ABSTRACT
Hand, foot and mouth disease was mainly caused by EV-A71 virus. The main antigen structure of VP1 region of EV-A71 was easily varied. Here, we investigated the seroprevalence of EV-A71 based on a large group of healthy individuals in Beijing, China, in order to study the effectiveness of EV-A71 vaccine in a real-world setting. BrCr and the clinical strain isolated from the Chinese mainland in 2008 (“vaccine strain:”CMU4232/BJ/CHN/2008), EV-A71 C4 epidemic strains isolated in 2010, 2013, and 2016, were tested for neutralizing antibodies (NtAb) in every year. Phylogenetic tree analysis of the EV-A71 strains above, as well as amino acid composition homologous sequence analysis were applied. The “vaccine strain” has 83.0% homology with FY23, H07 and FY7VP5. It belongs to the same branch of C4a as 10 C4, 13 C4 and 16 C4, and differs from the amino acid sites 283 and 293 of 16 C4. Compared with “vaccine strains,” there was a significant difference between the 50–59 years old age group when the NtAb titer of 16 C4 strain was 1:512-1:1024. Our results suggest that changes in the functional epitopes of NtAb caused by amino acid 283 and 293 loci in EV-A71 strains may affect the production of neutralizing antibodies.
KEYWORDS:
Introduction
Hand, Foot and Mouth Disease (HFMD) was first reported in New Zealand in 1957. Although HFMD is typically a self-limited disease, severe complications may occur including brain-stem encephalitis, acute flaccid paralysis and aseptic meningitis.Citation1,Citation2 It has been implicated in a series of outbreaks across the Asia-Pacific region since the early 20th century.Citation3–7 The largest Asia-Pacific epidemic occurred in China in 2008. According to data from the Chinese Center for Disease Control and Prevention, 24.64 million cases of HFMD were reported in mainland China among which 3,700 were fatal from 2008 to February 2022.
Enterovirus-A71 (EV-A71) was the dominant pathogen in severe and fatal HFMD cases.Citation8 Human EV-A71 is a group of genetically diverse viruses belonging to the genus Enterovirus species A, family Picornaviridae.Citation9 The emergence of the EV-A71 epidemic in the Asia-Pacific region has been associated with the circulation of different genetic lineages (genotypes B3, B4, C1, C2, and C4) that appear to be undergoing rapid evolutionary changes.Citation10 From March 2008 to June 2009, more than 600,000 cases of HFMD and 126 deaths were reported in Fuyang City, Anhui Province, PRC. Subsequently, it was reported that the epidemic spread rapidly to other areas. This was a new epidemic caused by recombinant EV-A71 C4a.Citation11 Then we assume that the EV-A71 outbreak may be caused by the infection of the epidemic strain and the vaccine are of different genotypes, which are phylogenetically unmatched.
Although there is no effective medicine for EV-A71 treatment at present, EV-A71 inactivated vaccine independently developed in China has been approved to appear on the market in December 2015, which can effectively prevent HFMD caused by EV-A71.Citation12,Citation13 However, it is not known whether the widespread vaccination of EV-A71 inactivated vaccine would cause the serotype change of domestic EV-A71 epidemic strains, or the mutation and recombination would lead to the generation of new pathogenic strains. When humans are infected with EV-A71, protective neutralizing antibodies will be generated. The long-term existence of antibodies in the body can effectively protect the body from re-infection,Citation14 and neutralizing antibodies can effectively evaluate the immune effect of vaccines. In the long-term existence of EV-A71, mutations in amino acid sites may occur, whether there is a change in the protective effect of neutralizing antibodies produced by vaccination is unknown.
Therefore, the difference of amino acids between the three EV-A71 epidemic strains and the “vaccine strain” was analyzed, and the effects of serum neutralizing antibodies in healthy people were monitored as well. It is of great significance for prevention and treatment of EV-A71 epidemic, which plays a certain guiding role for EV-A71 vaccine vaccination.
Methods
Serum samples
In this study, the serum samples were collected from 200 healthy human in 2016, including 80 males and 120 females, categorized into five age groups: 15 for aged 10–19, 60 for aged 20–29, 51 for aged 30–39, 45 for aged 40–49, 29 for aged 50–59. The number of males and females in each group and the average age of each group are shown in . The study was approved by the ethics committee of the Sixth Medical Center of PLA General Hospital, Beijing, China ().
Table 1. Demographic characteristics of the study participants.
EV-A71 cells and viruses
The RD cells (human embryo rhabdomyosarcoma) were cultured in DMEM containing 4.5 g/L glucose, L-glutamine, and sodium pyruvate (Corning, 10-013-CVR, USA), supplemented with 10% FBS (Corning, 35-076-CV, USA), 100 IU of penicillin, and 100 μg of streptomycin per ml. The cells were incubated at 37°C with 5% CO2. The “vaccine strain”,the three epidemic strains and BrCr that harvested from RD were cultured by freezing and thawing three times and were stored at −80°C. The titers of the virus stocks were tested using a modified plaque-forming assay and determining the CCID50.
The BrCr strain was derived from the American 1969 isolate, “vaccine strain” was a clinical isolate from China in 2008, 10 C4 strain (2010 years), 13 C4 strains (2013 years) and 16 C4 strains (2016 years) were all isolated from Chinese clinical isolates of corresponding years, all above are provided by the Centers for Disease Control (CDC).
Neutralization assays
The neutralizing potency of the EV-A71 antibody was manipulated by following standard protocols for microplate neutralization assays with some modifications.Citation15 Initially, the serum sample was diluted to 1:4, inactivated at 56°C for 30 minutes, and stored in a refrigerator at 4°C overnight. The serum was diluted from 1:4 to 1:1028 at the beginning of the experiment, and 50 μL of virus strain (CCID50) was mixed and incubated at 37°C for 2 hours. Finally, the RD cell suspension (2 x 105 cells/mL) was added to the mixture. The plates were then placed in a 36°C CO2 incubator for 7 days and the potential cytopathic effect was determined under the microscope. Virus back titrations were established for each experiment. This test is considered valid if the virus back titration shows 32–320 TCID50/well. The lowest dilution observed in >50% of the cytopathic effect was considered to be the anti-titer of the serum sample, and a titer >1:8 was considered an antibody positive cutoff.Citation16–18
Data availability
Thirty-three VP1 sequences of enterovirus types determined in this study have been deposited in the GenBank database under the accession numbers BrCr, FY23, FY7VP5, H07. The nearly VP1 genome sequences of EV-A71 strains associated with “vaccine strains”, 10 C4 (GS 2010–13 T), 13 C4 (GS 2013–009) and 16 C4 (GS 2016–001).
Statistical analysis
Chi-square test and t-test were applied for statistical analysis. The antibody titer of >1:8 was considered to be positive. The geometric mean titers (GMT) of antibody titers and 95% confidence intervals (CI) were calculated. Paired t-tests were performed for comparing cross-reactive neutralizing antibodies (NtAb) titers because we measured the serum neutralizing antibody titers against the five EV-A71 viruses for each people. Statistical analyses were performed using SPSS, version 20.0 (SPSS Inc., Chicago, IL, USA). P-values less than 0.05 were regarded as statistically significant.
Results
Phylogenetic tree analysis and difference of amino acid between five strains
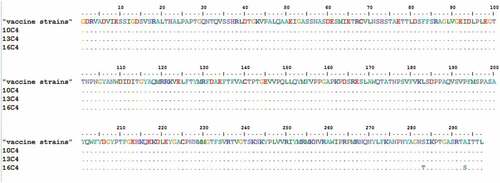
Representative strains isolated from China and other countries in 1998–2017, including “vaccine strains,” FY23, FY7VP5, and H07, were clustered in the C4a genotype. The VP1 genome sequences of “vaccine strains” from this study showed the closest genetic relationship to those of FY23, FY7VP5 and H07 from mainland China shared 97.9%–99.2% nucleotide sequence. 99.8%-100.0% amino acid sequence identity with the closest strains. “vaccine strains” had great similarity in VP1, VP2 and VP3 (). The VP1 of “vaccine strains” sequence was identical to amino acid of the three inactivated vaccine strains (FY23, FY7VP5, H07) in China, except four sites of VP1 (). The “vaccine strains” for this research were isolated from the 2008 clinical strain, and were analyzed as the “vaccine strain” in this study.
Figure 1. Neighbor-joining phylogenetic trees for EV-A71 complete VP1 sequences (891 bp). The nucleotide substitution model used was the p-distance model. One thousand bootstrap replicates were used for construction of the phylogenetic trees; values >70% are shown. The scale bar represents a genetic distance of 0.02 nucleotide substitutions per site.The symbol “■” indicates 2008 clinical isolates; “●”indicates 2010, 2013, 2016 Chinese clinical isolates and BrCr-USA-1970 strain;“▲”indicates three strains of vaccine produced in China, the reference sequences are labeled with GenBank accession no./country/year.
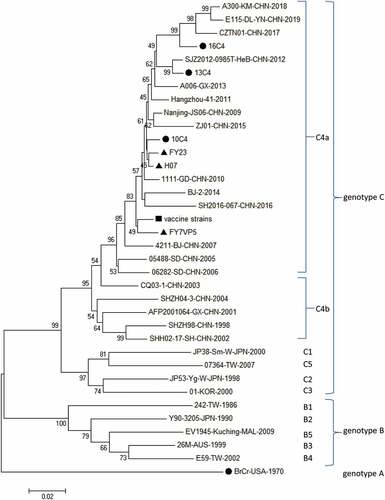
Figure 2. Variations were found in EV-A71 (VP1) of our study. ‘‘·’’ indicates matching to the FY23,FY7VP5,H07.
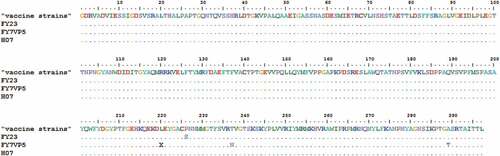
The phylogenetic tree of 33 EV-A71 strains from China and other countries were constructed, including BrCr (BrCr-USA-1970), and belong to the A genotype, which are consistent with previous research findings. The amino acid homology alignment analysis of VP1 of EV-A71 showed that the prototype strain BrCr (type A) were distinctive from other strains, in which 18 amino acid sites were found their positions has changed. The nucleotide homology and amino acid homology of the five strains were within 76.8–97.8% and 93.8–100.0% respectively ().
Figure 3. Variations were found in EV-A71 (VP1) of our study. ‘‘·’’ indicates matching to the BrCr-USA-1970.
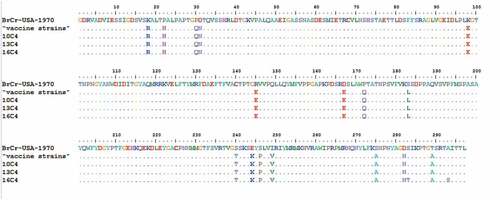
The sequence homology difference was 15.0% and the same branch was found of “vaccine strains,” 10 C4, 13 C4 and 16 C4 in this study. The four strains were clustered in the C4a genotype. The nucleotide homology and amino acid homology of the four strains were within 96.2–97.8% and 99.3–100.0%, respectively. The 297 amino acid homology alignment analysis of EV-A71-VP1 showed that “vaccine strains” and three epidemic strains (10 C4, 13 C4, 16 C4) have two amino acid positions and 54 nucleotide position mutations, and only nucleotide variations of 847 and 877 sites cause amino acid changes, corresponding to 283 and 293 sites, respectively. Still others are meaningless mutations. For the 16 C4 sequences, a substitution of aspartic acid (S) toasparagine (T) at residue 283 (S283T) and a substitution of aspartic acid (A) toasparagine (S) at residue 293 (A293S) were exhibited ().
Gender difference of five EV-A71 strains at the same age
No gender-specific difference was found in male and female neutralizing antibody GMT (p > .05). The GMT of BrCr NtAb was lower than other strains. It shows the feasibility of the experimental method ().
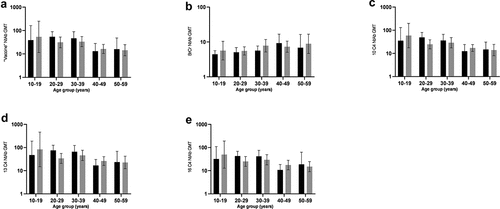
Figure 5. Age-Related GMT values of NtAb against five EV-A71 strains in different sexes in healthy individuals in Beijing, 2016. The y-axis represents the percentage of the NtAb GMT; the x-axis represents different ages. (a) vaccin, (b) BrCr, (c) 10 C4, (d) 13 C4 and (e)16 C4 among seropositive participants. Black, male; gray, female; NtAb, neutralizing antibody; GMT, geometric mean titers.
Seroprevalence and positive GMT values of five EV-A71 strains NtAb in each age group
The seroprevalence of “vaccine strain” NtAb was about 80.0% for all age groups. The highest seroprevalence of “vaccine strain” NtAb was 90.2% for 30–39 years old, The NtAb rates of 40–49 and 50–59 were 73.3% and 75.9%, respectively (). The positive GMT of the “vaccine strain” was at its highest in age group 10–19 (67.5, 95% CI: 28.5–160.0), and the positive GMT reduced gradually with the increase of age. There are significant differences between 10–19 and 20–29 (56.8, 95% CI: 41.0–78.6, p < .001), 30–39 (50.3, 95% CI: 34.9–72.5, p < .001) and 40–49 (24.9, 95% CI: 16.9–36.9, p = .002). The positive GMT was at its lowest in age group 50–59 (22.6, 95% CI: 13.7–37.3). There was no statistical difference between 50 and 59 years old and other age groups (22.6, 95% CI: 13.7–37.3, p > .05) ().
Table 2. Age-dependent seroprevalence and positive GMT values of five EV-A71 strains neutralizing antibodies in healthy individuals in Beijing, 2016.
10 C4, 13 C4, 16 C4 strains, the seroprevalence of three strains NtAb was about 80.0% in all groups, The lowest positive in age group of 10–19 in the 10 C4 (86.7%), 13 C4 (86.7%), 16 C4 (86.7%), but 20–29 in the BrCr (18.3%). Then, it gradually increased, and peaked (88.2%, 96.1%, 88.2%, 42.2%) in 30–39, and decreased slightly (80.0%, 91.1%, 82.2%, 42.2%) in 40–49, and then gradually increased at the age of 50–59 (82.8% and 93.1%) in 10 C4 and 13 C4, but it is still going down in 16 C4 (72.4%) and BrCr (37.9%), GMT of 10 C4, 13 C4, 16 C4 was at its lowest in age group 50–59. Significant differences were detected by pairwise comparison between age groups of 20–29 and 50–59 (10 C4, p = .000; 13 C4, p = .003; 16 C4, p = .000), 30–39 and 50–59 (13 C4, p = .005; 16 C4, p = .001), 40–49 and 50–59 (10 C4, p = .015; 16 C4, p = .015). However, there was statistically significant difference in BrCr between each group except 10–19 and 50–59 ().
Correlation analysis of NtAb GMT between “vaccine strain” and other four strains
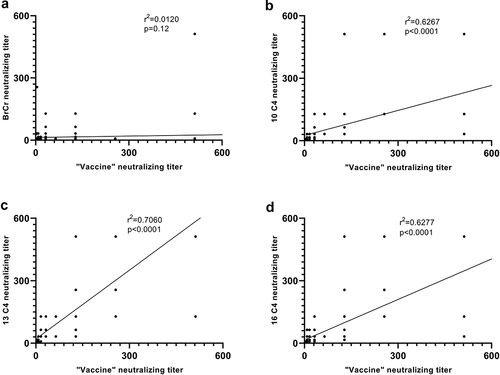
EV-A71 NtAb titers between 10 C4, 13 C4, 16 C4 and “vaccine strain” were strongly and positively correlated (r = 0.6267, p < .0001; r = 0.7060, p < .0001; r = 0.6277, p < .0001). No correlation was found between the titers against the “vaccine strain” and BrCr (r = 0.0120, p = .12) ().
Figure 6. Scatterplot representation of correlation between neutralizing antibody titers (NT50) against “vaccine strains” and four strains. (a) Scatterplot for samples seroneutralization-positive against both “vaccine strains” and BrCr strain. (b) Scatterplot for samples seroneutralization-positive against both “vaccine strains” and 10 C4 strain. (c) Scatterplot for samples seroneutralization-positive against both “vaccine strains” and 13 C4 strain. (d) Scatterplot for samples seroneutralization-positive against both “vaccine strains” and 16 C4 strain.
Titer distribution of NtAb in seropositive individuals and age-dependent immunity to EV-A71 infections
To analyze the immunity level, three NtAb titer ranges were defined: 1:8-1:32 (low), 1:64-1:256 (medium), and 1:512-1:1024 (high). Our analysis showed that the distribution of low and high EV-A71 NtAb titers among the different age groups was inconsistent ().
Figure 7. Age-stratified distribution of NtAb titers against. Antibody titers from 10-19-year, 20-29-year, 30-39-year, 40-49-year and 50-59-year of age are shown. The y-axis represents the percentage of the population with a given antibody concentration; the x-axis represents different ages. (a) vaccin, (b) BrCr, (c) 10 C4, (d) 13 C4 and (e)16 C4 among seropositive participants. NtAb, neutralizing antibody. GMT, geometric mean titers.
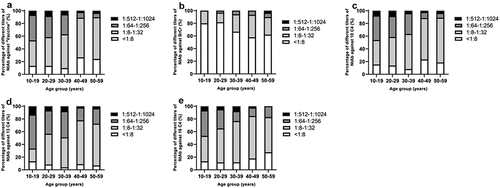
The distributions of NtAb titers in “vaccine strain” are shown in . For the high NtAb titer, the percentages of three groups (aged 30–39 and 40–49 levels) were <5.0%, while those of the remaining age groups were all >5.0%, and the proportion of the titer 1:512-1:1024 among “vaccine strain”-seroprevalence also decreased with age (p < .05).
The distributions of NtAb titers in BrCr are presented in . In the age group of 10–19 none of the NtAb showed titers in the range of 1:64-1:256 and 1:512-1:1024. On the contrary, for the higher NtAb titer, the highest percentage (3.45%) was found in the older age group (50–59). The distributions of NtAb titers in 10 C4, 13 C4 and 16 C4 are illustrated in . The younger children presented higher NtAb titers, which is similar to the trend observed in case of “vaccine strain.” However, the NtAb titers in 10 C4, 13 C4 and 16 C4 were in medium and high ranges, mostly in 1:8-1:32 or 1:64-1:256. Furthermore, in age between 50 and 59, none of the NtAb titer was of 1:512-1:1024 (p < .05) in 16 C4.
Discussion
The EV-A71 genotype C4 first appeared in 1998 and caused the latest large-scale outbreak in China in 2008. It is estimated that the incidence of HFMD in China is 1–2 per 1,000 people, and the number of deaths reported per year is 350–900, mainly young children. Since then, China has required clinicians and hospitals to report clinical cases of HFMD to the National Legal Infectious Disease Surveillance System (NNIDSS) within 24 hours of diagnosis.Citation19 From 2008 to 2018, China CDC registered a total of 20,537,199 cases of HFMD. In 2018, the incidence of hand, foot and mouth disease in China occurred 169.4129/100,000, and the mortality rate was 0.0025/100,000. Considering the changing trend of HFMD epidemics, we suggest that vaccine strategies for hand, foot and mouth disease should be closely monitored.
In December 2015, China approved three new inactivated EV-A71 vaccine (FY23, FY7VP5, H07) for HFMD,Citation20 vaccine measures starting in 2016 have reduced the total number of patients in 2016 and 2017 by 17.0% and 22.0%, respectively.Citation21 It has been reported that the immunization induced by the inactivated EV-A71 vaccine has good persistence within 5 years after the initial vaccination.Citation22 Furthermore, a phase III clinical trial of inactivated diploid enterovirus has been reported to demonstrate satisfactory safety.Citation23 However, between 1986 and 2008, the full-length genomic sequence of Taiwan’s popular EV-A71 was internally and recombined, and dominant genotype changes from B to C or C to B occurred at least three times.Citation24 Subtypes of EV-A71 subtypes were observed over time, but most people did not know their appearance and extinction at the exact time.Citation25 Therefore, continuous monitoring of the EV-A71 gene is recommended, including monitoring genetic evolution and antigenic changes, monitoring serum neutralizing antibodies, and contributing to the development of the EV-A71 vaccine.
China, however, has seen persistent predominance of subgenogroup C4 over the past 15 years, and represents more exception than the rule. The introduction of three monovalent C4 vaccines in China may however drive strain replacement and lead to a shift in EV-A71 genogroup or subgenogroup dominance, as seen with other infectious diseases.Citation26 At present, based on the sequence analysis of the VP1 gene, people have a deep understanding of the evolution and molecular epidemiology of enterovirus circulating strains. Studies have shown that in the Asia-Pacific countries, there has been a shift between enterovirus EV-A71 genotypes B and C.Citation27,Citation28 Phylogenetic tree analysis shows that the prototype strain BrCr (genotype A) is the most different strain from others. The “vaccine strain” and the three epidemic strains belong to the C4a genotype, and the “vaccine strain” has a certain homology with the inactivated vaccine strain in mainland China. Although the identified EV-A71 sequences belonged to a single subgenogroup across our study period, novel amino acid changes are also detected when compared to the sequences reported in previous molecular epidemiological studies. Additionally, we identify amino acid residue variations associated with major neutralization/antigenic epitopes. In this study, site variation, nucleotide variations at positions 847 and 877 caused amino acid changes (corresponding to amino acids 283 and 293, respectively) and other nonsense mutations. In addition, polymorphic loci at S283T and A293S in VP1 region have been previously reported.Citation29,Citation30 It has been reported that the VP1 mutation is a major determinant of the immunogenicity of EV-A71, and that single amino acid variation in VP1 can lead to the breadth and efficacy of immune responses against major EV-A71 isolates, as well as the sensitivities of EV-A71 to heterologous neutralization difference.Citation31 In our research, the NtAb titer ranges of 16 C4 was significantly lower than 13 C4 and BrCr [GMT (95% CI): p = .003 vs. p = .000]. In particular, the EV-A71 vaccine had a high mutation rate and frequent recombination, so it was important that we cannot use the same vaccine candidate invariably.Citation32
The GMT range of neutralizing antibody BrCr increased with the increase of age, but “vaccine strain,” contrary to the three strains, was on a downward course. In general, there was no significant difference between male and female among the five strains (p > .05). There was no difference between male and female, which indicated that the neutralization method was accurate to detect neutralizing antibody in serum. The results further highlight that inclusion of EV-A71 in the HFMD vaccine was a suitable strategy in vaccine development.
At present, the main method of serological epidemiology research is microneutralization experiment, which is a cross-sectional study of serum antibody positive rate and neutralization antibody titer of different age groups, as well as a retrospective or prospective study of multi-time sampling. According to the change of serum antibody positive rate and neutralizing antibody titer, epidemic characteristics of disease and population immune status can be scientifically evaluated. The positive rate of BrCr serum was less than 50.0%, because BrCr was isolated in 1970, and the infection in the current population was limited. The seropositive rate was high for those of 50–59 years old, and no significant difference was found among all age groups. The high proportion of samples positive for BrCr in the age group of 50–59 could be explained by two hypotheses. This age group was most probably naturally infected during their childhood and most likely with genotypes A, C, which were circulating during the 1950s to 1970s.Citation33 The immunity raised by natural infection during childhood of the age group of 50–59 could provide better cross-neutralization against different genotypes than that induced by vaccination, as seen for rotavirus and mumps virus.Citation34
But “vaccine strain” was different. Fewer individuals were found to be positive against the “vaccine strains” in the 40–49 and 50–59 age groups than other younger age groups (20–29 and 30–39), and there was a significant difference (p < .05). Studies have shown that the more variable P1 region, coding for the viral capsid proteins was believed to undergo mutations more frequently, particularly at the VP1 region, as interactions with antibodies and host cell receptors require the virus to evolve quickly in order to evade the host immune system, while maintaining its ability to bind to its host receptors.Citation35 Mutations occur at high rates in RNA viruses due to the lack of proofreading activity in the RdRp, which leads to approximately 1 × 10−4 substitutions per nucleotide copied.Citation36 Such high mutation rate allows EV-A71 to adapt rapidly to selection pressures, which select for beneficial mutations.Citation37 It can be inferred that the epitope of the C4 strain functional protein has changed in 16 years,Citation38,Citation39 and the epitope on the surface protein may make the immune response anti-infective. The type has a certain degree of cross-neutralization.Citation40 It is concluded that changes in important functional epitopes of this strain caused changes in the titer of neutralizing antibodies, and epitopes on this surface protein may reduce the immune response against infection.Citation41
In accordance with the divergent trend of the major HFMD serotype, the overall EV-A71 seropositive rate exhibited a fluctuating downward trend, which also prompted the higher risk of EV-A71 infection over the next several years. High seroprevalence and percentages of high-level EV-A71 NtAbs confirmed a documented EV-A71 “silent” epidemic in the 2008 post-HFMD epidemic period. Our study revealed that older than younger had higher NtAb seroprevalences against BrCr and “vaccine strain.” For BrCr, seropositivity rates and NtAb titer ranges increased with age; however, these rates and titer ranges were generally low. This finding indicates that very few people were exposed to BrCr. For “vaccine strain,” the seropositivity rates and higher NtAb titer ranges showed a decreasing trend with age, indicating that younger people are being continuously exposed to EV-A71 over time, and they often experience repetitive infections. Younger people may have increased chance of having been exposed. This serological finding suggests that EV-A71 is the major pathogen of HFMD and that its inclusion in vaccines is suitable. For “vaccine strain,” in comparison to other serotypes, higher seropositivity rates and higher NtAb titer ranges were observed in the age group of 50–59. Although EV-A71 causes limited damage in adults, the high level of titer also suggests that these individuals were infected with the virus at a young age, making early vaccination meaningful. In the 16 C4 group, there was no higher NtAb titer in 50–59 group. Thus, it is necessary to supervise the antigenic evolution of EV-A71 and update the vaccine in time like influenza viruses.Citation42–47 Although the relationship between antibody titers and protection against reinfection remains unknown, high NtAb levels are always considered to indicate recent infection. In our study, the highest percentage of high level EV-A71 NtAbs was in the group of 10–19 and 20–29. As such, monitoring surveillance of changes within the VP1 region of EVs may allow for the early identification of epidemiological and clinical changes, as well as the identification of potential novel vaccine targets.Citation48
Limitations
In this study, there is an absence of serum in 0–10-year-old children, and therefore studies on EV-A71 vaccine have certain limitations. The five strains were clustered in C4a Genotype, although it had an impact on evaluating the effectiveness of vaccine, the neutralizing antibody titer of C4 genotype could also be evaluated.
Conclusion
In conclusion, the VP1 protein plays a major role in the infectivity, replication and virulence of EVs and is the primary target for neutralizing antibodies. Our study revealed that with the passage of time, the amino acid of EV-A71 differed, and this difference was observed in the neutralization experiment of serum in 2016. In summary, if we use the vaccine produced by the isolated strains in 2008, the protective effect of the current vaccine has an impact.
Authors’ contributions
All authors contributed to the study conception and design. Material preparation, manuscript finalization and data analysis were performed by Yiwei Ding, Project supervision was performed by Zhihai Han. All authors read and approved the final manuscript.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Sixth Medical Center of PLA General Hospital, Beijing [No. 201,907,100].
Acknowledgements
We would like to thank all study subjects and their parents/guardians/relatives who agreed to participate in this study. The language of this manuscript has been checked by a native speaker of English.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data Availability statement
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ishimaru Y, Nakano S, Yamaoka K, Takami S. Outbreaks of hand, foot, and mouth disease by enterovirus 71. High incidence of complication disorders of central nervous system[J]. Arch Dis Child. 1980;55:1–10.
- Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71[J]. Lancet Infect Dis. 2010;10:778–790.
- Chan LG, Parashar UD, Lye MS, Ong FGL, Zaki SR, Alexander JP, Ho KK, Han LL, Pallansch MA, Suleiman AB, et al. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. For the outbreak study group[J]. Clin Infect Dis. 2000;31(3):678–683.
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance[J]. FEMS Microbiol Rev. 2002;26:91–107.
- Qiu J. Enterovirus 71 infection: a new threat to global public health?[J]. Lancet Neurol. 2008;7:868–869.
- Xu J, Qian Y, Wang S, Serrano JMG, Li W, Huang Z, Lu S. EV71: an emerging infectious disease vaccine target in the far East?[J]. Vaccine. 2010;28:3516–3521.
- Van Tu P, Thao N, Perera D, Truong KH, Tien NTK, Thuong TC, How OM, Cardosa MJ, McMinn PC. Epidemiologic and virologic investigation of hand, foot, and mouth disease, Southern Vietnam, 2005[J]. Emerg Infect Dis. 2007;13:1733–1741.
- Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al. An inactivated enterovirus 71 vaccine in healthy children[J]. N Engl J Med. 2014;370(9):829–837.
- van der Sanden S, Koopmans M, Uslu G, van der Avoort H. Epidemiology of enterovirus 71 in the Netherlands, 1963 to 2008[J]. J Clin Microbiol. 2009;47:2826–2833.
- Chang P, Chen S, Chen K, The Current Status of the Disease Caused by Enterovirus 71 Infections: Epidemiology, Pathogenesis, Molecular Epidemiology, and Vaccine Development[J]. IJERPH.2016;13(9):890.
- Zhang Y, Zhu Z, Yang W, Ren J, Tan X, Wang Y, Mao N, Xu S, Zhu S, Cui A, et al. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China[J]. Virol J. 2010;7(1):94.
- Li JX, Mao QY, Liang ZL, Ji H, Zhu F-C. Development of enterovirus 71 vaccines: from the lab bench to phase III clinical trials[J]. Expert Rev Vaccines. 2014;13:609–618.
- Mao QY, Wang Y, Bian L, Xu M, Liang Z. EV71 vaccine, a new tool to control outbreaks of Hand, Foot and Mouth Disease (HFMD)[J]. Expert Rev Vaccines. 2016;15:599–606.
- Yamazaki K, Okuno Y. Genetic diagnosis and molecular epidemiological analyses of hand, foot and mouth disease which prevailed in osaka prefecture in 2000][J]. Kansenshogaku Zasshi. 2001;75:909–915.
- Ooi EE, Phoon MC, Ishak B, Chan S-H. Seroepidemiology of human enterovirus 71, Singapore[J]. Emerg Infect Dis. 2002;8:995–997.
- Li W, Yi L, Su J, Lu J, Ke C, Zeng H, Guan D, Ma C, Zhang W, Xiao H, et al. Seroprevalence of human enterovirus 71 and coxsackievirus A16 in Guangdong, China, in pre- and post-2010 HFMD epidemic period[J]. PLoS One. 2013;8(12):e80515.
- Zeng M, El KN, Tu S, Ren P, Xu S, Zhu Q, Mo X, Pu D, Wang X, Altmeyer R, et al. Seroepidemiology of enterovirus 71 infection prior to the 2011 season in children in Shanghai[J]. J Clin Virol. 2012;53(4):285–289.
- Zhu Z, Zhu S, Guo X, Wang J, Wang D, Yan D, Tan X, Tang L, Zhu H, Yang Z, et al. Retrospective seroepidemiology indicated that human enterovirus 71 and coxsackievirus A16 circulated wildly in central and southern China before large-scale outbreaks from 2008[J]. Virol J. 2010;7(1):300.
- Xing W, Liao Q, Viboud C, Zhang J, Sun J, Wu JT, Chang Z, Liu F, Fang VJ, Zheng Y, et al. Hand, foot, and mouth disease in China, 2008-12: an epidemiological study[J]. Lancet Infect Dis. 2014;14(4):308–318.
- Li T, Wang H, Lu Y, Li Q, Chen C, Wang D, Li M, Li Y, Lu J, Chen Z, et al. Willingness and influential factors of parents to vaccinate their children with novel inactivated enterovirus 71 vaccines in Guangzhou, China[J]. Vaccine. 2018;36(26):3772–3778.
- Huang SW, Cheng HL, Hsieh HY, Chang C-L, Tsai H-P, Kuo P-H, Wang S-M, Liu C-C, Su I-J, Wang J-R, et al. Mutations in the non-structural protein region contribute to intra-genotypic evolution of enterovirus 71[J]. J Biomed Sci. 2014;21(1):33.
- Hu Y, Zeng G, Chu K, Zhang J, Han W, Zhang Y, Li J, Zhu F. Five-year immunity persistence following immunization with inactivated enterovirus 71 type (EV71) vaccine in healthy children: a further observation[J]. Hum Vaccin Immunother. 2018;14(6):1517–1523.
- Zhang W, Kong Y, Jiang Z, Li C, Wang L, Xia J. Comprehensive safety assessment of a human inactivated diploid enterovirus 71 vaccine based on a phase III clinical trial[J]. Hum Vaccin Immunother. 2016;12(4):922–930.
- Huang SW, Hsu YW, Smith DJ, Kiang D, Tsai HP, Lin KH, Wang SM, Liu CC, Su IJ, Wang JR. Reemergence of enterovirus 71 in 2008 in Taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008[J]. J Clin Microbiol. 2009;47(11):3653–3662.
- Noisumdaeng P, Sangsiriwut K, Prasertsopon J, Klinmalai C, Payungporn S, Mungaomklang A, Chokephaibulkit K, Buathong R, Thitithanyanont A, Puthavathana P. Complete genome analysis demonstrates multiple introductions of enterovirus 71 and coxsackievirus A16 recombinant strains into Thailand during the past decade[J]. Emerg Microbes Infect. 2018;7(1):214.
- Martcheva M, Bolker BM, Holt RD. Vaccine-induced pathogen strain replacement: what are the mechanisms?[J]. J R Soc Interface. 2008;5:3–13.
- Ooi MH, Wong SC, Podin Y, Akin W, Del Sel S, Mohan A, Chieng CH, Perera D, Clear D, Wong D, et al. Human enterovirus 71 disease in Sarawak, Malaysia: a prospective clinical, virological, and molecular epidemiological study[J]. Clin Infect Dis. 2007;44(5):646–656.
- Premanand B, Kiener TK, Meng T, Tan YR, Jia Q, Chow VT, Kwang J. Induction of protective immune responses against EV71 in mice by baculovirus encoding a novel expression cassette for capsid protein VP1[J]. Antiviral Res. 2012;95(3):311–315.
- Le TV, Nguyen VT, Nguyen QH, Pham DT. Molecular epidemiology analysis of enterovirus 71 strains isolated in Dak Lak, Vietnam, 2011-2016[J]. J Med Virol. 2019;91(1):56–64.
- van der Sanden S, van der Avoort H, Lemey P, Uslu G, Koopmans M. Evolutionary trajectory of the VP1 gene of human enterovirus 71 genogroup B and C viruses[J]. J Gen Virol. 2010;91(Pt 8):1949–1958.
- Chang J, Li J, Wei W, Liu X, Liu G, Yang J, Zhang W, Yu XF. Determinants of EV71 immunogenicity and protection against lethal challenge in a mouse model[J]. Immunol Res. 2015;62(3):306–315.
- Li L, He Y, Yang H, Zhu J, Xu X, Dong J, Zhu Y, Jin Q. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, people’s republic of China[J]. J Clin Microbiol. 2005;43(8):3835–3839.
- Hanna-Wakim R, Yasukawa LL, Sung P, Arvin AM, Gans HA. Immune responses to mumps vaccine in adults who were vaccinated in childhood[J]. J Infect Dis. 2008;197(12):1669–1675.
- Desselberger U, Huppertz HI. Immune responses to rotavirus infection and vaccination and associated correlates of protection[J]. J Infect Dis. 2011;203:188–195.
- Yip CC, Lau SK, Zhou B, Zhang MX, Tsoi HW, Chan KH, Chen XC, Woo PC, Yuen KY. Emergence of enterovirus 71 “double-recombinant” strains belonging to a novel genotype D originating from southern China: first evidence for combination of intratypic and intertypic recombination events in EV71[J]. Arch Virol. 2010;155(9):1413–1424.
- Domingo E, Wain-Hobson S. The 30th anniversary of quasispecies. Meeting on ‘Quasispecies: past, present and future’[J]. EMBO Rep. 2009;10:444–448.
- Domingo E, Martin V, Perales C, Escarmis C. Coxsackieviruses and quasispecies theory: evolution of enteroviruses[J]. Curr Top Microbiol Immunol. 2008;323:3–32.
- Han X, Ying XL, Zhou SL, Han T, Huang H, Jin Q, Yang F, Sun QY, Sun XX. Characterization of the enterovirus 71 P1 polyprotein expressed in pichia pastor as a candidate vaccine[J]. Hum Vaccin Immunother. 2014;10(8):2220–2226.
- Chen Y, Li C, He D, Cheng T, Ge S, Shih JW, Zhao Q, Chen PJ, Zhang J, Xia N. Antigenic analysis of divergent genotypes human enterovirus 71 viruses by a panel of neutralizing monoclonal antibodies: current genotyping of EV71 does not reflect their antigenicity[J]. Vaccine. 2013;31(2):425–430.
- Liu L, Mo Z, Liang Z, Zhang Y, Li R, Ong KC, Wong KT, Yang E, Che Y, Wang J, et al. Immunity and clinical efficacy of an inactivated enterovirus 71 vaccine in healthy Chinese children: a report of further observations[J]. BMC Med. 2015;13:226.
- Huang KY, Lin JJ, Chiu CH, Yang S, Tsao KC, Huang YC, Lin TY. A potent virus-specific antibody-secreting cell response to acute enterovirus 71 infection in children[J]. J Infect Dis. 2015;212(5):808–817.
- Lei D, Griffiths E, Martin J. WHO working group meeting to develop WHO recommendations to assure the quality, safety and efficacy of enterovirus 71 vaccines[J]. Vaccine. 2020;38:4917–4923.
- Anderson CS, McCall PR, Stern HA, Yang H, Topham DJ. Antigenic cartography of H1N1 influenza viruses using sequence-based antigenic distance calculation[J]. BMC Bioinform. 2018;19(1):51.
- Du X, Dong L, Lan Y, Peng Y, Wu A, Zhang Y, Huang W, Wang D, Wang M, Guo Y, et al. Mapping of H3N2 influenza antigenic evolution in China reveals a strategy for vaccine strain recommendation[J]. Nat Commun. 2012;3:709.
- Lee MS, Chen JS. Predicting antigenic variants of influenza A/H3N2 viruses[J]. Emerg Infect Dis. 2004;10:1385–1390.
- Lu C, Cai Z, Zou Y, Zhang Z, Chen W, Deng L, Du X, Wu A, Yang L, Wang D, et al. FluPhenotype-a one-stop platform for early warnings of the influenza a virus[J]. Bioinf. 2020;36(10):3251–3253.
- Luksza M, Lassig M. A predictive fitness model for influenza[J]. Nature. 2014;507:57–61.
- Zhou Y, Van Tan L, Luo K, Liao Q, Wang L, Qiu Q, Zou G, Liu P, Anh NT, Hong NT, et al. Genetic variation of multiple serotypes of enteroviruses associated with hand, foot and mouth disease in Southern China[J]. Virol Sin. 2021;36(1):61–74.