ABSTRACT
During the Covid-19 pandemic, the urgent need for safe and effective vaccines has led to many vaccine trials, implying fast and extensive recruitment of volunteers. In France, until 2020, vaccine clinical research participants were usually recruited locally, through center-based pools of volunteers, and local communication plans. Covireivac is a French public online platform launched on 10/01/2020 that enables national, large-scale recruitment of volunteers for Covid-19 vaccine studies. On the Covireivac website, all adult participants registered online, gave their informed consent, and filled out two online forms with information on their identity, health status (comorbidities, treatments), and known exposure to SARS-CoV-2. Since July 2021, volunteers could mention if their children are interested in participating in a Covid-19 vaccine trial. The objective of this work is to describe Covireivac’s volunteer characteristics registered from 10/01/2020 to 11/02/2022. To identify independent volunteer characteristics associated with a period of registration we performed a multivariate logistic regression. Among 54,424 registrations, 52,391 (96%) were analysed; 61% were male (n = 31,893), median age was 50 y; 13% (n = 6586) were healthcare workers. At registration, 15,879 volunteers (33%) reported at least one comorbidity, among whom 16% (n = 7349) were obese and 17% (n = 8346) had hypertension. Most volunteers registered during the first month (n = 35,876, 66%). The Covireivac platform allowed quick and large recruitment of potential volunteers for Covid-19 vaccine trials and could be used on a larger scale for vaccine trials in France. It could facilitate recruitment in vaccine trials and provide sponsors with better visibility of the recruitment capacities of clinical research centers.
Introduction
During the Covid-19 pandemic, the urgent need for effective vaccines at a large scale has led to many vaccine trials worldwide, implying quick and extensive recruitment of volunteers. In France, until 2020, vaccine clinical research participants were usually recruited locally; each center had its recruitment strategy and eventually a pool of available volunteers. An innovative recruitment plan through a digital national platform, Covireivac, was implemented (www.covireivac.fr) during the Covid-19 pandemic. This platform was set up in June 2020 by the Innovative Clinical Research Network in Vaccinology (I-REIVAC), a French clinical research network dedicated to research in vaccinology, with the support of the French Clinical Research Infrastructure Network (F-CRIN), and the French National Agency for Research on AIDS, hepatitis, and emerging infectious diseases (ANRS/MIE), an autonomous agency of National Institute for Health and Medical Research (Inserm). The main objective of Covireivac was to offer optimal conditions for academic or industrial vaccine trials against Covid-19 implementation in France. The project aimed to coordinate French stakeholders of vaccine research into a unique national task force to evaluate and validate Covid-19 vaccine clinical trial feasibility. Covireivac includes a computerized volunteer platform to facilitate inclusion in Covid-19 vaccine trials, a network of 32 clinical investigation centers associated with their biological resource laboratories, and an immune-monitoring laboratories network. This platform was created, launched, and provided first participants within a few months. The objective of this work is to describe the population that has been recruited and to provide guidelines and lessons learned from this experience.
Methods
Covireivac platform overview
Covireivac was set as an open, online platform to enroll potential participants. Registered users logged in to a personal account and received updated information on (i) Covid-19 vaccines, their development, and safety; (ii) clinical trial conduct (i.e., description of vaccine trial phases, actors, regulatory requirements); (iii) Covid-19 vaccine trials conducted in France; (iv) how to participate in a Covid-19 vaccine trial; and (v) the Covireivac project, networks, and coordination team. All information published on the website was validated by a group of experts from the College of General Practitioners teachers (CNGE), French Ministry for Solidarity and Health, French Public Health Agency (Santé Publique France), and F-CRIN.
Covireivac platform was launched on October 1, 2020, through a free advertising campaign to media and general practitioners, with a target of 25,000 registrations. Covireivac occasionally communicates in the mass media through press releases, press conferences, or interviews. Media communication topics were mainly starts of Covireivac trials, specific recruitment needs, or first results of Covireivac trials. Moreover, newsletters were sent regularly to volunteers, providing information on vaccine trials conducted by Covireivac (i.e., beginning, amendments, recruitments, results, etc.) but also answers to frequently asked questions.
Data collection
All adults aged 18 y or more could register directly on the website. After receiving information on the platform and clinical trials, volunteers provided identifying information and a verified e-mail and phone number to give online explicit informed consent. Registration was not possible for volunteers with incomplete contact and identity data. No financial incentive was offered for enrollment. Volunteers then logged in to their accounts and filled out a form providing information on (i) risk factors of exposure to SARS CoV-2 (e.g., daily use of public transportation/healthcare workers/interaction with more than 10 people per day) and past medical history of Covid-19; (ii) health status at registration: current smoking habits, body mass index (BMI), being affected by one or more diseases (diabetes, treated high blood pressure, treated heart and/or respiratory diseases, treated renal failure, solid organ transplantation, autoimmune disease, cancer under treatment, ongoing chemical therapy including immunosuppressive therapy); (iii) pregnancy or breastfeeding; (iv) other information (health insurance affiliation, how the participant heard about Covireivac project, willingness to participate in non-Covid-19 clinical vaccine trials, previous participation in a trial). Volunteers with a BMI between 25 and 29 kg/m2 were classified as overweight, and those with a BMI of 30 kg/m2 or more were classified as obese. Region of residence was defined according to the “Région” (French district) of residence: Center of France (Center-Val de Loire, Auvergne – Rhône-Alpes), East of France (Bourgogne-Franche-Comté, Grand Est), North of France (Normandie, Hauts-de-France), Paris region (Ile-de-France), South of France (Occitanie, Provence-Alpes-Côte d’Azur, Corse), West of France (Nouvelle-Aquitaine, Pays de la Loire, Bretagne). As there were no investigation centers in the overseas territories, no volunteers from these regions registered.
During the first 8 months, a free hotline was set up, and third-party registration was available on the platform to help volunteers register.
Since July 5, 2021, an upgrade allowed volunteers to mention if their children (<2 y, 2–6 y, 7–12 y, and 13–17 y) were interested to participate in a Covid-19 vaccine trial. Adults could also register without volunteering for themselves but only for their children.
The management of the platform was done with the Voozanoo software from Epiconcept which follows Food and Drug Administration guidance for computerized systems used in clinical trials. The hosting, provided by Epiconcept, was certified ISO 27,001 HDS. In the platform, each patient was assigned an identification number consisting of the hash of the preferred contact data (telephone or e-mail). This unique identifier was used throughout the platform to identify the patient. To access the platform, users (volunteers, research workers, or administrators) needed to connect through the use of HTTPS protocol for network connection as well as two authentication modalities: the use of a one-time password and password authentication. Different types of rights were attributed to each platform user which limits his access to the platform. Platform administrators in Covireivac were the only members to have access to all data. Each volunteer could only access their data. In each investigation center, a person responsible for the data was identified and assigned personal access codes to the platform. Each data modification on the platform was traced by the system to guarantee the traceability of accesses.
Population and statistical methods
Characteristics of volunteers registered from October 1, 2020 (platform opening) to November 2, 2021, were analyzed. We excluded volunteers without essential baseline information: incomplete identity information (full name or surname, mailing address, e-mail, or phone number to be contacted), no health insurance (as required in France to participate in a clinical trial), or participants already included in a clinical trial. Among duplicated or triplicated registrations (defined by the same last name, first name, and date of birth), only the most recent registrations were kept (according to the date and time of registration). Descriptive statistics were performed on all variables. Continuous variables were reported as medians and interquartile range (IQR) according to distribution. Categorical variables were reported as numbers and percentages. To compare the characteristics of volunteers registered before and after the launch of the Covid-19 vaccination in France (December 27, 2020) we performed univariate analyses: a Student’s t-test or Wilcoxon rank-sum test for continuous variables and the Fisher test of Chi-Square when appropriate for categorical variables. A two-sided P value <.05 was considered statistically significant. Interactions were tested between working as a healthcare worker and (i) gender, (ii) age, and (iii) history of Covid-19. To identify independent volunteer characteristics associated with the period of registration (before or after the launch of the French Covid-19 vaccination campaign), we performed a multivariate logistic regression stratified on gender. Explanatory variables with a p-value of ≤.20 in univariate analysis were included in the final model. Analyses were performed using STATA® software Version 15.1 (Stata Corporation, College Station, Texas, USA).
Ethics and data protection
The platform is compliant with the general data protection regulation (GDPR); the database was declared to the Commission Nationale de l’Informatique et des Libertés (CNIL – French National Data Protection Authority) by Inserm. Following French law and as the data have not been collected and used for research purposes, the Covireivac platform project was not submitted to an ethics committee. Registration was effective only after receiving online explicit informed consent.
Results
Characteristics of registered volunteers
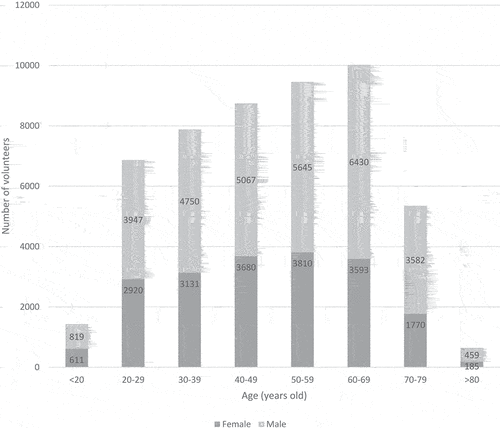
On November 2, 2021, 54,424 volunteers registered on the Covireivac platform, and 52,391 (96%) were included in the analysis. Among them, more than 35,000 volunteers registered 1 month after the opening (October 2020), and 75% of them registered during the first week (). Reasons for non-inclusion were uncomplete identifying information (n = 1), no health insurance (n = 934), volunteers already included in a clinical trial (n = 113), and duplicate or triplicate registrations (n = 962). Volunteer characteristics are shown in . Sixty-one percent (n = 31,893/52,391) were male; the overall ratio of men/women was 1.6 and increased with age, from 1.3 in volunteers under 20 y, to 2.5 for those over 80 y ().
Figure 1. Covireivac’s volunteer platform registration per week from October 1, 2020 to November 2, 2021.*
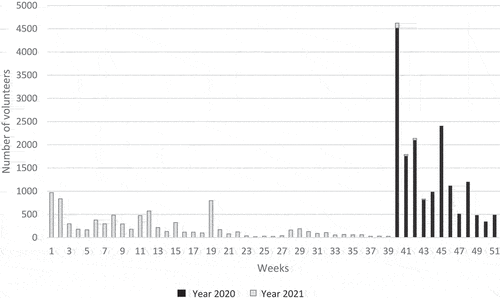
Table 1. Characteristics of volunteers registered on Covireivac platform from October 7, 2020 to November 2, 2021.
The median age was 50 y (IQR: 35–63 y). A quarter of volunteers were aged 65 y and older (26%; n = 13,665/50,399) and only 1% (n = 644/50,399) were more than 80 y. A quarter of volunteers (25%, n = 13,418/52,391) were living in the Paris area. Healthcare workers represented 13% of the volunteers (n = 6,586/49,180). While only 3% of volunteers (n = 1,245/41,500) had previous experience with a clinical trial, 69% were willing to participate in clinical trials other than Covid-19 vaccine trials. This willingness decreased with age, from 77% for those under 20 y, to 50% for those over 80 y.
Thirty-one percent of volunteers were overweight (31%, n = 14,442/46,173) and 16% were obese (n = 7,349/46,173). A total of 32% (n = 15,059/47,172) of volunteers declared having at least one comorbidity: the main comorbidity was treated hypertension (17.1%, n = 8346), followed by treated respiratory disease (10.2%, n = 4994) and heart disease (6.8%, n = 3,326). The proportion of volunteers with at least one comorbidity increased with age from 13% for those under 45 yto more than 60% after 70 y. At registration 7.2% of volunteers (n = 3,489/48,957) had a history of Covid-19.
Since July 5, 2021, 308 children were mentioned as volunteers by their parents: 2% (n = 7) were less than 2 y, 24% (n = 74) were between 2 and 6 y, 51% (n = 157) were between 7 and 12 y, and 23% (n = 70) were between 13 and 17 y.
Multivariate analysis
Most volunteers registered before the opening of the Covid-19 vaccine campaign in France (79%, n = 41,694) on December 27, 2020, and 8,705 volunteers (21%) registered after. We compared the characteristics of volunteers registered before (early registration) and after the opening (late registration) (). Compared to early registered, late registered were younger (47 vs. 49 y, p < .001), more frequently women (48% vs. 37%, p < .001), less likely to be healthcare workers (11% vs. 14%, p < .001), and were more exposed to Covid-19 (9% vs. 7%, p < .001). Moreover, fewer volunteers were willing to participate in other trials (63% vs. 67%, p < .001), particularly in volunteers aged over 45 y. An interaction was found between working as a healthcare worker and gender. We identified four independent characteristics associated with late registration: (i) younger age and (ii) occupation (less likely to be health workers) were consistent in both genders, (iii) obesity (less obesity) in men and (iv) more comorbidities in women ().
Table 2. Comparison of characteristics of COVIREIVAC volunteers registered before and after the launch of the French Covid-19 campaign, univariate analysis (December 27, 2020).
Table 3. Comparison of characteristics of COVIREIVAC volunteers registered before and after the launch of the French Covid-19 campaign, multivariate analysis (December 27, 2020).
Discussion
We describe here the implementation of the first online recruitment platform coupled with an information website on vaccine trials and Covid-19 vaccines in France. For the last years, experts worldwide have raised concerns against the rise of vaccine hesitancy in Europe,Citation1 and especially in France.Citation2,Citation3 Hence, researchers were concerned about volunteer willingness to participate in vaccine trials,Citation4 in a country where healthy volunteer participation in clinical trials is very low.Citation5 However, this platform was a success and far exceeded the recruitment target of 25,000 volunteers, without any advertisement other than communication throughout the media and no financial incentive. Although vaccine hesitancy is of major concern in France,Citation3 the platform succeeded to register more than 300 parents and children within 6 months.
In the USA, ongoing trials promoted by American federal agencies, academic or industrial sponsors, are listed on several websites on which volunteers can find contacts of the investing centers (e-mail or phone number). Hence, the National Institute of Allergy and Infectious Diseases (NIAID) implemented the “COVID-19 Prevention Network” (COVPN), which aims to recruit volunteers for large-scale Covid-19 vaccines or monoclonal antibodies recruited almost 600,000 volunteers in the US in April 2021.Citation6,Citation7 In February 2020, in Europe, VACCELERATE a clinical research network for the coordination and conduct of Covid-19 vaccine trials was implemented. Twenty-nine national partners took part in the network. As a part of the project, volunteer registries were implemented in eight countries. In December 2021, more than 13,000 volunteers were registered on this platform (https://www.vaccelerate.eu/volunteer-registry/index.html). Even if the Covireivac project and Inserm are part of Vaccelerate, French volunteers did not register on the same platform, as the European platform was implemented later.
Recruitment for vaccine trial volunteers in France is usually local. Clinical centers have their own healthy volunteers database and launch recruitment campaigns in local media or through healthcare workers’ networks, as they are part of hospitals. In 1992, in response to the human immunodeficiency virus (HIV) pandemic, a healthy volunteers network was implemented by ANRS for HIV-related vaccine trials.Citation8,Citation9 Calls for application for this network were made through various targeted media, and 4,259 applicants were selected after a long selection process. To recruit volunteers with no identified risk factors of HIV, each volunteer had to write a letter of interest, undergo clinical and biological examinations, and be interviewed by a psychologist or psychiatrist. These recruitment methods did not allow the large and quick recruitment needed for vaccine trials in a time of pandemic whereas the Covireivac platform recruited more than 40,000 volunteers in just a few weeks.
More men than women (61% vs. 39%) are registered on Covireivac. This gender balance was found in a US study on willingness to participate in vaccine-related clinical trials in the US,Citation10 but also in an English and a French survey on willingness to participate in Covid-19 vaccine-related trials.Citation11,Citation12 According to the French National Institute of Statistics and Economic Studies (INSEE), the age of Covireivac volunteers are similar to that of the overall French population in 2021, except for volunteers over 70 y: 12% of volunteers over 70 y vs. 20% in the French population, 10% of volunteers aged 65–69 y vs. 7%, 38% of volunteers aged 45–64 y vs. 34%, 40% of volunteers aged under 45 y vs. 39%.Citation13 This is consistent with a study conducted in Belgium on recruitment barriers for prophylactic vaccine trials showing that the very elderly participants (over 85 y) are the most difficult to recruit.Citation14 Moreover, an English survey exploring the key factors related to perceptions and participation in vaccination trials showed that people over 70 y were less willing to participate in a vaccine trial.Citation12 Barriers to recruiting older adults may be multiple, but the computer-based Covireivac recruitment might not be adequate for elderly people even if a free hotline was set up and third-party registration was available on the platform. But third-party registration was only used by nine volunteers. More volunteers under 45 y registered after the launch of the French vaccination campaign. It reflects the evolution of the French Covid-19 vaccination policy, which advised vaccine first for elderly people (institutionalized, then over 75 y and between 65 and 74 y).Citation15
A high proportion of healthcare workers registered on the Covireivac platform even if calls were made through national nonspecialized media. According to INSEE, the health sector represents 7% of workers in France, whereas 13% of health workers registered on Covireivac.Citation16 A French study on intention to participate in a Covid-19 vaccine clinical trial in 2020 corroborates that healthcare workers were more prone to participate in a Covid-19 vaccine trial than non-healthcare workers (50.5% vs. 45.4%, p < .005).Citation11
Covireivac volunteers appear to have fewer comorbidities than the general French population. Only 30% of volunteers stated having at least one chronic disease, whereas in 2017, according to INSEE, 40% of the overall French population aged over 17 y stated having a chronic disease.Citation17 This could be explained as the list of diseases on the Covireivac form being restrictive. But, according to a cross-sectional study conducted in 2016–2017 in Atlanta (USA) in people over 60 y, participants with a better health status were more likely to participate in a clinical trial.Citation10 A French study and a German study conducted during the Covid-19 pandemic highlighted that volunteers with comorbidities were more likely to participate in Covid-19 vaccine trials.Citation11,Citation18 This finding may be explained by the pandemic context, as Covid-19 was perceived as an acute threat in persons with comorbidities.Citation19
Only 8,705 volunteers registered after the launch of the French Covid-19 vaccination campaign (vs. 41,694 in the three previous months). As some volunteers could have registered on the platform to get early access to Covid-19 vaccines the registration decrease can be explained by the availability of Covid-19 vaccines in 2021. But it could also be explained by the large variety of licensed vaccines in France and around the world,Citation20 but also the lack of communication campaign for volunteer recruitment after the platform launch.
The platform allowed large-scale recruitment of healthy volunteers. Hence, this kind of recruitment is only suitable for large clinical trials including healthy volunteers such as vaccine trials or preventive treatment trials. It did not allow recruitment of specific populations such as elderly volunteers with specific comorbidities or pregnant women highlighting the need to have several types of recruitment depending on the type of the study population: through patient association, focused media campaigns, or general practitioners.
Overall, the Covireivac platform was a great success since the number of volunteers registered in 3 months was almost twice the number expected. It allowed quick and large recruitment of healthy volunteers, with different profiles matching inclusion criteria expected for industrial or academic sponsored trials. This recruitment method could be used on a larger scale for vaccine trials in France, even in a non-crisis context. It could facilitate recruitment and provide sponsors with better visibility of the recruitment capacities of clinical research centers.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The state of vaccine confidence 2016: global insights through a 67-country survey. EBioMedicine. 2016;12:1–7. doi:10.1016/j.ebiom.2016.08.042.
- Verger P, Fressard L, Collange F, Gautier A, Jestin C, Launay O, Raude J, Pulcini C, Peretti-Watel P. Vaccine hesitancy among general practitioners and its determinants during controversies: a national cross-sectional survey in France. EBioMedicine. 2015;2(8):891–897. doi:10.1016/j.ebiom.2015.06.018.
- Peretti-Watel P, Verger P, Raude J, Constant A, Gautier A, Jestin C, Beck F. Dramatic change in public attitudes towards vaccination during the 2009 influenza A(H1N1) pandemic in France. Eurosurveillance. 2013;18(44):20623. doi:10.2807/1560-7917.ES2013.18.44.20623.
- Peretti-Watel P, Seror V, Cortaredona S, Launay O, Raude J, Verger P, Fressard L, Beck F, Legleye S, L’Haridon O. A future vaccination campaign against COVID-19 at risk of vaccine hesitancy and politicisation. Lancet Infect Dis. 2020;20(7):769–770. doi:10.1016/S1473-3099(20)30426-6.
- Detoc M, Launay O, Dualé C, Mutter C, Le Huec J-C, Lenzi N, Lucht F, Gagneux-Brunon A, Botelho-Nevers E. Barriers and motivations for participation in preventive vaccine clinical trials: experience of 5 clinical research sites. Vaccine. 2019;37(44):6633–6639. doi:10.1016/j.vaccine.2019.09.048.
- Coronavirus Clinical Studies [Internet]. [accessed 2021 Dec 29]. https://www.coronaviruspreventionnetwork.org.
- Andrasik MP, Broder GB, Wallace SE, Chaturvedi R, Michael NL, Bock S, Beyrer C, Oseso L, Aina J, Lucas J. Increasing black, indigenous and people of color participation in clinical trials through community engagement and recruitment goal establishment. PLoS One. 2021;16(10):e0258858. doi:10.1371/journal.pone.0258858.
- Fillieule O. Logics of commitment in vaccine trials of the ANRS cohorts 1992-2001. J Acquir Immune Defic Syndr. 2018;79(Suppl 1):S59–68. doi:10.1097/QAI.0000000000001809.
- Durier C, Desaint C, Launay O. Social and behavioral consequences of participation in HIV preventive vaccine trials in the ANRS COHVAC cohort. J Acquir Immune Defic Syndr. 2018;79(Suppl 1):S37–50. doi:10.1097/QAI.0000000000001807.
- Raheja D, Davila EP, Johnson ET, Deović R, Paine M, Rouphael N. Willingness to participate in vaccine-related clinical trials among older adults. Int J Environ Res Public Health. 2018 Aug;15(8):1743. doi:10.3390/ijerph15081743.
- Detoc M, Bruel S, Frappe P, Tardy B, Botelho-Nevers E, Gagneux-Brunon A. Intention to participate in a COVID-19 vaccine clinical trial and to get vaccinated against COVID-19 in France during the pandemic. Vaccine. 2020;38(45):7002–7006. doi:10.1016/j.vaccine.2020.09.041.
- Sethi S, Kumar A, Mandal A, Shaikh M, Hall CA, Kirk JMW. The UPTAKE study: implications for the future of COVID-19 vaccination trial recruitment in UK and beyond. Trials. 2021;22(1):296.
- Population par sexe et groupe d’âges | Insee [Internet]. [accessed 2021 Dec 23]. https://www.insee.fr/fr/statistiques/2381474#figure1_radio1.
- Harrington L, Van Damme P, Vandermeulen C, Mali S. Recruitment barriers for prophylactic vaccine trials: a study in Belgium. Vaccine. 2017 Dec;35(48):6598–6603. doi:10.1016/j.vaccine.2017.10.041.
- Laura Z. Stratégie de vaccination contre le Sars-Cov-2. 2020;58.
- Emploi par activité − Tableaux de l’économie française | Insee [Internet]. [accessed 2021 Dec 28]. https://www.insee.fr/fr/statistiques/4277675?sommaire=4318291#titre-bloc-3.
- État de santé de la population − France, portrait social | Insee [Internet]. [accessed 2021 Dec 28]. https://www.insee.fr/fr/statistiques/4238405?sommaire=4238781.
- Shamsrizi P, Kramer FJ, Addo MM, Fathi A. Characterization of individuals interested in participating in a phase I SARS-CoV-2 vaccine trial. Vaccines (Basel). 2021;9(10):1208. doi:10.3390/vaccines9101208.
- Laires PA, Dias S, Gama A, Moniz M, Pedro AR, Soares P, Aguiar P, Nunes C. The association between chronic disease and serious COVID-19 outcomes and its influence on risk perception: survey study and database analysis. JMIR Pub Health Surveill. 2021;7(1):e22794. doi:10.2196/22794.
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397(10278):1023–1034. doi:10.1016/S0140-6736(21)00306-8.