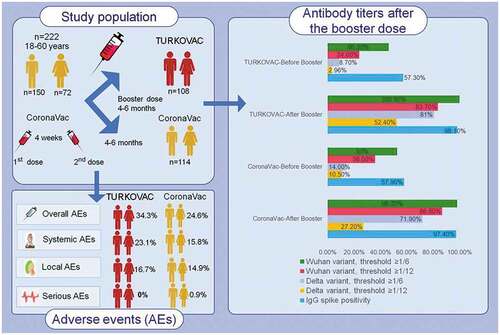
ABSTRACT
Protective neutralizing antibody titers reduce in time after COVID-19 vaccinations, as in individuals who have had COVID-19. This study aimed to evaluate the safety and immunogenicity of CoronaVac and TURKOVAC vaccines used as a booster dose after CoronaVac primary vaccination. This double-blind, randomized, controlled, phase II, multicenter study included healthy male and female adults (18–60 years) who were vaccinated with two doses of CoronaVac vaccine and did not exceed the duration of at least 90 days and a maximum of 270 days from the second dose of vaccination. Among 236 eligible volunteers, 222 were recruited for randomization between July 12, 2021 and September 10, 2021; 108 and 114 were randomized to the TURKOVAC and CoronaVac arms, respectively. The primary endpoint was adverse events (AEs) (ClinicalTrials.gov; Identifier: NCT04979949). On day 28, at the neutralizing antibody threshold of 1/6, the positivity rate reached 100% from 46.2% to 98.2% from 52.6% in the TURKOVAC and CoronaVac arms, respectively, against the Wuhan variant and the positivity rate reached 80.6% from 8.7% in the TURKOVAC arm vs. 71.9% from 14.0% in the CoronaVac arm against the Delta variant. IgG spike antibody positivity rate increased from 57.3% to 98.1% and from 57.9% to 97.4% in the TURKOVAC and CoronaVac arms, respectively. The TURKOVAC and CoronaVac arms were comparable regarding the frequency of overall AEs. Both vaccines administered as booster yielded higher antibody titers with acceptable safety profiles.
Plain Language Summary
What is the context?
The timing of the primary and booster doses for each vaccine differs.
We aimed to evaluate the safety and immunogenicity of CoronaVac and TURKOVAC vaccines used as homologous booster dose after CoronaVac primary vaccination.
What is new?
The neutralizing antibody titers against the Wuhan variant decreased below 1/6- the seropositivity threshold value- in more than 55% of the participants 4 months after administration of two doses of CoronaVac vaccine.
Immunogenicity was re-stimulated and the neutralizing antibody titers increased rapidly and markedly with the administration of the CoronaVac or TURKOVAC as a booster dose 4 months after the second dose.
While the increase in neutralizing antibodies against the Wuhan variant was similar with both CoronaVac and TURKOVAC, more antibodies developed against the Delta variant with TURKOVAC.
What is the impact?
With the Hybrid COV-RAPEL TR study, after the primary vaccination consisting of two doses of inactivated vaccine, antibody titers decreased in the long term; however, higher antibody titers are achieved than the primary vaccination after the booster dose administered after 4–6 month interval.
Booster application with TURKOVAC provides antibodies at least as much as the CoronaVac booster dose, with an acceptable safety profile.
GRAPHICAL ABSTRACT
Introduction
For fighting against the COVID-19 pandemic, many companies have developed vaccines. So far, inactivated vaccines, mRNA vaccines, and adenovirus vaccines have been used worldwide. Inactivated COVID-19 vaccines have shown good safety profiles and protect against COVID-19.1 In a prospective observational cohort conducted in Chile for investigating the effectiveness of inactivated SARS-CoV-2 vaccine, the adjusted vaccine effectiveness in fully immunized individuals were reported to range from 65.9% (for prevention of COVID-19) to 90.3% (for prevention of intensive care unit admission); the effectiveness was 86.3% for prevention of COVID-19-related death and 87.5% for prevention of hospitalization.Citation1 On the effectiveness of inactivated COVID-19 vaccines for primary vaccination, consistent results were reported from TürkiyeCitation2 and China.Citation3 As of 13 January 2021, Türkiye granted emergency use authorization to the CoronaVac vaccine (Sinovac Life Sciences Company, Beijing, China).Citation4,Citation5 In Türkiye, an inactivated SARS-CoV-2 vaccine -TURKOVAC- was produced by SBT Science and Biotechnologies and manufactured by Kocak Farma. The product is under development; its preclinical studies has been publishedCitation6,Citation7 and phase II studies have been completed. The phase III comparative efficacy and safety trial (NCT04942405) versus CoronaVac is ongoing. In December 2021, it is authorized for emergency use by the Turkish Medicines and Medical Devices Agency.
The protective effects of inactivated vaccines is usually observed 14 days after the second dose.Citation8 However, protective neutralizing antibody titers decrease significantly at 6 months in patients who have had COVID-19; a similar loss of efficacy can be considered likely to be observed after vaccination.Citation9,Citation10 It has been shown that with booster vaccination, higher antibody titers are achieved and protection lasts longer.Citation11,Citation12 Although there are no clear data on the timing, booster doses are recommended four to 6 months after the primary vaccination.
During the pandemic, both inactivated CoronaVac and BNT162B2 mRNA vaccines were used in primary vaccination in Türkiye. Individuals who have completed their two dose vaccination with inactivated vaccines, the application of a booster dose is recommended at the 6th month as of July 2021.
This study aimed to evaluate the safety and immunogenicity of CoronaVac and TURKOVAC vaccines used as homologous booster dose after the second dose of CoronaVac primary vaccination.
Patients and methods
This double-blind, randomized, controlled, phase II study was conducted to determine the safety and immunogenicity of booster doses of CoronaVac and TURKOVAC vaccines randomized at a 1:1 ratio to individuals who had two dose vaccination with CoronaVac in Türkiye (ClinicalTrials.gov, NCT04979949). The study was started on 12 July 2021 in Ankara, Türkiye with a single center and was continued with multi-centers. Healthy male or female adults aged 18–60 years, who were vaccinated with two doses of CoronaVac vaccine and did not exceed the duration of at least 90 days and a maximum of 270 days from the second dose of vaccination, were included (Table S1 in the Supplemental Appendix S1 for other inclusion and exclusion criteria). The clinical trial protocol (Supplemental Material-Study Protocol) and informed consent forms were approved by the Ethics Committee of Ankara City Hospital (No: E2-21-640, Date: 22 June 2021).
Participants were divided into two in a 1:1 ratio. It was planned to randomly recruit at least 111 participants for each vaccine arm. A total of 236 volunteers were screened; 222 volunteers were randomly assigned to receive a single dose of TURKOVAC or CoronaVac vaccine. Age and sex quotas were applied while selecting participants (Table S2 in the Supplemental Appendix S1). Randomization was performed with the Omega Research Randomization and Investigational Product Management System (Omega Interactive Voice Response Systems [IVRS]/Interactive Web Response Systems [IWRS]).
Pre-vaccine PCR testing and blood samples were taken to determine anti-spike antibody levels. Volunteers with positive pre-vaccine PCR test were excluded. Physical examination and vital checks were performed. A single dose of booster vaccine (TURKOVAC or CoronaVac) was injected into the upper arm deltoid muscle (preferably left) of the volunteers. Except the study pharmacist preparing the application product, the study team and volunteers, including the nurse who performed the vaccination, were blinded.
Blood was drawn for immunogenicity analysis at the study visit on day 0 (the day of vaccination or 1 day before the vaccination). Twenty-eight days (±2 days) after the booster, the amount of changes in SARS-CoV-2 neutralizing antibody and anti-spike protein IgG were evaluated in both groups. SARS-CoV-2 neutralizing antibody and anti-spike protein IgG measurements were repeated on day 84 for both vaccine arms. Volunteers were followed up at least to day 168 for side effect assessment. A virus neutralization test technique was used for SARS-CoV-2 neutralizing antibody detection. The neutralizing antibody threshold values against Wuhan and Delta variants were taken as 1/6 and 1/12 for assessments.Citation13
All participants were monitored for post-vaccine adverse events (AEs) and were asked to record any AEs using an electronic diary during the first 14-day follow-up period. Participants were also asked about both expected and undesirable side effects daily for 7 days using IVRS. AEs were recorded as local, systemic, and serious AEs. Undesirable AEs were defined as related or unrelated to study treatment based on criteria of causation, reasonable probability, temporal relationship, and alternative cause. Serious AEs were defined as an adverse reaction resulting in death, life-threatening, requiring or prolonging hospitalization, resulting in persistent or significant disability or reduced capacity. Serious AEs up to day 28 were recorded.
Vaccines used in the study
CoronaVac
COVID-19 Vaccine (Vero Cell) contains SARS-CoV-2 antigen as inactivated active ingredient and is available in pre-filled syringes or vials, dosage is 3 μg/0.5 mL per injection.
TURKOVAC
TURKOVAC was manufactured using the SARS-CoV-2 strain (hCoV-19/Turkiye/ERAGEM-001/2020 strain, GenBank accession number; MT327745.1 and GISAID; EPI_ISL_424366) isolated from a patient’s nasopharyngeal sample in the Kayseri City Training and Research Hospital, Kayseri, Turkiye.Citation14 The virus was cultivated in a Vero cell line for 72–96 h at multiplicities of infection of 0.05. The data about manufacturing of TURKOVAC have been presented in Phase 1/2 study of TURKOVAC (manuscript submitted for publication). In brief, TURKOVAC COVID-19 vaccine containing inactivated SARS-CoV-2 antigen as an active ingredient is in a white suspension. Dosage is 3 µg/0.5 mL per injection. Alum Gel (10% AlOH – InvivoGen, USA) was used as adjuvant. It is formulated from a 2% suspension to a final concentration of 0.05% (0.5 mg/dose). It does not contain any preservatives or stabilizers.
Viral isolation for microneutralization test
The Wuhan and Delta strains were used to detect the neutralizing antibody of vaccinated sera in order to evaluate the neutralization ability of vaccine immunization. Original SARS‐CoV‐2 viruses were isolated from a combined nose and throat swab sample obtained from COVID‐19 patients and amplified in Vero E6 (ATCC CRL‐1586) cells in the Biosafety Level (BSL‐3) laboratory. The virus was named for Wuhan strain as SARS‐CoV‐2/Türkiye/27/2020 and for Delta strain as hCoV-19/Türkiye/HSGM-B18515/2021 and was passaged twice in Vero E6 cells. Stock virus was harvested, divided, and stored at −80°C until use.
Vero E6 cell line were obtained from American Type Culture Collection (ATCC CRL-1586). Vero E6 cells were maintained using Modified Eagle’s medium (MEM Sigma M4655) supplemented with 10% heat‐inactivated fetal bovine serum (FBS) (Cegrogen A0500–3210) and 1% penicillin and streptomycin (Cegrogen P0100–790) in an incubator at 37°C under 5% CO2 and 99% relative humidity.Citation15
The virus name, accession ID, and date of the collection for the Wuhan strain are hCoV-19/Türkiye/HSGM-1192/2020, EPI_ISL_811143, and 2020, respectively. The virus name, accession ID, and date of collection for the Delta strain are hCoV-19/Türkiye/HSGM-B18515/2021, EPI_ISL_2958539, and 2021, respectively.
Titration of virus was performed in 96‐well microtiter plates (Greiner Cellstar, 96 well, F bottom, single packed/ 655,160) on Vero E6 cells at serial log10 dilutions and 10 times for each dilution factor to obtain a 50% tissue culture infectious dose (TCID50, diluted serum inhibiting 50% of infectiousness). Plates were observed for cytopathic effect (CPE) daily for 4 days (96 hours). The endpoint of viral dilution leading to CPE in 50% of the inoculated wells was calculated using the Reed‐Muench method.Citation16
Virus neutralization test technique (Microneutralization test)
Serum samples were studied by microneutralization test (MNT) at the General Directorate of Public Health, National Virology Reference Laboratory. Blood serum samples were inactivated at 56°C for 30 min. In 96‐well microplates, sera were serially diluted two-fold in duplicate starting at 1:2 in MEM (Sigma M4655) supplemented with 2% heat‐inactivated FBS (Cegrogen A0500–3210) and 1% penicillin and streptomycin (Cegrogen P0100–790). Then, sera mixed with the same volume of 100 TCID50 SARS-CoV-2 (approximately 1:100 diluted virus) propagated as described above and incubated at 37°C for 1 hour for neutralization. This step has been performed with Wuhan and Delta strains concurrently in different cell culture microplates. After incubation, 100 μL Vero E6 cells at a concentration of 2 × 105/mL in Dulbecco’s MEM supplemented with 2% heat-inactivated FBS and 1% penicillin/streptomycin were added to the virus – serum mixture, and plates were incubated for 96 h at 37°C under 5% CO2 and 99% relative humidity. Virus dilution was back titrated by replacing serum with medium in each experiment to determine the virus test dose. The neutralization endpoint titer was determined as the highest serum dilution inhibiting the virus infection in 50% of the inoculated wells.Citation17 The MNT titer ≥4 was considered positive. The test was checked for virus and cell control with a phase contrast cell culture microscope and was evaluated as positive when 100% of SARS-CoV-2-specific CPE was observed in the virus control section. Figure S1 in the Supplemental Appendix S1 shows the main steps of MNT.
While each center performed the SARS-CoV-2 PCR tests, anti-spike protein IgG tests were performed at the Ankara City Hospital Medical Microbiology Clinic Laboratory. SARS-CoV-2 neutralizing antibody tests were conducted in the National Virology Reference Laboratory of the Turkish Ministry of Health, General Directorate of Public Health.
Measurement of anti-spike IgG level
A fully automated two-step sandwich immunoassay using indirect chemiluminescent technology was performed to measure anti-spike IgG levels (Atellica IM sCOVG Assay, Siemens Healthineers, Erlangen, Germany). A direct relationship exists between the amount of SARS-CoV-2 IgG antibody present in the blood sample and the amount of relative light units detected by the system. The analytical assay range of 0.50–150.00 index is reported as nonreactive (<1.00 index) or reactive (≥1.00 index). The cutoff value of the test is ≥1 U/mL (1 index value) and the corresponding reference standard concentration for 1 U/mL is 21.80 BAU/mL.
Outcomes
The primary endpoint was to determine the number, severity, and rate of volunteers experiencing AEs and serious AEs. The secondary endpoint was the immunogenicity response at day 28 of the inactivated vaccine against SARS-CoV-2. This response was calculated on the geometric mean of the titer of SARS-CoV-2 neutralizing antibody and IgG antibody against the spike protein antigen, and seroconversion was evaluated as a 4-fold increase in this titer after vaccination.
Statistical analysis
Sample size calculation was based on the primary analysis of anti-spike protein IgG at day 28 post-vaccination using the following assumptions: 1) the minimum clinical difference to be detected is 1.75 times the difference in geometric mean concentration between the COVID-19 vaccine and the control arm, i.e. 0.243 on a log10 scale; 2) the standard deviation of the geometric mean concentration on the log10 scale is 0.4. Accordingly, the estimated sample size in each arm was 83 participants to achieve a 90% power at 1% significance level. Assuming approximately a drop-out rate of 25% for the primary analysis due to the positivity of baseline anti-nucleocapsid IgG or loss to follow-up, 111 volunteers were included in each study arm.
Data were analyzed using the PASW Statistics for Windows, Version 18.0. (SPSS Inc., Chicago, IL, USA). A p value of <.05 was considered statistically significant. Descriptive statistics were presented as numbers and percentages for categorical variables and mean, standard deviation, and median, and interquartile range (IQR) for numerical variables. Normality of variables was tested using visual (histogram and probability graphs) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk tests). The amount of SARS-CoV-2 neutralizing antibody and SARS-CoV-2 anti-spike protein IgG on days 0 and 28 in both vaccine arms were presented descriptively. The incidence of adverse reactions within 7 days post-vaccination and the incidence of serious AEs up to day 28 post-vaccination were expressed separately in the vaccine groups as descriptive statistics. The incidence of reactions was compared between the two study arms using the chi-square test for overall, systemic, and local AEs.
Results
Among 236 volunteers assessed for eligibility, 222 were recruited (). Accordingly, 108 and 114 were randomized to the TURKOVAC and CoronaVac arms, respectively. The characteristics of the volunteers are presented in .
Figure 1. Study flowchart.
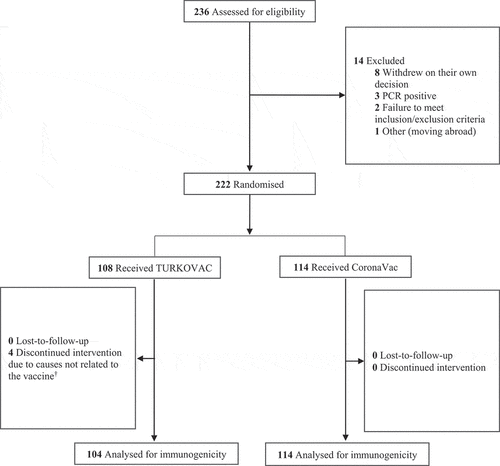
Table 1. Characteristics of the study participants.
The median neutralizing antibody titers were increased by 32 times for the Wuhan variant and by 12 times for the Delta variant in the TURKOVAC arm and by 4 times for the Wuhan variant and by 6 times for the Delta variant in the CoronaVac arm. Taking the neutralizing antibody threshold value against the Wuhan variant as 1/6, the positivity rate reached 100% from 46.2% in the TURKOVAC arm and to 98.2% from 52.6% in the CoronaVac arm on day 28 (). Taking the threshold value as 1/12, the positivity rate reached 83.7% from 24.0% in the TURKOVAC arm and to 86.8% from 36.0% in the CoronaVac arm. In the analysis of the neutralizing antibody response against the Delta variant, taking the threshold value as 1/6, the positivity rate increased to 80.6% from 8.7% in the TURKOVAC arm and to 71.9% from 14.0% in the CoronaVac arm on day 28 (). Taking the threshold value as 1/12, the positivity rate increased to 52.4% from 2.9% in the TURKOVAC arm and to 27.2% from 10.5% in the CoronaVac arm.
Figure 2. Neutralizing antibody positivity against the (a) Wuhan and (b) Delta variants, and (c) immunoglobulin G-Spike (IgG-S) positivity before and after booster doses in the two study arms.
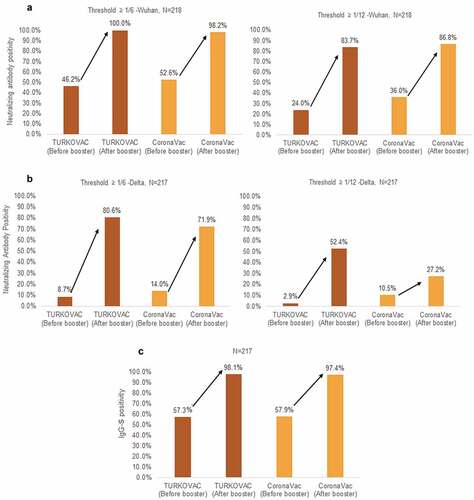
Evaluation of the IgG spike antibody positivity rate revealed that the rate increased from 57.3% to 98.1% in the TURKOVAC arm and from 57.9% to 97.4% in the CoronaVac arm (). The median spike specific (IgG-S) antibody level was 1.30 U/mL (0.1–150) before the booster dose and 26.69 U/mL (0.5–150 U/mL) after the booster dose in the TURKOVAC arm on day 28 (). The median IgG-S level was 1.30 U/mL (0.31–150 U/mL) before the booster dose and 12.38 U/mL (0.71–150 U/mL) after the booster dose in the CoronaVac arm on day 28 (). The IgG-S levels in BAU/mL for before and after booster doses in the TURKOVAC and CoronaVac arm are presented in the Supplemental Appendix 2 (Table S1, Figure S1 and Figure S2). The proportions of volunteers in whom a four times increase was observed in the IgG-S antibody levels on day 28 were 82.5% after the TURKOVAC booster dose and 64.9% after the CoronaVac booster dose.
Table 2. Change in spike-specific immunoglobulin G antibody levels before and after the booster doses.
The neutralizing antibody geometric mean titer (GMT) results are summarized in (95% confidence interval [CI]). For Wuhan variant, the GMT was 13.98 (10.77–18.13) before the booster dose and 33.97 (27.77–41.54) after the booster dose in the TURKOVAC arm; it was 16.58 (13.12–20.95) before the booster dose and 27.92 (23.37–33.35) after the booster dose in the CoronaVac arm. For Delta variant, the GMT was 12 (6.51–22.14) before the booster dose and 14.36 (11.83–17.43) after the booster dose in the TURKOVAC arm; it was 12 (9.09–15.84) before the booster dose and 10.49 (8.97–12.28) after the booster dose in the CoronaVac arm.
Table 3. Neutralizing antibody geometric mean titer results.
When the antibody responses were evaluated for sex, antibody formation against the Wuhan and Delta variants at 1/6 and 1/12 threshold values, and IgG-S antibody formation were close to each other in the female and male volunteers (Figures S3–S5 in the Supplemental Appendix S2).
When the volunteers were grouped for the age ranges of 18–29, 30–39, 40–49, and 50–60 years, the 28th day neutralizing antibody levels against the Wuhan variant and the frequency of occurrence of IgG-S antibody response were similar in all age groups in both groups (Figures S6 and S8 in the Supplemental Appendix S2). Neutralizing antibody positivity to the Delta variant was higher in the TURKOVAC arm in all age groups (Figure S7 in the Supplemental Appendix S2).
Figure S9–S13 in the Supplemental Appendix S2 show neutralizing antibody positivity against the Wuhan and Delta variants and IgG-S antibody responses in case the volunteers were grouped for the interval between the second dose of the primary vaccination and the booster-dose administration, as less than or more than 120 days, and less than or more than 180 days.
The frequencies of developing an antibody response to the Wuhan variant and IgG-S antibodies on the day 28 were close to each other in the TURKOVAC and CoronaVac arms, while the percentage of volunteers who developed an antibody response to the Delta variant was higher in the TURKOVAC arm than the CoronaVac arm, particularly for the threshold value of 1/12.
and Figures S14–S16 in the Supplemental Appendix S2 show frequency of AEs observed after TURKOVAC and CoronaVac booster vaccines and Table S2 in the supplemental Appendix S2 shows frequency of AEs according to sex. In terms of the frequency of overall AEs, there is no difference between the TURKOVAC and CoronaVac booster vaccine groups (37 [34.3%] vs. 28 [24.6%], p = .1124). In addition, when local and systemic AEs were examined separately, no difference was observed between the TURKOVAC and CoronaVac booster vaccine groups (18 [16.7%] vs. 17 [14.9%], p = .8616; 25 [23.1%] vs. 18 [15.8%], p = .1655). Regarding serious AEs, while no serious AEs were observed in the TURKOVAC arm, 1 (0.9%) subject in the CoronaVac arm experienced post-vaccine hospitalization due to COVID-19.
Table 4. Frequency of adverse events in the two study arms.
Discussion
The results of the study revealed that the neutralizing antibody titers against the Wuhan variant decreased below the seropositivity threshold value of one-sixth in more than 55% of the volunteers 4 months after administration of two doses of CoronaVac vaccine. However, with the administration of the third dose inactive vaccines (CoronaVac or TURKOVAC as a booster dose), immunogenicity was re-stimulated and the neutralizing antibody titers increased rapidly and markedly. While the CoronaVac and TURKOVAC arms did not differ regarding the increase in neutralizing antibodies against the Wuhan variant, more antibodies developed against the Delta variant in the TURKOVAC arm.
Administration of inactivated vaccines as a booster dose has demonstrated an acceptable and manageable reactogenicity profile, with no significant safety concerns.Citation18 Memory T cells play a critical role in viral infections. When antigen-specific memory T cells encounter the same antigen again, they proliferate rapidly, both exerting a cytotoxic effect against infected cells and stimulating the activation of antigen-specific B cell clones and thus the formation of neutralizing antibodies.Citation19 Accordingly, booster-dose applications may increase the induction capacity of the adaptive immune system.Citation20 It was previously reported in a study conducted on Macedonian healthcare workers that seropositive individuals yielded higher antibody levels than those of seronegative individuals after a single dose of BNT162b2Citation21 which may be suggestive for the efficiency of booster doses after primary vaccination and also supports our study regarding the rationale for the administration of booster doses.
The immune response produced particularly by inactivated vaccines may be weaker and short-lived compared to other vaccine groups. Waning of humoral response over time and need for a booster dose are also known for mRNA COVID-19 vaccines.Citation22 Therefore, intermittent booster doses are needed for strengthening and maintaining the protective effects of vaccines.Citation23 In a study of 355 volunteers, positive seroconversion rate of serum neutralizing antibody reached 88.5% 1 month after the second dose of inactivated SARS-CoV-2 vaccine. However, the seropositivity rate decreased to 48.5% in the same volunteers 8 months after the second dose.Citation24 In our study, at a threshold value of one-sixth for the Wuhan variant, following the second dose, the seropositivity rate regressed to 44.7% after 4 months and it was 48.6% after 6 months. After inactivated SARS-CoV-2 vaccination, the positive seroconversion rate was less than 50% 4–6 months after the second dose.
For inactivated vaccines that are considered insufficient against different strains, a booster dose may be necessary to maintain effective protection. In our study, similar responses were obtained for the neutralizing antibody levels produced by the TURKOVAC and CoronaVac vaccines against the Wuhan variant at titer threshold values of 1/6 and 1/12 (100% vs. 98.2%; 83.7% vs. 86.8%, respectively). However, the TURKOVAC arm had higher response rates than the CoronaVac arm for the Delta variant at titer threshold values of 1/6 and 1/12 (80.6% vs. 71.9%; 52.4% vs. 27.2, respectively). This finding can be resulted from the fact that the virus used in TURKOVAC vaccine was isolated in the later periods of the pandemic (March 2020) as compared with the one used in CoronaVac. However, this possibility is highly controversial, and no supportive data are available. The main reason for higher neutralizing antibody levels by TURKOVAC against the Delta variant may be associated with higher level of aluminum hydroxide adjuvant in TURKOVAC than in the CoronaVac.
The inverse correlation between neutralizing antibody titers and risk of reinfection has been demonstrated in more than one phase III study.Citation25 The BNT162b2 booster dose increases the neutralizing antibody levels by an average of 10 times compared to the antibody levels after the second dose.Citation26 In an inactivated vaccine study conducted on 355 volunteers, in 67 volunteers who received a booster dose (third dose) 8 months after the primary two dose vaccinations, the positive conversion rate decreased from 86.6% to 65.7% 8 months after the second dose, and increased to 95.5% after the booster dose.Citation24 In the light of these results, it should be kept in mind that higher antibody titers can be obtained after booster dose compared to primary. Our study was also supportive of the other studies and revealed that the positive conversion rate decreased to 46.2% at the threshold value of one-sixth against the Wuhan Variant, after a median 4.85 months after the second dose, and increased to 100% after the TURKOVAC booster dose. The positive conversion rate decreased to 52.6% for the one-sixth threshold value, after a median 4.5 months from the second dose, and increased to 98.2% after the CoronaVac booster dose.
The capacity of vaccines to create spike-specific antibodies also gains importance. It has been reported that spike-specific IgG antibody levels increased from an average of 710 U/mL to 40,000 U/mL with a single booster dose of BNT162b administered after 15 months in patients who have had COVID-19 infection.Citation27 In a study from Türkiye, in healthcare workers (mean age, 41 ± 10.9 years) who were administered with inactivated SARS-CoV-2 vaccines, the median IgG-S levels, which were found as 547.7 AU/mL (IQR: 756.7) after the second dose, increased to a median of 947.3 AU/mL (IQR: 1405.3) after the third booster dose.Citation28 In our study, the median IgG-S level, which was 1.30 (1.82) after a median 4.85 months after the second dose, increased to a median 26.69 (88.13) after the TURKOVAC booster dose. The median IgG-S level, which was 1.30 (2.38) after a median of 4.5 months after the second dose of CoronaVac vaccine, increased to 12.38 (19.04) after CoronaVac booster vaccination. In a study conducted in Israel, the frequency of infection was 11.3 times lower in volunteers who received a booster dose in addition to primary vaccination with BNT162b2 compared to those who did not [Booster group: 10,603,410 persons at risk—934 cases per day; Non-booster group: 5,193,825 persons at risk-4439 cases per day, 95% CI (10.4–12.3)].Citation29 Similarly, the frequency of severe disease was found to be less in the booster group [Booster group: 6,265,361 people at risk—29 cases per day; Non-booster group: 4,574,439 people at risk-294 cases per day, 95% CI (12.9–29.5)].Citation29
In our study, responses of male and female volunteers to vaccines did not significantly differ regarding variant types (Wuhan and Delta variant), antibody types, and titers. The booster vaccine responses to the Wuhan variant of both TURKOVAC and CoronaVac arms were similar for age groups. However, when the responses to the Delta variant were evaluated, the booster vaccine response was higher in the TURKOVAC arm than in the CoronaVac arm, especially after the age of 40 years. Additionally, this study demonstrated that antibody positivity was relatively higher when the time elapsed after vaccination was short, and the antibody loss was higher when the time elapsed after vaccination was long; however, antibody response with booster doses was similar in both study arms (Figures S9–S11 in the Supplemental Appendix S2).
An increase in the incidence of local and systemic adverse reactions has been reported with the second dose of mRNA vaccines as compared with the first dose.Citation18 In our study, in both TURKOVAC and CoronaVac arms, the most commonly reported adverse event was injection site pain and the most common systemic adverse event was fatigue.
The important limitations of our study include small sample size, inclusion of volunteers aged <60 years, and exclusion of special populations such as young adolescents, children, and pregnant women. Additionally, our study only examined the immune responses of healthy adults. Individuals who may be more susceptible to infection and at higher risk for severe diseases due to underlying additional medical conditions were not studied. Another limitation of the present study can be considered the lack of the results of neutralization assay of the Omicron variant. Finally, the vaccine response was evaluated with neutralizing antibody titers, only 1/6 and 1/12 neutralizing antibody threshold values were used, and no examination method was used to directly show T cell responses., all of which can also be considered limitations of the present study.
Conclusively, vaccination is the most effective, safe, economical, and rational approach to prevent infectious diseases today. In the SARS-CoV-2 pandemic, with the awareness that “no one is safe until everyone is safe,” it is important to sustain protective immunity until the epidemic is completely taken under control.Citation30 Booster doses will be needed.
Author contributions
IA and AK conceptualized and coordinated the study. AO and IA had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. AO, AE, IA, and AK drafted the article. IA and AK revised the manuscript critically for important intellectual content. All authors contributed to the acquisition of data. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
Anonymous participant data that support the findings of this study are available from the corresponding author upon reasonable request.
Role of the funder
The TUSEB provided funding for the present study; approved the final protocol, the manuscript, and gave permission to submit it for publication, but had no role in data collection, analysis, interpretation, or writing of the manuscript.
Supplemental Material
Download Zip (9.8 MB)Acknowledgments
The authors thank the Health Institutes of Türkiye (TUSEB) for funding the study. The authors also thank OMEGA CRO, Ankara, Türkiye for representing TUSEB and for their contribution to the correspondence between the study investigators, the ethics committee, and the Ministry of Health; additionally for performing the monitoring, management of the study sites, storage, and distribution of the consumables, provision of OpenClinica- an open source clinical trial software serving for the purpose of clinical data management and electronic data capture, developing electronic case report forms, the interactive web response system (IWRS), and the interactive voice response system (IVRS) and data management, statistical analyses, and overall project management, and manuscript formatting support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2122503.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Jara A, Undurraga EA, González C, Paredes F, Fontecilla T, Jara G, Pizarro A, Acevedo J, Leo K, Leon F, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385(10):1–10. doi:10.1056/NEJMoa2107715.
- Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22. doi:10.1016/S0140-6736(21)01429-X.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–92. doi:10.1016/S1473-3099(20)30843-4.
- Sharma O, Sultan AA, Ding H, Triggle CR. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11:585354. doi:10.3389/fimmu.2020.585354.
- Genetic Engineering & Biotechnology News. Sinovac biotech – CoronaVac; 2020 May 18. [accessed 2021 Nov 29]. https://www.genengnews.com/covid-19-candidates/covid-19-keeping-an-eye-on/sinovac-biotech/.
- Pavel STI, Yetiskin H, Uygut MA, Aslan AF, Aydın G, İ̇nan Ö, Kaplan B, Ozdarendeli A. Development of an inactivated vaccine against SARS CoV-2. Vaccines (Basel). 2021;9(11):1266. doi:10.3390/vaccines9111266.
- Pavel STI, Yetiskin H, Aydin G, Holyavkin C, Uygut MA, Dursun ZB, Celik İ, Cevik C, Ozdarendeli A. Isolation and characterization of severe acute respiratory syndrome coronavirus 2 in Turkey. PLoS One. 2020;15(9):e0238614. doi:10.1371/journal.pone.0238614.
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, Al Nusair M, Hassany M, Jawad JS, Abdalla J, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35–45. doi:10.1001/jama.2021.8565.
- Anand SP, Prévost J, Nayrac M, Beaudoin-Bussières G, Benlarbi M, Gasser R, Brassard N, Laumaea A, Gong SY, Bourassa C, et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep Med. 2021;2(6):100290. doi:10.1016/j.xcrm.2021.100290.
- Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384(1):80–82. doi:10.1056/NEJMc2032195.
- Zinkernagel RM. On natural and artificial vaccinations. Annu Rev Immunol. 2003;21(1):515–46. doi:10.1146/annurev.immunol.21.120601.141045.
- Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21(2):83–100. doi:10.1038/s41577-020-00479-7.
- Wang X, Guo X, Xin Q, Pan Y, Hu Y, Li J, Chu Y, Feng Y, Wang Q. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020;71(10):2688–94. doi:10.1093/cid/ciaa721.
- Pavel STI, Yetiskin H, Aydin G, Holyavkin C, Uygut MA, Dursun ZB, Celik İ, Cevik C, Ozdarendeli A, Li K. Isolation and characterization of severe acute respiratory syndrome coronavirus 2 in Turkiye. PLoS One. 2020;15(9):e0238614. doi:10.1371/journal.pone.0238614.
- Perera RA, Mok CK, Tsang OT, Lv H, Ko RL, Wu NC, Yuan M, Leung WS, Chan JM, Chik TS, et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Euro Surveill. 2020;25(16):2000421. doi:10.2807/1560-7917.ES.2020.25.16.2000421.
- Reed LJ, Muench H. A simple method of estimating fifty percent end points. Am J Hyg. 1938;27:493–97. doi:10.1093/oxfordjournals.aje.a118408.
- World Health Organization. Laboratory Procedures, Serological detection of avian influenza A(H7N9) infections by microneutralization assay; 2013 May 23. [accessed 2022 July 29]. https://www.who.int/publications/m/item/serological-detection-of-avian-influenza-a(h7n9)-infections-by-microneutralization-assay.
- Lee YW, Lim SY, Lee JH, Lim JS, Kim M, Kwon S, Joo J, Kwak SH, Kim EO, Jung J, et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Korean Med Sci. 2021;36(21):e153. doi:10.3346/jkms.2021.36.e153.
- Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12(2):136–48. doi:10.1038/nri3152.
- Choi A, Koch M, Wu K, Chu L, Ma L, Hill A, Nunna N, Huang W, Oestreicher J, Colpitts T, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–31. doi:10.1038/s41591-021-01527-y.
- Brnjarchevska Blazhevska T, Babačić H, Sibinovska O, Dobrevski B, Kirijas M, Milanovski G, Arsov T, Petlichkovski A. A single dose of BNT162b2 vaccine elicits strong humoral response in SARS-CoV-2 seropositive individuals. Allergy. 2022;77(1):296–98. doi:10.1111/all.15047.
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi:10.1056/NEJMoa2114583.
- Kara A. Genel İmmünizasyon Prensipleri. Cocuk Enfeksiyon Dergisi 2(Supp 1) . 2008;3–14. [Article in Turkish].
- Yue L, Xie T, Yang T, Zhou J, Chen H, Zhu H, Li H, Xiang H, Wang J, Yang H, et al. A third booster dose may be necessary to mitigate neutralizing antibody fading after inoculation with two doses of an inactivated SARS-CoV-2 vaccine. J Med Virol. 2022;94(1):35–38. doi:10.1002/jmv.27334.
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11. doi:10.1038/s41591-021-01377-8.
- Pfizer quarterly corporate performance — second quarter 2021; 2021 July 28 [accessed 2021 Nov 29]. https://investors.pfizer.com/events-and-presentations/event-details/2021/Pfizer-Quarterly-Corporate-Performance–Second-Quarter-2021/default.aspx.
- Lee HK, Knabl L, Knabl L, Kapferer S, Pateter B, Walter M, Furth PA, Hennighausen L. Robust immune response to the BNT162b mRNA vaccine in an elderly population vaccinated 15 months after recovery from COVID-19. medRxiv. 2021. Preprint 2021.09.08.21263284. doi:10.1101/2021.09.08.21263284.
- Keskin AU, Bolukcu S, Ciragil P, Topkaya AE. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J Med Virol. 2022;94(1):39–41. doi:10.1002/jmv.27350.
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. doi:10.1056/NEJMoa2114255.
- Lancet Infectious Diseases. COVID-19 vaccine equity and booster doses. Lancet Infect Dis. 2021;21(9):1193. doi:10.1016/S1473-3099(21)00486-2.
