ABSTRACT
It is well documented that COVID-19 vaccines greatly reduce the severity and complications of SARS-CoV-2 infection. However, it has been reported that COVID-19 related vaccines may induce or exacerbate autoimmune hematological disorders, for example, a decrease in platelet numbers characteristic of immune thrombocytopenia (ITP). To investigate this, we retrospectively reported, for the first time, the clinical characteristics of 42 ITP patients after COVID-19 vaccination in southwest China. Of the 42 patients, 28 patients were historically diagnosed ITP, and their platelet counts (PC) decrease mainly occurred after the first-dose vaccinations. The average PC after vaccination was 39.5 × 109/L and recovered to an average of 80.6 × 109/L after treatment. Efficacy of treatment was 90%, and only 10% maintained low PC at the third month of treatment. More interestingly, of the 42 patients, 14 were newly diagnosed ITP following vaccination. Of these 14 patients, 6 patients (43%) were found PC deterioration after the first vaccine dose, and 7 patients (50%) after the second dose. Fortunately, the peripheral PC of all 14 patients recovered significantly after treatment, and the average PC was 139.4 × 109/L, including 8 CRs (complete response) and 6 PRs (partial response). Notably, 9 of the 14 cases were found to have abnormal immune indices when thrombocytopenia diagnosed. No severe organ hemorrhage was found in either subgroup. These results are reassuring the vaccine safety for ITP patients, in that the risks of aggravating thrombocytopenia by COVID-19 vaccination do exist, but it was transient and can be effectively controlled through intensive clinical monitoring and management.
Introduction
The COVID-19 epidemic has swept the world now for over two years. With the development and clinical application of COVID-19 related vaccines, healthy populations have less chance of being affected by this disease and, if affected, have much less severity of clinical manifestations.Citation1 More than 100 kinds of vaccines have been developed against SARS-CoV-2 according to the World Health Organization (WHO).Citation2 In China, there are predominantly three kinds of vaccines being used, including adenovirus vaccine (one dose to complete a full vaccination), inactivated virus vaccine (two dose) and recombinant protein vaccine (three dose), with the latter two types of vaccines representing the most widely used in Chongqing, the largest city in southwest China. Inactivated-virus vaccines consist of the whole components of a virus but it lacks the ability to infect cells and to replicate itself, while the protein-based vaccine is made of a fragments of virus proteins or polysaccharides from recombinant protein, virus-infected cells, or virus-like particles.Citation3 Although full vaccination with any of these types of vaccines can effectively reduce complications from infection with SARS-CoV-2 substantially, such as pneumonia, hospitalization, and death, these vaccines may be associated with certain types of adverse events (AEs) depending on different designs of the vaccines.
The most common AEs are mild and include injection site reactions, fever, chills, fatigue, headache, and muscle and joint aches.Citation4,Citation5 However, there are also reports of some rare complications, such as interstitial pneumoniaCitation6 and immune thrombotic thrombocytopenia.Citation7 It has been reported that the SARS-CoV-2 virus can cause thrombocytopenia,Citation8 however, it is unclear if COVID-19 related vaccines may also cause or exacerbate thrombocytopenia, in healthy people and in ITP patients, respectively. Here, we report the characteristics of both ITP patients and healthy individuals diagnosed with thrombocytopenia at our hospital in Chongqing City, China, after COVID-19 vaccination.
Data and methods
Patient data
Data was collected and analyzed from admitted thrombocytopenia patients after vaccinated with a COVID-19 related vaccine, who visited our outpatient department before 31 October 2021. This study was approved by the ethics committees of Xinqiao Hospital.
Diagnostic criteria
ITP is diagnosed according to the following criteria: (1) platelet counts (PC)< 100 × 109/L by routine blood test at least twice, without abnormal morphology of blood cells; (2) With or without clinical manifestations such as skin hemorrhage and ecchymosis, and (or) mucosal hemorrhage and visceral hemorrhage; (3) usually no spleen enlargement; (4) Exclusion of other secondary thrombocytopenia, such as hypoplastic leukemia, aplastic anemia with thrombocytopenia as the primary hematologic abnormality, hereditary thrombocytopenia, secondary to other immune diseases or infection.Citation9,Citation10
Classification criteria
Newly diagnosed ITP refers to patients within 3 months of diagnosis. Persistent ITP describes patients with ITP lasting between 3 and 12 months from diagnosis. This category includes patients not achieving spontaneous remission or those unable to maintain the therapeutic effect after stopping treatment for 3 to 12 months from diagnosis. Chronic ITP describes patients with ITP lasting for more than 12 months.Citation9
Bleeding severity scoring
Bleeding scoring is carried out in accordance with the Chinese ITP diagnosis and treatment guidelines.Citation9,Citation10
Treatment methods and efficacy evaluation
When patients included in this analysis met the criteria to receive treatment, their treatment was conducted based on the relevant ITP guidelines of ChinaCitation9 and the experience of our center,Citation11–13 including traditional Chinese medicine (TCM), corticosteroids (dexamethasone and prednisone), TPO receptor agonists (TPO-RAs), cyclosporine, rapamycin (sirolimus), IVIg, etc.
Efficacy evaluation
Complete response (CR) indicates platelet count higher than 100 × 109/L and absence of bleeding was achieved following treatment. Partial response (PR) indicates PC between 30 × 109/L and 100 × 109/L, at least doubling of the baseline count, and absence of bleeding. Stable disease (SD) indicates platelet count higher than 30 × 109/L and absence of bleeding, but less than doubling of the baseline count. No response (NR) indicates a platelet count lower than 30 × 109/L.Citation10
Statistical analysis
Statistical analysis was performed using SPSS 22.0 and GraphPad Prism 6. The enumeration data is expressed as percentage (%), and the measurement data is expressed as (× ± s). ANOVA (normal distribution) or nonparametric test (non-normal distribution) for continuous variables and the Chi-square test was used to analyze the clinical features of patients. P < .05 was statistically significant.
Results
Patient data
Among the 42 ITP patients, 32 (76%) were female, and 10 (24%) were male. The age span was 14–87 years old with a median age of 50 years old. There were 28 patients (67%) with ITP diagnosed prior to COVID-19 vaccination, while 14 patients (33%) were newly diagnosed ITP patients claiming normal PC before vaccination. Notably, only 2 of the 14 patients were able to provide pre-vaccination platelet test results, while the other 12 patients were unable to confirm normal platelet count prior to vaccination. For those with preexisting ITP (persistent and chronic ITP), their duration from the first historical ITP diagnosis to the observation end time (until October 31st, 2021) was as follows: 1 patient experienced three months of ITP, 1 patient five months, 3 patients nine months, and the rest of the 23 patients (23/28, 82%) had ITP more than one year, with the longest having a history of ITP for 23 years.
The effect of COVID-19 vaccination on patients with historically diagnosed ITP
Among the 28 ITP patients with previously diagnosed ITP, 26 patients exhibited a further reduction of platelet counts, albeit to different extents, following vaccination. Compared with the platelet number before vaccination (with the most recent laboratory results ranging from 7 to 30 days prior to vaccination), 11 patients (39%) exhibited a PC further decreasing by more than 60%; 6 patients (22%) exhibited a PC further decreasing by 40–60%; 5 patients (18%) exhibited a PC further decreasing by 20–40%; 4 patients (14%) exhibited a PC decline of less than 20% (). There were two exceptions, one refractory ITP patient had PC as low as 7 × 109/L both before and after vaccination. The other ITP patient who was under rapamycin administration showed PC recovery from 86 × 109/L to 127 × 109/L after vaccination, and the vaccination did not disturb the therapeutic effect of rapamycin. Prior to vaccination, 17 patients did not receive medication to treat their ITP, while 5 were treated with TCM, 3 with prednisone, 1 with Eltrombopag, and 2 with rapamycin. As for impact of vaccination on organ bleeding, severe organ bleeding was not found in any patient; 6 people (22%) had mild bleeding, and all of their bleeding scores were less than 3 ().
Figure 1. The effect of COVID-19 vaccination on patients with historically diagnosed ITP. a: Analysis of the degree of platelet decline in ITP patients after vaccination, compared with the platelet base level before vaccination. b: Proportion of ITP patients with platelet deterioration after the first, second and third dose. c and d: Response to therapy in ITP patients with PC decline after vaccination.
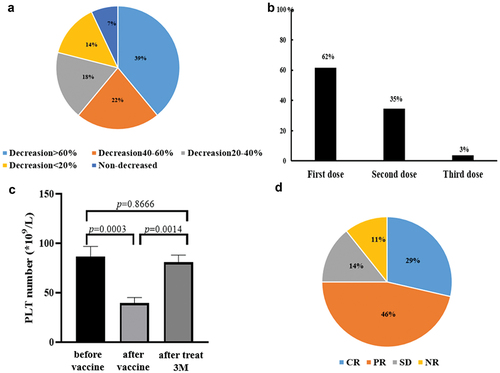
Table 1. The effect of COVID-19 vaccination on patients with historically diagnosed ITP.
Of the 28 patients, 26 patients were vaccinated with inactivated virus vaccine (two doses) and two were vaccinated with recombinant protein vaccine (three doses). PC of 16 patients (62%, 16/26) were found to decrease after the first dose, while PC of 9 patients (35%, 9/26) were found to have decreased after the second dose, and only one patient (3%) exhibited platelet deterioration after the third dose ().
In the 28 patients, the average PC before vaccination was 86.4 × 109/L, decreased to 39.5 × 109/L post-vaccination, and recovered to 80.6 × 109/L after treatment (). After the identification of PC drop and subsequent intervention, the total efficacy of intervention was about 90%, while 10% of patients with ITP still exhibited reduced PC at the end of three-month treatment ().
The effect of COVID-19 vaccination on newly diagnosed ITP patients claiming previous normal platelet counts
Of the 42 patients, 14 had never been diagnosed with ITP and claimed that their platelet counts were normal; however only 2/14 could provide laboratory results confirming normal PC. Because the actual number of platelets before vaccination could not be provided, but they had not been yet diagnosed with ITP prior to vaccination, for this analysis we assume the PC count to higher than 100 × 109/L. Of these 14 individuals, all had thrombocytopenia after vaccination, with a mean PC 38.07 × 109/L. After 3 months of treatment, the platelets of all patients recovered significantly, and the average PC was 139.4 × 109/L, including 8 CRs and 6 PRs (), while the lowest PC recovered to 51 × 109/L.
Figure 2. The effect of COVID-19 vaccination on new diagnosed ITP patients claiming previous normal PC. a: After 3 months of treatment, the platelets of all patients recovered significantly to 139.4 × 109/L. b: Response to therapy in ITP patients with PC decline after vaccination. ORR (CR+PR) is 100%. c: Proportion of ITP patients with platelet deterioration after the first, second and third dose.
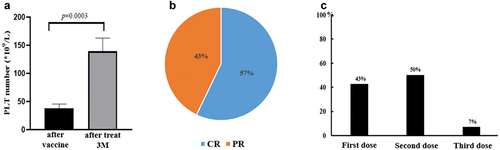
Among the 14 patients, 10 were vaccinated with inactivated virus vaccine (two doses) and four with recombinant protein vaccine (three doses). Six patients (43%) exhibited PC decrease after the first dose vaccine, 7 patients (50%) were found to have decrease of PC after the second vaccine, and one patient (7%) platelet deteriorated even after the third-dose vaccine (). Likewise, no severe organ hemorrhage was found. There were five people (5/14, 36%) had mild bleeding, with all the bleeding scores less than 3 (). It is worth noting that 9 of the 14 cases were found to have abnormal immune status at the time when thrombocytopenia diagnosed. Among them, seven had abnormal antinuclear antibody spectrum, two had abnormal thyroid function, and 1 had hepatitis B antigen positive ().
Table 2. The effect of COVID-19 vaccination on newly diagnosed ITP patients claiming previous normal platelet counts.
Discussions
The administration of COVID-19 related vaccines has greatly reduced the spread and severe outcomes associated with infection with the SARS-CoV-2 virus. The precise platelet dynamics in previously diagnosed ITP patients and “healthy” individuals diagnosed with ITP after COVID-19 vaccination is currently unknown. Although there have been a handful of case reports of thrombocytopenia after COVID-19 vaccination,Citation14–16 our article describes the characteristics of platelet counting in these ITP populations for the first time in southwest China. To date, this is the largest number of case report of COVID-19 vaccine-induced platelet deterioration.
COVID-19 vaccines are a major concern for ITP patients who fear that vaccination might exacerbate their thrombocytopenia. Among the 28 ITP patients in this study, 26/28 patients exhibited platelet count decreased to different degrees, which fortunately, responded well to platelet-specific treatment. After 3-months of treatment, the ORR (CR+PR+SD) was 89%, and only about 11% were non-responders. After vaccination, 29% (8/28) of patients had PC less than 20 × 109/L, 21% (6/28) of patients had slight organ hemorrhage, and no severe bleeding events were found.
Therefore, we suggest that ITP patients, especially during a period of active disease, need to be vaccinated with careful caution or postpone the vaccination. Once inoculated, it is necessary to increase frequency and intensity of patient management, including closely monitoring routine blood tests, and actively intervening once platelet reduction getting worse. In our center, some ITP patients received no treatment after vaccination and their platelets recovered on their own, while other patients with chronic ITP did not experience platelet deterioration after receiving the COVID-19 vaccines. Taken together, we cannot simply say that all ITP patients must or must not be vaccinated against COVID-19 vaccines. We do suggest, however, that ITP patients receive enhanced disease monitoring after vaccination.
As a reminder, all 28 patients who visited our clinic were from southwest area of China. We do not have the data of the total ITP population vaccinated and the extent of platelet exacerbation in all ITP patients after vaccination. As one of the largest hematological disease centers in southwest China, based on an annual incidence rate of 5 ITP cases per 100,000 adults, using data from the Chongqing Region (population 30,000,000 inhabitants), we roughly estimate that COVID-19 vaccines worsened ITP would only occur in 1.8% of the population, which is consistent with the 1.5–3.0% reported internationally.Citation17
Of note in this study, we observed 14 cases of ITP diagnosed post-vaccine in self-declared “healthy” people. However, we found that 9 of the 14 cases were found to have an abnormal immune index when thrombocytopenia was diagnosed (). It is possible that the vaccine led to this immune response or, more likely, that these patients’ abnormal immune condition possibly made them more prone to COVID-19 vaccine-related thrombocytopenia. In the latter case, they would not be considered truly “healthy” at the time of vaccination, but rather may have had undiagnosed thrombocytopenia. In addition, limitation exists in our paper. All 14 patients claimed that the platelets were normal before receiving the vaccine, but they had not undergone routine blood tests, so the actual PC before vaccination was not evidenced. It is possible that these individuals did have thrombocytopenia to a certain extent, but they have not undergone regular physical examinations to be diagnosed earlier. After three months of treatment, the PC of all the 14 patients were able to recover to more than 50 × 109/L successfully. As a result, the authors suggest that, for healthy people, there is less concern about the occurrence of thrombocytopenia after the administration of COVID-19 related vaccines. Our second suggestion is that platelets and immune indicators (antinuclear antibody spectrum, thyroid function, etc.) should be closely monitored and managed after vaccination, for the population at higher risk of developing immune-mediated disease.
Another feature of this study is that we observed and compared the incidence of thrombocytopenia after receiving the first, second and third dose of COVID-19 vaccines. For the historically diagnosed ITP population, platelet decline after the first dose of the vaccine, accounted for 62%, and receiving the second dose for 35%. It hints that ITP should be actively managed as early as the first injection. In “healthy” people, the morbidity of platelet deterioration after the first dose and the second dose were 43% and 50% respectively, with no significant difference (). It is suggested that close observation is required after each dose vaccine. On the other hand, assuming that 30 million people in Chongqing all received 2 doses of inactivated vaccine, then the incidence of ITP after vaccination was 0.7 per million doses (42 patients/60 million doses), which is lower than the reporting rate of immune thrombocytopenia after receipt of mRNA COVID-19 vaccines,Citation18 but needs more clinical data to confirm.
A recent report summarized a series of case reports of ITP patients after COVID-19 vaccination,Citation19 including 22 patients in total, and most patients experienced ITP or thrombocytopenia after first-dose COVID-19 vaccination. Platelets of the 21 patients were improved after treatment with glucocorticoid and IVIG, except one case, which is consistent with our report that thrombocytopenia caused by COVID-19 vaccination has a relatively good response rate. Our report further subgrouped ITP population into two parts, pointing out that preexisting ITP patients are more prone to platelet deterioration after the first dose, while de novo ITP patients may develop thrombocytopenia both after the first and the second dose of vaccines. Compared with other case reports, the 42 patients in our report had low bleeding scores, and organ bleeding was mostly manifested in skin or mucosa. In addition, we found that the treatment response rate of preexisting ITP patients was not as good as that of newly diagnosed ITP patients (ORR 89% vs 100%). Finally, as mentioned above, for de novo ITP patients after COVID-19 vaccination, we must comprehensively check their antinuclear antibody spectrum, thyroid function and other immune indicators. It may be that the patients themselves have immune abnormalities (not found before), and vaccination is just a triggering event, evoking the occurrence of ITP, which is also an unexpected discovery of this study.
Because the incidence of SARS-CoV-2 vaccine-related ITP is low, the clinical characteristics and treatment outcomes for this kind of ITP, comparing with non-vaccine induced ITP, remains inconclusive. Based on our observations of these 42 cases and other reports in the literature, we believe that SARS-CoV-2 vaccine-related ITP are expectedly responsive to the standard or modified treatment of ITP. Clinical bleeding tendency is not high, and recovery is generally good. In 10 of 42 patients, platelets recovered on their own even without treatment ( , ). Of course, more clinical evidence is needed to validate our conclusions. Notably, it is suggested that rituximab is best preferentially avoided in the initial treatment regimen since it may take up to 6⁓8 weeks to produce a response and may also impair the protective effect of the COVID-19 vaccine.Citation20
In conclusion, COVID-19 vaccination may have a significant effect on platelet count in preexisting ITP patients and certain individuals with previously undiagnosed ITP. Our results demonstrate that close monitoring of platelet count after COVID-19 vaccination is important for patients historically diagnosed with ITP, especially after the first dose. The results are very reassuring for ITP patients that the risks of aggravated thrombocytopenia specifically due to getting COVID-19 vaccine are small but non-negligible. Decreases in platelet count following vaccine administration did occur, but the disease could be successfully managed and most decreases were transient and responded well to treatment.
Author contributions
Yimei Feng contributed to the design and conceptualization of the research, design of data analyses, interpretation of data, collection of data, and writing of the manuscript. YQ contributed collection of data. KC, ZZ, YG and XZ edited this report. Xi Zhang and Yimei Feng funded the work. All authors contributed to the article and approved the submitted version.
Disclosure statement
Author Kaniel Cassady is now employed by Regeneron Pharmaceuticals. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Zeng QL, Lv YJ, Liu XJ, Jiang ZY, Huang S, Li WZ, Yu ZJ. Clinical characteristics of Omicron SARS-CoV-2 variant infection after non-mRNA-based vaccination in China. Front Microbiol. 2022;13(901826). doi:10.3389/fmicb.2022.901826.
- Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect. 2022;28(2):1–7. doi:10.1016/j.cmi.2021.10.005.
- Mingot-Castellano ME, Butta N, Canaro M, Solano MDGD, Sanchez-Gonzalez B, Jimenez-Barcenas R, Pascual-Izquierdo C, Caballero-Navarro G, Urena LE, Gonzalez-Lopez TJ, et al. COVID-19 vaccines and autoimmune hematologic disorders. Vaccines. 2022;10(6):961. doi:10.3390/vaccines10060961.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi:10.1056/NEJMoa2035389.
- Park JY, Kim JH, Lee IJ, Kim HI, Park S, Hwang YI, Jang SH, Jung KS. COVID-19 vaccine-related interstitial lung disease: a case study. Thorax. 2021;77(1):102–104. doi:10.1136/thoraxjnl-2021-217609.
- Schultz NH, Sorvoll IH, Michelsen AE, Munthe LA, Lund-Johansen F, Ahlen MT, Wiedmann M, Aamodt AH, Skattor TH, Tjonnfjord GE, et al. Thrombosis and thrombocytopenia after ChAdox1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi:10.1056/NEJMoa2104882.
- Singh S, Sharma R, Singh J, Jain K, Paul D. Thrombocytopenia in COVID-19: focused summary of current understanding of mechanisms and clinical implications. J Pediatr Hematol Oncol. 2021;43(7):243–248. doi:10.1097/MPH.0000000000002264.
- Liu XG, Bai XC, Chen FP, Cheng YF, Dai KS, Fang MY, Feng JM, Gong YP, Guo T, Guo XH, et al. Chinese guidelines for treatment of adult primary immune thrombocytopenia. Int J Hematol. 2018;107(6):615–623. doi:10.1007/s12185-018-2445-z.
- Hemostasis Group. Association CSoHCM. Chinese guideline on the diagnosis and management of adult primary immune thrombocytopenia (version 2020). Zhonghua Xue Ye Xue Za Zhi. 2020;41(8): 617–623. in Chinese. doi:10.3760/cma.j.issn.0253-2727.2020.08.001.
- Feng YM, Xiao YS, Yan HJ, Wang P, Zhu W, C K, Zou Z, Wang K, Chen T, Quan Y. Sirolimus as rescue therapy for refractory/Relapsed immune thrombocytopenia: results of a single-center, prospective, single-arm study. Front Med. 2020;7:110. doi:10.3389/fmed.2020.00110.
- Zhang C, Liu HF, Chen XH, Gao L, Gao L, Liu Y, Kong PY, Sun AH, Zhang X. Is splenectomy necessary for immune thrombocytopenic purpura? The role of rituximab in patients with corticosteroid resistance in a single-center experience. Clin Ther. 2014;36(3):385–388. doi:10.1016/j.clinthera.2014.01.017.
- Feng Y, Chen X, Cassady K, Zou Z, Yang S, Wang Z, Zhang X. The role of mTOR inhibitors in hematologic disease: from bench to bedside. Front Oncol. 2020;10:611690).
- Dijk W, Schutgens REG. Relapse of immune thrombocytopenia after COVID-19 vaccination. Eur J Haematol. 2022;108(1):84–85. doi:10.1111/ejh.13713.
- Kuter DJ. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br J Haematol. 2021;195(3):365–370. doi:10.1111/bjh.17645.
- Shah SRA, Dolkar S, Mathew J, Vishnu P. COVID-19 vaccination associated severe immune thrombocytopenia. Exp Hematol Oncol. 2021;10(1):42. doi:10.1186/s40164-021-00235-0.
- Kragholm K, Sessa M, Mulvad T, Andersen MP, Collatz-Christensen H, Blomberg SN, Lippert F, Mikkelsen S, Leutscher P, Melgaard D, et al. Thrombocytopenia after COVID-19 vaccination. J Autoimmun. 2021;123(102712):102712. doi:10.1016/j.jaut.2021.102712.
- Welsh KJ, Baumblatt J, Chege W, Goud R, Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the vaccine adverse event reporting system (VAERS). Vaccine. 2021;39(25):3329–3332. doi:10.1016/j.vaccine.2021.04.054.
- Shonai T, Kimura F, Watanabe J. Severe immune thrombocytopenia after COVID-19 vaccination: two case reports and a literature review. Intern Med. 2022;61(10):1581–1585. doi:10.2169/internalmedicine.9177-21.
- Sivaramakrishnan P, Mishra M. Vaccination-Associated immune thrombocytopenia possibly due to ChAdox1 nCov-19 (Covishield) coronavirus vaccine. BMJ Case Rep. 2022;15(3):e249237. doi:10.1136/bcr-2022-249237.
