.
The HPV-4 and HPV-9 cLIAs are fully validated assays that have been widely used to support regulatory filings for the qHPV and 9vHPV vaccines around the world. The HPV-4 and HPV-9 cLIAs are based on the same general principle and have shown good agreement between assays for detection of HPV6/11/16/18.Citation22 However, the two assays have not been calibrated against each other. Although the same designation is used for the unit of measurement in both assays, HPV-4 cLIA mMU/mL and HPV-9 cLIA mMU/mL are different units of measurement and cannot be directly compared between tests or individual HPV types.
Safety follow-up
In Study V501–029, injection-site and systemic adverse events (AEs) and serious AEs (SAEs) were collected on Days 1–15 following each qHPV vaccine dose using daily vaccination report cards (VRCs). Deaths and vaccine-related SAEs were collected for the entire duration of the study.
In Study V501–125, SAEs occurring within 30 days after each qHPV vaccine dose were actively solicited via follow-up telephone calls on Day 7 and Day 30 after each dose.
In Study V503–002, injection-site and systemic AEs were recorded on VRCs on Days 1–15 following each 9vHPV vaccine dose. SAEs were collected from Day 1 through 6 months post-dose 3. Deaths and vaccine-related SAEs were collected for the entire duration of the study.
Data analysis
Immunogenicity was evaluated in the per-protocol immunogenicity (PPI) populations from Study V501–029 and V503–002, which included participants who (1) received all three study vaccine doses within acceptable day ranges, (2) had Month 7 serology results within prespecified day ranges, (3) were seronegative at Day 1 to the appropriate HPV type and (for participants 16–26 years of age only) PCR-negative to the appropriate HPV type from Day 1 through Month 7, and (4) had no other protocol violations that could interfere with the evaluation of immunogenicity. To be included in the PPI for HPV6 or HPV11, participants needed to be seronegative by cLIA to both HPV6 and HPV11 at baseline (because of extensive cross-reactivity due to the high amino acid sequence identity [93%] between the HPV6 and HPV11 L1 proteins);Citation20 to be included in the type-specific PPIs for all other HPV types, participants needed to be negative only for the HPV type being analyzed.
Immunogenicity data were summarized as geometric mean titers (GMTs) and percentages of participants who seroconverted, with seroconversion defined as a change in serostatus from seronegative to seropositive by 4 weeks post-vaccine dose 3, for the qHPV (Study V501–029) and 9vHPV (Study V503–002) vaccine HPV types. In Study 029, exact 95% confidence intervals (CI) are provided for seroconversion percentages; for GMTs, 95% CI are provided based on the asymptotic t-distribution. For the subgroup analysis of Study V503–002, GMTs and corresponding 95% CI were estimated using an analysis of variance model with log anti-HPV as the response and vaccination group as the fixed effect. In addition, analysis of covariance model of log anti-HPV titers via a mixed effects model methodology was used to estimate GMTs and associated 95% CI.
The V501–029 study was designed to enroll approximately 110 participants; if no serious vaccine‐related AEs were observed, it could be concluded that the rate of serious vaccine-related AEs would be <3.3% with a 95% probability. The safety assessment in the V501–125 study was based on 500 doses of vaccines as required by the Indian health authorities. For the V503–002 study, the safety assessment included the subgroup of participants enrolled in India. In all three studies, safety was analyzed in all participants who received at least one dose of qHPV or 9vHPV vaccine and had follow-up data. Safety data were summarized descriptively as numbers of events and proportions of participants reporting AEs.
Results
Participants
The qHPV vaccine Phase 3 clinical trial in India (Study V501–029) enrolled 110 girls 9–15 years of age between 3 May 2007 and 4 February 2008 (last participant visit). All participants received the first dose of qHPV vaccine, and 108 (98.2%) completed the three-dose vaccination series (). Baseline demographics are summarized in .
Figure 1. Participant disposition for Indian participants in the qHPV vaccine (Study V501–029) (a) and 9vHPV vaccine (Study V503–002) (b) clinical trials.
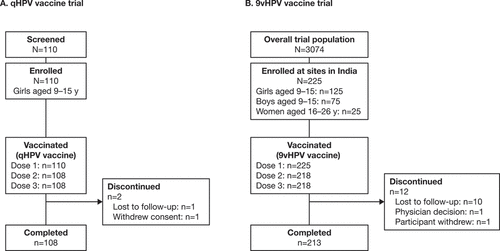
Table 2. Participant baseline characteristics for Study V501–029 and V503–002 (Indian participants).
The qHPV vaccine post-marketing surveillance study (Study V501–125) enrolled 188 female participants (24 adolescents aged 9–17 years [mean: 14.0 years (y); SD: 2.4 y] and 164 adults aged 18–45 years [mean: 28.0 y; SD: 6.6 y]) between 29 January 2016 and 30 June 2018 (last participant visit). All participants received at least one dose of qHPV vaccine, 171 (91.0%) received two doses, and 149 (79.3%) received all three doses. Among 36 participants who discontinued vaccination or withdrew from the study, none withdrew due to AEs.
Of 3074 participants in the global 9vHPV study (Study V503–002),Citation16 225 were enrolled at sites in India (125 girls, 75 boys, and 25 young women). Overall, 218 Indian participants completed the three-dose 9vHPV vaccination series (). Baseline demographics for participants enrolled in India are presented in .
Immunogenicity
Among Indian girls aged 9–15 who received qHPV vaccine in Study V501–029, robust anti-HPV6/11/16/18 responses were observed at Month 7 (). Anti-HPV6/11/16/18 GMTs were similar to those observed in a prior international qHPV vaccine pivotal efficacy, immunogenicity, and safety study in young women conducted outside of India ().Citation23 Across the qHPV vaccine HPV types, ≥97.0% of Indian girls underwent seroconversion within 1 month of the last qHPV vaccine dose ().
Table 3. Summary of anti-HPV6/11/16/18 GMTs and seroconversion at Month 7 in Indian girls receiving qHPV vaccine in Study V501–029 and the overall population of young women from Study V501–015 (per-protocol immunogenicity populationa).
Among Indian participants who received 9vHPV vaccine in Study V503–002, most girls (≥98.1%) and boys (100%) seroconverted to each of the nine HPV types by Month 7 (Supplementary Table S1). In Indian girls and boys, anti-HPV GMTs generally declined after Month 7 through Month 36, with the sharpest decreases between Month 7 and Month 12 and more gradual declines thereafter (). GMTs at Month 7, 12, 24, and 36 for each of the 9vHPV vaccine HPV types in Indian girls and boys were similar to or higher than those observed in young women from the 9vHPV vaccine pivotal efficacy studyCitation13 (). Most Indian girls (96.8%–100%) and boys (94.0%–100%) remained seropositive to each of the 9vHPV vaccine HPV types at Month 36 (Supplementary Table S1). All Indian young women (100%) enrolled in Study V503–002 seroconverted to each of the 9vHPV vaccine HPV types by Month 7, and GMTs at Month 7 for each HPV type were similar to or higher than those observed in the entire cohort of young women enrolled in V503–002 (). Of note, no additional timepoints were available since immunogenicity analyses in women 16–26 years of age enrolled in study V503–002 were conducted only at Month 7.Citation16
Table 4. Summary of anti-HPV6/11/16/18/31/33/45/52/58 GMTs through Month 36 in Indian girls and boys receiving 9vHPV vaccine in Study V503–002 versus young women from the pivotal 9vHPV vaccine efficacy study (Study V503–001) (per-protocol immunogenicity populationa).
Table 5. Summary of anti-HPV6/11/16/18/31/33/45/52/58 GMTs and seroconversion at Month 7 in Indian young women versus the entire cohort of young women in Study V503–002 (per-protocol immunogenicity populationa).
Safety
Among 108 Indian girls who received qHPV vaccine in Study V501–029 and had available follow-up, 63 (58.3%) reported AEs on Days 1–15 after any qHPV vaccination (). A total of 50 participants (46.3%) reported injection-site AEs, most commonly injection-site pain (42.6%) and tenderness (23.1%). These AEs were mostly mild or moderate in intensity; there were two cases of severe injection-site pain. Systemic AEs were reported by 35 participants (32.4%), including 26 (24.1%) with AEs considered vaccine-related. The most common systemic AEs were pyrexia (23.1%), nasopharyngitis (7.4%), and headache (4.6%). There were no SAEs or deaths, and no participants discontinued due to AEs.
Table 6. Summary of AEs in Indian girls (aged 9–15 years) from the qHPV vaccine study (Study V501–029) (Safety population).
In Study V501–125, 188 participants received a total of 500 doses of qHPV vaccine in routine clinical practice. No SAEs were reported.
Among Indian 9vHPV vaccine recipients in Study V503–002 with available follow-up, 85/122 girls (69.7%), 42/72 (58.3%) boys, and 14/25 women (56.0%) reported AEs on Days 1–15 after any 9vHPV vaccination (). The most common AEs were injection-site reactions, which were reported by 81 girls (66.4%), 40 boys (55.6%), and 14 women (56.0%) and were mostly mild or moderate in severity. Pyrexia was the most common systemic AE, which was reported by 14 girls (11.5%), eight boys (11.1%), and five women (20.0%). Vaccine-related systemic AEs were reported by 14 girls (11.5%), seven boys (9.7%), and five women (20.0%). Most systemic AEs were mild or moderate in intensity. One SAE was reported: an Indian participant enrolled in the cohort of women became pregnant (last menstrual period 12 days post-dose 1) and was hospitalized at approximately 35 weeks gestation for severe intrauterine growth restriction and fetal distress; an emergency C-section was performed; the participant delivered the baby and was discontinued from the study per physician decision. This SAE was considered not related to the study vaccine. There were no vaccine-related SAEs, deaths, or discontinuations due to an AE among Indian participants.
Table 7. Summary of AEs in Indian girls (aged 9–15 years), boys (aged 9–15 years), and young women (aged 16–26 years) from the 9vHPV vaccine study (Study V503–002) (Safety population).
Discussion
The 9vHPV vaccine has become available in India in 2021, which creates more options for prevention of HPV-related cancers and diseases. Therefore, this report of the local studies of the qHPV and 9vHPV vaccine development programs is timely and should provide a useful reference for public health stakeholders and health-care providers. We report immunogenicity and safety data from three HPV vaccine clinical studies that included female and male Indian participants aged 9–45 years. Two of the studies provided data to support registration of qHPV and 9vHPV vaccine in India, and one study was conducted as a qHPV vaccine post-licensure commitment. Both vaccines elicited robust immune responses, with no unexpected safety findings in Indian participants.
In both the qHPV vaccine and 9vHPV vaccine immunogenicity and safety studies, seroconversion percentages were high (≥97%) among Indian participants for each of the vaccine-covered HPV types at 1 month after the last vaccine dose. In the qHPV vaccine study, anti-HPV6, 11, 16, and 18 antibody GMTs were similar to historic values observed in the qHPV vaccine arm from a qHPV vaccine efficacy study in young women. Among 9vHPV vaccine recipients, anti-HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 antibody GMTs in Indian girls and boys were similar to or higher than those observed in young women from the pivotal 9vHPV vaccine efficacy study.Citation13 Within the 9vHPV vaccine study (V503–002), Indian young women generated similar or higher antibody responses as the overall population of young women from 17 countries located in five continents from the same study. Together, these results suggest that both vaccines elicit sufficient antibody responses to provide protection against infection and disease caused by the vaccine-targeted HPV types in Indian participants. Moreover, durable antibody responses were observed in Indian girls and boys through Month 36, similar to in the overall V503–002 study population.Citation16 Durable effectiveness and sustained immunogenicity were observed for up to 10 and 8 years, respectively, in long-term follow-up extensions of the global qHPV and 9vHPV vaccine studies in girls and boys 9–15 years of age.Citation24,Citation25 Long-term follow-up extension of qHPV and 9vHPV vaccine efficacy studies also demonstrated durable efficacy and persistent antibody responses through up to 14 years (qHPV vaccine) and 8 years (9vHPV vaccine) of follow-up in womenCitation26,Citation27 and 10 years of follow-up in men (qHPV vaccine).Citation28 The generally similar antibody responses observed at Month 7 for Indian versus global clinical trial participants and, for the 9vHPV vaccine, through Month 36 in the Indian girls and boys compared with young women in the 9vHPV vaccine pivotal efficacy studyCitation13 suggest that vaccination induces antibody levels sufficient to provide high-level efficacy and long-term protection in Indian populations. In a meta-analysis of immunogenicity of the 9vHPV vaccine, it was noted that it would be important to further evaluate immunogenicity of the vaccine in certain regions, including South Asia.Citation14 This report will contribute to filling this data gap.
The antibody levels assessed by cLIA are expressed in mMU/mL, which is consistent with previously published literature and global regulatory documents. World Health Organization international antibody units (IU) have only been established for HPV16 and HPV18 but are not available for other HPV types. A conversion factor from mMU/mL in the HPV-4 cLIA to IU/mL has been proposed in a previous publication.Citation29
The qHPV and 9vHPV vaccines were generally well tolerated in Indian clinical trial participants. No participant died during the studies, and there were no vaccine-related SAEs or discontinuations due to an AE. Consistent with the known safety profiles of the vaccines, the most common AEs were injection-site events, which were mainly mild or moderate in intensity.Citation30–32
Strengths of the studies reported herein include the consistent methods of evaluation of immunogenicity (i.e., HPV-4 and HPV-9 cLIA) and safety (e.g., use of VRCs) with global studies. Study limitations include the relatively small sample sizes in Study V501–029 and Study V501–125 and the small number of Indian participants included in Study V503–002. However, the overall consistency between the immunogenicity data in Indian and international clinical trial participants suggests that the findings from previous global studies can be extended to Indian populations. While vaccine efficacy was not directly assessed in Indian clinical trial participants, GMTs in Indian participants were similar to or higher than GMTs in historical efficacy trials; this suggests that the qHPV and 9vHPV vaccines generate antibody levels in the Indian population sufficient to induce protective efficacy against vaccine HPV type-related infection and disease.
The WHO, Advisory Committee on Immunization Practices, and Indian Academy of Pediatrics recommend a two-dose HPV vaccine schedule (with ≥6 months between doses) for individuals who receive the first dose before 15 years of age.Citation33–35 The two-dose schedule has been widely adopted: for instance, the 9vHPV vaccine is licensed as a two-dose schedule in 9–14-year-olds in over 80 countries (Merck & Co., Inc., Rahway, NJ, USA; data on file). Studies conducted outside of India have demonstrated that two-dose regimens of qHPV vaccine in girls 9–13 years of ageCitation36,Citation37 and 9vHPV vaccine in girls and boys 9–14 years of ageCitation38,Citation39 elicit HPV antibody levels that are non-inferior to those observed in young women 16–26 years of age (i.e., the population in whom vaccine efficacy has been demonstrated). Given the generally similar immunogenicity for both vaccines across various geographic regions,Citation12,Citation14 it is reasonable to expect that the global results with two-dose schedules can be extrapolated to Indian populations.
In summary, three-dose regimens of qHPV and 9vHPV vaccines elicit robust antibody responses in Indian girls aged 9–15 years. Similarly, high antibody responses were also observed in Indian boys (aged 9–15 years) and young women (aged 16–26 years) who received the 9vHPV vaccine. Both vaccines were well tolerated in Indian clinical trial participants, with no unexpected safety signals. These results support the implementation of broad HPV vaccination programs in India.
Supplemental Material
Download Zip (27.3 KB)Acknowledgments
The authors thank the study participants, investigators, and study site personnel. Medical writing assistance, under the direction of the authors, was provided by Erin Bekes, PhD, for CMC AFFINITY, a division of IPG Health Medical Communications, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Disclosure statement
Suzanne M. Garland reports fees for board membership and travel support from Merck, consulting fees from Merck and Sanofi Pasteur, lecture fees from Merck, Sanofi Pasteur, and GlaxoSmithKline, and grant support from Merck, GlaxoSmithKline, and CSL Behring.
Manjula Anagani has no interests to declare.
Neerja Bhatla has received research funding through her Institute from MSD, GlaxoSmithKline, and Digene/Qiagen Inc.
Sukanta Chatterjee has no interests to declare.
Sanjay Lalwani has no interests to declare.
Cecil Ross has no interests to declare.
Thomas Group is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock/stock options in the Company.
Jianxin Lin is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock/stock options in the Company.
Alain Luxembourg is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock/stock options in the Company.
Anuj Walia is a former employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock/stock options in the Company.
Yingmei Tu is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock/stock options in the Company.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2105067.
Additional information
Funding
References
- Lacey CJN, Lowndes CM, Shah KV. Chapter 4: burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24(Supplement 3):1–9. doi:10.1016/j.vaccine.2006.06.015.
- de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8(2):e180–190. doi:10.1016/S2214-109X(19)30488-7.
- Bruni L, Albero G, Serrano B. ICO/IARC information centre on HPV and cancer (HPV information centre). Human papillomavirus and related diseases in India. Summary Report; 2021 Oct 14 [accessed 2022 Jan 14]. https://hpvcentre.net/statistics/reports/IND.pdf.
- Arbyn A, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, and Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi:10.1016/S2214-109X(19)30482-6.
- Luxembourg A, Moeller E. 9-Valent human papillomavirus vaccine: a review of the clinical development program. Expert Rev Vaccines. 2017;16(11):1119–1139. doi:10.1080/14760584.2017.1383158.
- de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–670. doi:10.1002/ijc.30716.
- Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, Barr E, Haupt RM, and Joura EA. Natural history of genital warts: Analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J Infect Dis. 2009;199(6):805–814. doi:10.1086/597071.
- Barr E, Gause CK, Bautista OM, Railkar RA, Lupinacci LC, Insinga RP, Sings HL, and Haupt RM. Impact of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like particle vaccine in a sexually active population of North American women. Am J Obstet Gynecol. 2008;198(3):261.e1–261.e11. doi:10.1016/j.ajog.2007.09.001.
- Majewski S, Bosch FX, Dillner J, Iversen O-E, Kjaer SK, Muñoz N, Olsson S-E, Paavonen J, Sigurdsson K, Bryan J et al. The impact of a quadrivalent human papillomavirus (types 6, 11, 16, 18) virus-like particle vaccine in European women aged 16 to 24. J Eur Acad Dermatol Venereol. 2009;23(10):1147–1155. doi:10.1111/j.1468-3083.2009.03266.x.
- Perez G, Lazcano-Ponce E, Hernandez-Avila M, García PJ, Muñoz N, Villa LL, Bryan J, Taddeo FJ, Lu S, Esser MT et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like-particle vaccine in Latin American women. Int J Cancer. 2008;122(6):1311–1318. doi:10.1002/ijc.23260.
- Tay EH, Garland S, Tang G, Nolan T, Huang L-M, Orloski L, Lu S, Barr E. Clinical trial experience with prophylactic HPV 6/11/16/18 VLP vaccine in young women from the Asia-Pacific region. Int J Gynaecol Obstet. 2008;102(3):275–283. doi:10.1016/j.ijgo.2008.03.021.
- Giuliano AR, Lazcano-Ponce E, Villa L, Nolan T, Marchant C, Radley D, Golm G, McCarroll K, Yu J, Esser MT et al. Impact of baseline covariates on the immunogenicity of a quadrivalent (types 6, 11, 16, and 18) human papillomavirus virus-like-particle vaccine. J Infect Dis. 2007;196(8):1153–1162. doi:10.1086/521679.
- Huh WK, Joura EA, Giuliano AR, Iversen O-E, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143–2159. doi:10.1016/S0140-6736(17)31821-4.
- Petersen LK, Restrepo J, Moreira ED, Jr., Iversen O-E, Pitisuttithum P, Van Damme P, Joura EA, Olsson S-E, Ferris D, Block S et al. Impact of baseline covariates on the immunogenicity of the 9-valent HPV vaccine – a combined analysis of five phase III clinical trials. Papillomavirus Res. 2017;3:105–115. doi:10.1016/j.pvr.2017.03.002.
- Mugo N, Ansah NA, Marino D, Saah A, and Garner EIO. Evaluation of safety and immunogenicity of a quadrivalent human papillomavirus vaccine in healthy females between 9 and 26 years of age in Sub-Saharan Africa. Hum Vaccin Immunother. 2015;11(6):1323–1330. doi:10.1080/21645515.2015.1008877.
- Van Damme P, Olsson SE, Block S, Castellsague X, Gray GE, Herrera T, Huang L-M, Kim DS, Pitisuttithum P, Chen J et al. Immunogenicity and safety of a 9-valent HPV vaccine. Pediatrics. 2015;136(1):e28–39. doi:10.1542/peds.2014-3745.
- Luxembourg A, Moreira ED, Jr., Samakoses R, Kim K-H, Sun X, Maansson R, Moeller E, Christiano S, and Chen J. Phase III, randomized controlled trial in girls 9-15 years old to evaluate lot consistency of a novel nine-valent human papillomavirus L1 virus-like particle vaccine. Hum Vaccin Immunother. 2015;11(6):1306–1312. doi:10.1080/21645515.2015.1009819.
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). ICH: E 6 (R2): guideline for good clinical practice - step 5. [ accessed 2022 July 1]. https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice.
- Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12(8):959–969. doi:10.1128/CDLI.12.8.959-969.2005.
- Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10(1):108–115. doi:10.1128/cdli.10.1.108-115.2003.
- Smith JF, Kowalski R, Esser MT, Brown MJ, Bryan JT. Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil: a vaccine for human papillomavirus types 16, 18, 6 and 11. Hum Vaccin. 2008;4(2):134–142. doi:10.4161/hv.4.2.5261.
- Roberts C, Green T, Hess E, Matys K, Brown MJ, Haupt RM, Luxembourg A, Vuocolo S, Saah A, Antonello J. Development of a human papillomavirus competitive luminex immunoassay for 9 HPV types. Hum Vaccin Immunother. 2014;10(8):2168–2174. doi:10.4161/hv.29205.
- The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915–1927. doi:10.1056/NEJMoa061741.
- Olsson SE, Restrepo JA, Reina JC, Pitisuttithum P, Ulied A, Varman M, Van Damme P, Moreira ED, Jr., Ferris D, Block S et al. Long-term immunogenicity, effectiveness, and safety of nine-valent human papillomavirus vaccine in girls and boys 9 to 15 years of age: Interim analysis after 8 years of follow-up. Papillomavirus Res. 2020;10:100203. doi:10.1016/j.pvr.2020.100203.
- Ferris DG, Samakoses R, Block SL et al. 4-Valent human papillomavirus (4vhpv) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140(6):e20163947. doi:10.1542/peds.2016-3947.
- Kjaer SK, Nygård M, Sundström K, Dillner J, Tryggvadottir L, Munk C, Berger S, Enerly E, Hortlund M, Ágústsson ÁI et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi:10.1016/j.eclinm.2020.100401.
- Kjaer S, Nygard M, Sundström K, Munk C, Berger S, Dzabic M, Fridrich KE, Waldstrøm M, Sørbye SW, Bautista O et al. Long-term effectiveness of the 9-valent human papillomavirus vaccine in Scandinavian women: interim analysis after 8 years of follow-up. Hum Vaccin Immunother. 2021;17(4):943–949. doi:10.1080/21645515.2020.1839292.
- Goldstone SE, Giuliano AR, Palefsky JM, Lazcano-Ponce E, Penny ME, Cabello RE, Moreira ED, Jr., Baraldi E, Jessen H, Ferenczy A et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2022;22(3):413–425. doi:10.1016/S1473-3099(21)00327-3.
- Brown D, Müller M, Sehr P, Pawlita M, Seitz H, Rubio I, Antonello J, Radley D, Roberts C, Saah A. Concordance assessment between a multiplexed competitive Luminex immunoassay, a multiplexed IgG Luminex immunoassay, and a pseudovirion-based neutralization assay for detection of human papillomavirus types 16 and 18. Vaccine. 2014;32(44):5880–5887. doi:10.1016/j.vaccine.2014.08.004.
- Moreira ED, Jr., Block SL, Ferris D, Giuliano AR, Iversen O-E, Joura EA, Kosalaraksa P, Schilling A, Van Damme P, Bornstein J et al. Safety profile of the 9-valent HPV vaccine: A combined analysis of 7 Phase III clinical trials. Pediatrics. 2016;138(2):e20154387. doi:10.1542/peds.2015-4387.
- Phillips A, Patel C, Pillsbury A, Brotherton J, Macartney K. Safety of human papillomavirus vaccines: an updated review. Drug Saf. 2018;41(4):329–346. doi:10.1007/s40264-017-0625-z.
- Block SL, Brown DR, Chatterjee A, Gold MA, Sings HL, Meibohm A, Dana A, Haupt RM, Barr E, Tamms GM et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) 1 virus-like particle vaccine. Pediatr Infect Dis J. 2010;29(2):95–101. doi:10.1097/INF.0b013e3181b77906.
- World Health Organization (WHO). Human papillomavirus vaccines: WHO position paper. Wkly Epidemiol Rec. 2017;92(19):241–268.
- Meites E, Kempe A, and Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–1408. doi:10.15585/mmwr.mm6549a5.
- Kasi SG, Shivananda S, Marathe S, Chatterjee K, Agarwalla S, Dhir SK, Verma S, Shah AK, Srirampur S, Kalyani S et al. Indian Academy of Pediatrics (IAP) Advisory Committee on Vaccines and Immunization Practices (ACVIP): recommended immunization schedule (2020–21) and update on immunization for children aged 0 through 18 years. Indian Pediatr. 2021;58(1):44–54. doi:10.1007/s13312-021-2096-7.
- Dobson SRM, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–1802. doi:10.1001/jama.2013.1625.
- Donken R, Dobson SRM, Marty KD, Cook D, Sauvageau C, Gilca V, Dionne M, McNeil S, Krajden M, Money D et al. Immunogenicity of 2 and 3 doses of the quadrivalent human papillomavirus vaccine up to 120 months postvaccination: follow-up of a randomized clinical trial. Clin Infect Dis. 2020;71(4):1022–1029. doi:10.1093/cid/ciz887.
- Iversen OE, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Nur Azurah AG, Fong SM et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. 2016;316(22):2411–2421. doi:10.1001/jama.2016.17615.
- Bornstein J, Roux S, Petersen LK, Huang L-M, Dobson SR, Pitisuttithum P, Diez-Domingo J, Schilling A, Ariffin H, Tytus R et al. Three-year follow-up of 2-dose versus 3-dose HPV vaccine. Pediatrics. 2021;147(1):e20194035. doi:10.1542/peds.2019-4035.
