ABSTRACT
All EU countries have introduced Human papilloma virus (HPV) vaccination for adolescent girls and many countries are expanding the strategy to include adolescent boys. There is uncertainty about the cost-effectiveness and epidemiological impact of a gender-neutral HPV vaccination strategy. Here we present the results of an economic model adapted for Spain. Five vaccination strategies were compared from the Spanish healthcare system perspective, combining two vaccines (4-valent and 9-valent) in a gender-neutral or girls-only programme in a dynamic population-based model with a discrete-time Markov approach. Costs and benefits were discounted at 3%. The benefits of immunization were measured with quality-adjusted life years (QALYs), which are achieved by reducing the incidence of diseases attributable to HPV. Incremental cost-effectiveness ratio (ICER) was compared with the willingness-to-pay threshold in Spain. The two most effective strategies were compared: gender-neutral 9-valent vaccination vs. girls-only 9-valent vaccination, resulting in an ICER of € 34,040/QALY, and an important number of prevented cases of invasive cancers and anogenital warts. The sensitivity analysis revealed that gender-neutral 9-valent vaccination would become cost-effective if protection against oropharyngeal and penile cancers was included or if the price per dose decreased from €45 to €28. The gender-neutral 9-valent HPV vaccination in Spain offers more benefits than any other modeled strategy, although in the conservative base case it is not cost-effective. However, certain plausible assumptions would turn it into an efficient strategy, which should be borne in mind by the decision makers together with equity and justice arguments.
Plain Language Summary
What is the context?
Human papillomavirus (HPV) is a group of viruses that causes sexually transmitted diseases, including certain cancers. European countries offer HPV vaccination to adolescent girls.
Many countries have also introduced the vaccination in adolescent boys.
There are doubts about whether it is worth vaccinating adolescents of both genders.
What this study adds?
We estimated costs and benefits of the vaccination in Spain, comparing two types of vaccine, only in girls and in both genders. This analysis considered indirect protection of vaccinated people to unvaccinated ones.
It seems that the benefits of HPV vaccination in all adolescents do not compensate the costs.
However, when we included likely protection against additional cancers or we reduced the price per vaccine dose, it would be worth vaccinating all adolescents in Spain.
We also stated ethical arguments in favor of HPV vaccination for both genders.
What is the impact?
HPV vaccination in adolescent boys and girls in Spain will prevent more HPV-related diseases.
For certain scenarios, HPV vaccination in both genders would be worth in Spain.
Ethically, vaccinating also boys would be fair, equitable and would not discriminate a part of the population.
Introduction
Human papillomavirus (HPV) is a group of viruses causing skin or mucous membrane growths and is often sexually transmitted. More than 80% of sexually active individuals will get infected during their lifetime.Citation1 Most HPV infections are asymptomatic and transient. However, persistent high-risk HPV infection can lead to precancerous lesions at the site of infection in a small percentage of people, and can cause cancer of the cervix, vulva, vagina, penis, anus, and head and neck.Citation2 High-risk genotypes 16 and 18 are the most involved in these cancers. Low-risk HPV infection, particularly genotypes 6 and 11, can cause anogenital warts.Citation3
Three vaccines are currently authorized by the European Medicines Agency (EMA) for the prevention of various HPV-related diseases: 2-valent vaccine (Cervarix), 4-valent (Gardasil) and 9-valent (Gardasil 9). All three vaccines are licensed for the prevention of cervical cancer, as well as precancerous lesions of the cervix, vulva, vagina, and anus. Only the 4-valent and 9-valent vaccines are also indicated to prevent genital warts.Citation4
All EU countries have introduced HPV vaccination in girls but more and more countries are expanding the strategy to include boys. However, there is uncertainty about the cost-effectiveness of the gender-neutral vaccination strategy. A systematic review of the economic evaluations showed large variations in results and revealed that the cost-effectiveness ratio of vaccination of boys depends to a large extent on parameters that are specific for each country, such as vaccination coverage or the price of the vaccine.Citation5 In Spain, adolescent girls are being vaccinated since 2008, but adolescent boys have not been included in the program by now. Only one economic evaluation of gender-neutral vaccination was developed for Spain and it was funded by one of the vaccine manufacturers.Citation6
The aim of this study was to determine the cost-effectiveness of extending HPV vaccination in Spain to include boys, given that the adolescent female population is already being vaccinated.
Methods
Evaluation overview
Gender-neutral vaccination was compared with two possible alternatives: vaccination of girls only and a scenario with no vaccination. Currently in Spain, girls are being vaccinated at 12 years of age. As a simplification, those who get vaccinated outside the recommended age range (catch-up program) were not taken into account, although the system allows it. There were five strategies being compared: 1) no vaccination; 2) girls-only 4-valent vaccination; 3) gender-neutral 4-valent vaccination; 4) girls-only 9-valent vaccination; 5) gender-neutral 9-valent vaccination.
The benefits of immunization were measured with quality-adjusted life years (QALYs). The benefit is achieved by reducing the incidence of diseases attributable to HPV infection. On the other hand, possible adverse effects reduce these benefits. In the base case, the following diseases attributable to HPV were considered: cervical intraepithelial neoplasia (CIN), cervical cancer, anal cancer, vulvar intraepithelial neoplasia (VIN), vulvar cancer, vaginal intraepithelial neoplasia (VaIN) and recurrent respiratory papillomatosis (RRP). Head and neck and penile cancers were only included in a scenario analysis, since the effectiveness of the vaccine in these diseases has not yet been sufficiently demonstrated.Citation4
Costs and benefits were discounted at 3%, as recommended by the economic evaluation guidelines in Spain.Citation7 The model applied a lifetime horizon following the cohort up to a maximum age of 99 years.
Economic model description
The cost-effectiveness model is an adaptation of a population-based model developed by the Irish HTA agency, HIQA (Health Information and Quality Authority), in 2018 as part of its report on the extension of HPV vaccination to boys.Citation8 The Irish model was based on the model described by Chesson et al., originally developed to evaluate the 4-valent vaccine in the United States,Citation9 and subsequently adapted by the Norwegian HTA agency.Citation10
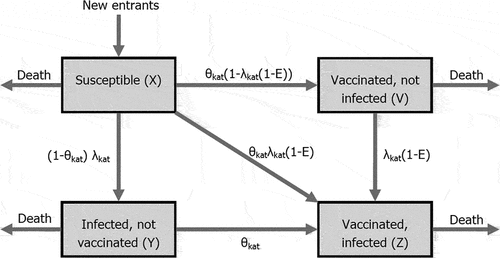
The model used a discrete-time Markov approach. Population moves between five mutually exclusive states: susceptible; vaccinated and not infected; infected and not vaccinated; vaccinated and infected; and dead (). The probabilities of transition between states depend on the age and sex of the individual, the annual probability of receiving the HPV vaccine, the annual probability of contracting HPV infection, and the effectiveness of the vaccine against HPV infection.
Figure 1. Model structure (reprinted from HIQA, Citation2018)Citation8.
The total benefits and costs of the strategies to be compared were calculated by performing 10,000 simulations (due to the computational burden of running the model, the scenario analyses were based on 3,000 simulations). Random values of individual parameters were used in each simulation. The summary of the iterations provides an estimate of the average costs and benefits, as well as the uncertainty associated with these values.
The original US model was developed as a deterministic model in Microsoft Excel. The Irish probabilistic model was built in R 3.4.2, and adapted for Spain in R 4.0.3. All assumptions are listed in Supplementary material.
Epidemiological parameters
The model used epidemiological data from Spain. The original values of the Irish model were maintained only for those parameters where there were no local data or because of their global nature, such as the efficacy of the vaccines or the probability of becoming infected.
The population modeled was all 12-year-old adolescents (girls and boys) in Spain: according to the National Institute of Statistics (INE), as of 1 January 2020, there were 245,693 12-year-old girls and 262,266 12-year-old boys. The model assumed a stable population and the natural reduction of the population occurs in time through the mortality rate according to age. The model did not incorporate future migration or projected birth rates.
Data on incidence of anogenital warts and invasive cancers in Spain, as well as the proportions attributable to HPV, were taken from the HPV-related database, HPV Information Center, managed by the Catalan Institute of Oncology (ICO) and the International Agency for Research on Cancer (IARC).Citation11 Survival data for invasive cancers in Spain were obtained from the EUROCARE-5 study.Citation12 For individuals who do not suffer from invasive cancer, the model assumed a life expectancy of the general Spanish population, estimated by the INE (data from 2018).Citation13
Parameters related to the vaccine
Regarding the vaccine coverage, the model required historical girls’ vaccination rates and a projection of the rate into the future. For current and future coverage, the model was based on the official coverage values for women in 2018, in which 79.4% of 12-year-old adolescents had received at least two doses of the vaccine.Citation14 It was assumed that the coverage for girls in Spain will remain at 80% in the coming years. Regarding vaccination coverage in boys, an experience from Australia with gender-neutral vaccination was used: coverage in boys was 87.7% of coverage in girls on average between 2014 and 2016.Citation15 This proportion was adopted for Spain, resulting in an expected coverage of 70.2% for boys.
The model included the incidence of adverse effects potentially attributable to vaccination. About 69% of vaccinated persons experience a mild effect such as pain at the injection site,Citation8 and the ratio of mild adverse effects is somewhat higher for the 9-valent vaccine.Citation16 The incidence of serious effects, that is, those requiring hospitalization, was estimated by the Norwegian HTA agency at 81 cases per 1 million vaccinations,Citation10 and the same ratio between the 9-valent and 4-valent vaccine was applied.
Data on the efficacy of the vaccine against persistent infection were obtained from clinical trials with the 4-valent vaccine versus placebo and the 9-valent vaccine versus the 4-valent vaccine ().Citation17–19 The efficacy of two doses in adolescents aged 12 years was assumed based on the non-inferiority of the immune response compared to three doses of the vaccine in young adult women and men, in which efficacy has been demonstrated. The 4-valent vaccine was assumed not to offer cross-protection against HPV genotypes other than 6, 11, 16, and 18. Vaccine efficacy was incorporated into the model as relative risk reductions. For HPV types 6, 11, 16, and 18 the 4-valent and 9-valent the vaccines were assumed to be equally effective. Data on vaccine efficacy versus clinical outcomes are included in Supplementary material.
Table 1. Relative risk (RR) of persistent HPV infection after vaccination.
Health-related quality of life
The QALYs gained were estimated based on the health-related quality of life of the general population in Spain, measured using the EQ-5D-5 L questionnaire in the National Health Survey 2011/12.Citation20 Disutility due to illnesses and adverse effects were collected from the literature and applied to the Spanish general population utilities (Supplementary material).
Cost parameters
Adopting the Spanish NHS perspective, only healthcare costs related to the vaccine (doses, administration, adverse effects) and those related to the treatment of HPV-attributable diseases were included. All costs were expressed in Euros 2020. As far as possible, sources from Spain were sought and the costs of previous years were updated to 2020 ().Citation8–21–Citation27
Table 2. Cost data included in the HPV vaccination model.
According to vaccination statistics from 2018, 88.8% of adolescents who received the first dose also received the second one. Although the model assumed that subjects with only one dose did not benefit from the effects of the vaccine, the cost of the first dose and its administration was reflected in the final result.
Cost of mild and severe adverse effects were taken into account. It was assumed that 5% of adolescents presenting mild adverse effects, such as pain, local swelling or redness, would be attended by their GP. Costs of severe adverse events were assumed to be similar to those of an anaphylactic reaction with emergency room visit and a short hospital stay.
Treatment costs of HPV-attributable diseases were obtained from the eHealth healthcare cost database and other literature sources.Citation28 For those costs that reported only hospital treatment, costs of follow-up medical visits in the following 5 years were added. In the case of intraepithelial neoplasms and anogenital warts, there were no follow-up visits, but it was assumed that, in 38% of cases of CIN1 and 71% of cases of CIN2/3, an additional cytology at one year would be required, at a cost of € 58.95 [95%CI: 48.36–71.64].
Outcome measures
Once the costs and benefits of all the strategies to be compared had been calculated, they were ordered according to their results. The incremental cost-effectiveness ratio (ICER) was calculated to compare the cost-effectiveness of the included strategies. A strategy was considered to be cost-effective compared to another if the ICER was lower than the value of willingness-to-pay threshold, which in Spain has been estimated at € 25,000/QALY.Citation29
A base case was estimated using the expected values of the model parameters, while probabilistic and deterministic sensitivity analyses were carried out to quantify decision uncertainty. Probability distribution functions in the probabilistic sensitivity analysis are the following: lognormal for costs, probability of HPV acquisition, relative risks and duration of HPV-attributable diseases; beta for uptake and incidence rates and disutility; and Dirichlet for proportion of events attributable to HPV. The deterministic sensitivity analysis addresses plausible scenarios beyond the base case, modifying key conceptual parameters.
Results
Base case results
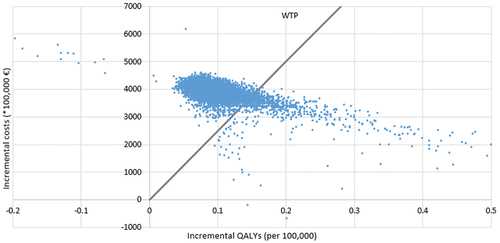
The results are presented in relation to the vaccination strategy for adolescent girls with the 4-valent vaccine, which is close to the current situation in Spain. In , the strategies to be compared have been ordered according to their benefits for the population. In terms of cost-effectiveness, non-vaccination and all 4-valent vaccination strategies would remain dominated, in other words, more expensive and less effective than another strategy. Two remaining strategies forming the cost-effectiveness frontier were the girls-only 9-valent (dominant strategy: cheaper and more effective than the rest) and gender-neutral 9-valent vaccination. The ICER between these two options was calculated at € 34,040/QALY ().
Table 3. Costs and benefits of HPV vaccination strategies (in relation to vaccination of girls with the 4-valent vaccine). Strategies are sorted by QALYs.
Table 4. Incremental cost-effectiveness ratio (ICER) of the two most effective strategies.
The probabilistic analysis showed that the gender-neutral 9-valent strategy would be cost-effective (below the willingness-to-pay threshold) in only 8.2% of cases ().
Epidemiological impact
The greater protection against diseases associated with the 9-valent vaccine and the herd immunity associated with a gender-neutral program leads to a lower incidence of HPV attributable diseases. shows the numbers of cases prevented by switching from girls-only 4-valent vaccination to girls-only 9-valent or to gender-neutral 9-valent vaccination. Switching to a 9-valent vaccine is expected to lead to substantial reductions in cases of precancerous lesions in the short and medium term, while the impact on invasive cancers will take longer to be seen. Vaccination of girls with the 9-valent vaccine would not offer greater protection against anogenital warts than the program with the 4-valent vaccine. However, by extending vaccination to a gender-neutral program, men acquire protection against anogenital warts.
Table 5. Cumulative number of cases averted after changing the HPV immunization strategy.
Scenario analyses
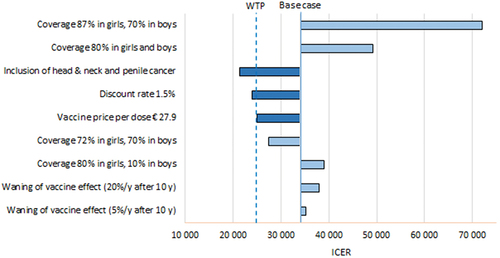
Values of key parameters were modified to simulate plausible situations in the Spanish context (). The gender-neutral 9-valent vaccination compared to girls-only 9-valent vaccination would become a cost-effective strategy (ICER below € 25,000) if the protection against head and neck and penile cancer was added (average risk reduction of 50%; ICER € 21,323/QALY), or if vaccine price per dose was reduced to at least € 27.9. The ICER is also highly sensible to the discount rate. At a discount rate of 0%, 6% (not shown in ) and 1.5%, the ICER fell to € 13,284/QALY, increased enormously to € 151,611/QALY, and was reduced to € 23,994/QALY (becoming a cost-effective strategy), respectively. Variations in coverage among boys did not have much impact on the ICER, while the coverage among girls was crucial. Waning effect (5–20% reduction of effectiveness after 10 years from vaccination) had only a marginal influence on the ICER.
Discussion
A gender-neutral 9-valent HPV vaccination program would be more effective than the current only-girls program. Extending the program to include boys could prevent additional cases of intraepithelial lesions, invasive cancers and anogenital warts in both men and women. The ICER of 9-valent gender-neutral vaccination compared to girls-only 9-valent strategy was € 34,040/QALY, which is above the willingness-to-pay threshold for Spain of € 25,000.Citation29 However, and in line with other published models, this result is highly sensitive to several key parameters, whose variation can change the direction of the results, and which are discussed below.
Protection against head and neck and penile cancers
Current evidence of the efficacy of HPV vaccine in men is limited to the prevention of persistent HPV infections, genital warts, and precursor lesions of anal cancer (anal intraepithelial neoplasia) with the 4-valent vaccine. There is no meaningful estimate of vaccine efficacy for penile intraepithelial lesions and there is no direct evidence of efficacy against anal, penile, and head and neck cancers.Citation4 However, it seems biologically plausible that reducing exposure to HPV would reduce the incidence of associated head and neck cancers and this effect is expected to be demonstrated in future studies. It should also be borne in mind that there is evidence of an increase in HPV-associated head and neck cancers and that its incidence is as high as that of cervical cancer, whose mortality diminished in Spain due to the screening program.Citation2,Citation30 From the ethical perspective, the assumption that the extension of immunity acquired by girls to boys is enough to reduce the risk and would exclude men who have sex with men (MSM) has been described as a heteronormative view.Citation31
As expected, when the analysis includes vaccine protection against penile and head and neck cancers, the cost-effectiveness of gender-neutral vaccination improves. With an average risk reduction of 50% of these cancers, the ICER fell below the cost-effectiveness threshold (€ 21,323/QALY), making it a cost-effective strategy. In the probabilistic sensitivity analyses, the gender-neutral 9-valent strategy would increase from 8.2% (base case) to 43.61% the cases of cost-effective strategy (Supplementary material).
Discount rate of costs and benefits
A highly influential parameter is the discount rate for the benefits of the vaccine. In Spain, the 3% discount is recommended for both costs and benefits,Citation7 and this percentage was used in the base case of the model. The problem with preventive interventions is that most of the costs are incurred at the beginning, and therefore are not affected by the discount, but the benefits occur in a more or less distant future, as is the case of the vaccine (cases of prevented cancer), and are therefore substantially affected by the discount. Our model shows that by lowering the discount rate by half (1.5%), the gender-neutral vaccination becomes a cost-effective strategy (ICER € 23,994/QALY).
In the United Kingdom, the JCVI (Joint Committee on Vaccination and Immunization) refused the extension of the HPV vaccine to boys in 2016, based on results of a model with a discount rate of 3.5%.Citation32 In 2018, the same body reviewed the decision and admitted a rate of 1.5% for benefits, in accordance with the UK economic evaluation guidelines for public health interventions with long-term health benefits.Citation33 This improved the cost-effectiveness of gender-neutral vaccination, which was finally recommended by the JCVI.Citation34 Some authors have criticized this change of the universally accepted rules as unethical and favoring a politically popular but not efficient decision.Citation35
Other parameters
The price of the vaccine has been falling over the last 12 years, thanks to the change from the three-dose to two-dose schedule, but also due to public negotiation between the industry and the national health authorities. Prices for the HPV vaccine used in national immunization programs are substantially lower than officially listed prices. A study recently analyzed the prices negotiated by the health authorities of 15 European countries and observed that the price of the 2- or 4-valent vaccine has dropped from € 100 to € 30 in the last 10 years and in the case of the 9-valent vaccine, the average price was € 50 per dose.Citation36 This means that the negotiated prices can be up to four times lower than officially listed prices. From published studies it can be seen that the decrease in the price of the vaccine improves the cost-effectiveness of gender-neutral vaccination. Our model used the negotiated prices in Spain. Maintaining the rest of the parameters’ values from the base case, the gender-neutral 9-valent vaccine would be a cost-effective strategy in Spain if its price fell from € 45 to € 27.9 per dose.
The influence of vaccination coverage of girls on the cost-effectiveness of gender-neutral vaccination has been demonstrated by numerous studies. However, the context of HPV immunization has changed in recent years with the drop in vaccine prices and the inclusion of other HPV-related diseases in the analyses, and consequently, the relation between cost-effectiveness and the coverage rate could be modified. The model showed how coverage among boys has little influence on the cost-effectiveness of the gender-neutral strategy, while coverage among girls is crucial. This is because in the absence of including penile and head-and-neck cancers, most of the health benefits accrue to females. It could be said that vaccination of girls protects the individual and the herd, whereas in boys it primarily protects the herd.
Most published models assume that the duration of the effect of the vaccine is lifetime.Citation5 To date, vaccine efficacy studies have not suggested a decrease in protection over time. However, the longest follow-up of available clinical trials is 14 years,Citation37 and efficacy could theoretically decrease after that time. The results suggest that waning would have a limited impact on the cost-effectiveness of gender-neutral vaccination. It should be borne in mind that even with decreasing efficacy, the period of protection coincides with the period of maximum risk of contracting HPV infection.
Comparison with other studies
Relevant studies for comparison with our results are those that evaluated the 9-valent vaccine. The systematic review by Linertová et al. reported five studies meeting this criterium;Citation5 three industry-funded dynamic transmission models adapted for Spain,Citation6 Italy,Citation38 and Germany,Citation39 a dynamic population-based model within an HTA report from Ireland, and a dynamic transmission model to evaluate the extension of the HPV vaccine to boys in UK. 34
Gender-neutral vaccination with the 9-valent vaccine compared to vaccination of only girls with the same vaccine was found to be cost-effective only in Italy.Citation38 Despite using a negotiated (reduced) price in our study and in Ireland,Citation8 gender-neutral vaccination was not cost-effective. In the UK the model included head and neck cancer prevention in the base case,Citation34 but gender-neutral vaccination was accepted only when considering a discount rate of 1.5% for benefits, in line with our results and the results of the other mentioned models. An in-depth analysis of the differences between models and the most influential parameters can be found in Linertová et al.Citation5
Limitations
The economic model for this report was based on a model developed for Ireland, which in turn was based on Norwegian and US models. The model uses discrete times, modeling annual transitions between a limited number of mutually exclusive states. Other models have used a larger number of possible states and the continuous time method. However, the simplifications of our model reduce the amount of data required to feed the model and allow a completely probabilistic approach.
Despite the fact that most of the key parameters were adapted to Spain, there were other variables for which specific values for our context were not available and values from other countries or data from the calibration process of the original model were used. It was assumed that people who do not meet the two-dose schedule have no protection against HPV infection. The hypothesis that a single dose could provide the same efficacy as both doses is currently being tested.Citation40,Citation41 The assumption of no vaccine efficacy against head-and-neck and penile cancers is conservative and does not favor gender-neutral immunization. Finally, the model assumes a heterosexual population and does not simulate the MSM population. The effects of the vaccine in this population are assumed to be identical to those in the heterosexual population, but in reality, the MSM population would get protection in a gender-neutral program.
Conclusions
Gender-neutral vaccination in Spain, using the 9-valent vaccine, offers more benefits than any other modeled strategy. In comparison with the girls-only vaccination it would not cost-effective in the conservative base case. However, a decrease of the vaccine price or the inclusion of vaccine effectiveness against additional health outcomes would make it a cost-effective option. In addition, there are strong arguments in favor regarding the ethical considerations of justice, equity and nondiscrimination.
Author contributions
All authors contributed substantively to this manuscript: model adaptation (RL, CGF, JM), parameters search (RL, CGF), internal validation (CGF, CT), drafting the manuscript (RL, CGF, JM), critical revision of the manuscript (CGF, JM, CT) and final approval (all authors).
Supplemental Material
Download MS Word (1.7 MB)Acknowledgements
The authors gratefully acknowledge the support of Arantzazu Arrospide.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2127983
Additional information
Funding
References
- Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41:1–9.
- Serrano B, Brotons M, Bosch FX, Bruni L. Epidemiology and burden of HPV-related disease. Best Pract Res Clin Obstet Gynaecol. 2018;47:14–26.
- de Sanjosé S, Serrano B, Tous S, Alejo M, Lloveras B, Quirós B, Clavero O, Vidal A, Ferrándiz-Pulido C, Pavón MÁ, et al. Burden of human papillomavirus (HPV)-related cancers attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr. 2018;2(4).
- European Centre for Disease Prevention and Control. Guidance on HPV vaccination in EU countries : focus on boys, people living with HIV and 9-valent HPV vaccine introduction. [Internet]. Stockholm; 2020. https://www.ecdc.europa.eu/en/publications-data/guidance-hpv-vaccination-eu-focus-boys-people-living-hiv-9vHPV-vaccine.
- Linertová R, Guirado-Fuentes C, Mar Medina J, Imaz-Iglesia I, Rodríguez-Rodríguez L, Carmona-Rodríguez M. Cost-effectiveness of extending the HPV vaccination to boys: a systematic review. J Epidemiol Community Health. 2021;75:910–16.
- De La Fuente J, Hernandez Aguado JJ, Martín MS, Boix PR, Cedillo S, López N. Estimating the epidemiological impact and cost-effectiveness profile of a nonavalent HPV vaccine in Spain. Hum Vaccin Immunother. 2019;5515:1–13.
- López Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, Puig-Junoy J. Propuesta de guía para la evaluación económica aplicada a las tecnologías sanitarias. Gac Sanit. 2010;24:154–70.
- Health Information and Quality Authority (HIQA). Health technology assessment (HTA) of extending the national immunisation schedule to include HPV vaccination of boys. Dublin: Health Information and Quality Authority; 2018.
- Chesson HW, Ekwueme DU, Saraiya M, Dunne EF, Markowitz LE. The cost-effectiveness of male HPV vaccination in the United States. Vaccine. 2011;29:8443–50.
- Jiménez E, Torkilseng EB, Klemp M. Cost-effectiveness of HPV-vaccination of boys aged 12 in a Norwegian setting. Oslo: Knowledge Centre for the Health Services at The Norwegian Institute of Public Health (NIPH); 2015.
- Bruni L, Albero G, Serrano B, Mena M, Collado JJ, Gómez D, Muñoz J, Bosch F, and de Sanjosé S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human papillomavirus and related diseases in Spain. Summary report 2021 Oct 22.
- Baili P, Di Salvo F, Marcos-Gragera R, Siesling S, Mallone S, Santaquilani M, Micheli A, Lillini R, Francisci S, Hackl M, et al. Age and case mix-standardised survival for all cancer patients in Europe 1999-2007: results of EUROCARE-5, a population-based study. Eur J Cancer. 2015;51(15):2120–29.
- Instituto Nacional de Estadística (INE). Proyecciones de población - Últimos datos.2019 [accessed 2019]. https://www.ine.es/INEbase/DemografíINEbase/DemografíA y población/Cifras de población y Censos demográficos/proyecciones de población/Últimos datos.
- MSCBS. Cobertura de vacunación de la primera y segunda dosis de VPH en mujeres. 2018 [accesssed 2019]. https://www.sanidad.gob.es/en/profesionales/saludPublica/prevPromocion/vacunaciones/calendario-y-coberturas/coberturas/docs/Todas_las_tablas2018.pdf.
- Australian Government Department of Health. Historical data from the national HPV vaccination program register.2019 [accesssed 2019]. https://www.health.gov.au/resources/collections/historical-data-from-the-national-hpv-vaccination-program-register.
- Costa APF, Cobucci RNO, da Silva JM, da Costa Lima PH, Giraldo PC, Gonçalves AK. Safety of human papillomavirus 9-valent vaccine: a meta-analysis of randomized trials. J Immunol Res. 2017;2017:3736201.
- Villa LL, Costa RLR, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Høye J, Steinwall M, Riis-Johannessen G, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95(11):1459–66.
- Giuliano AR, Palefsky JM, Goldstone S, Moreira Jr ED, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. Erratum appears N Engl J Med. 2011 Apr 14;364(15):401–11.
- Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2014;372(8):711–23.
- Cabasés Hita JM, Sánchez Iriso E, Ollo López A, Errea Rodríguez M. Encuesta Nacional de Salud. España 2011/12. Calidad de vida relacionada con la salud en adultos: EQ-5D-5L [Internet]; 2014. https://www.msssi.gob.es/estadEstudios/estadisticas/encuestaNacional/encuestaNac2011/informesMonograficos/CVRS_adultos_EQ_5D_5L.pdf.
- Soler Soneira M, Olmedo Lucerón C, Sánchez-Cambronero Cejudo L, Cantero Gudino E, Limia Sánchez A. El Coste de vacunar a lo largo de toda la vida en España. Rev Esp Salud Publica. 2020;94:18–20.
- Diaz M, Moriña D, Rodríguez-Salés V, Ibáñez R, Espinás JA, de Sanjosé S. Moving towards an organized cervical cancer screening: costs and impact. Eur J Public Health. 2018;28:1132–38.
- Mar J, Errasti J, Soto-Gordoa M, Mar-Barrutia G, Martinez-Llorente JM, Domínguez S, García-Albás JJ, Arrospide A. Valoración Del coste económico del cáncer colorrectal según estadio tumoral. Cir Esp. 2017;95:89–96.
- López N, Gil-de-Miguel Á, Pascual-García R, Ramón Y Cajal JM, Gil-Prieto R. Hospitalization burden associated with malignant neoplasia and in situ carcinoma in vulva and vagina during a 5-year period (2009–2013) in Spain: an epidemiological study. Papillomavirus Res. 2018;5:80–86.
- Gil-Prieto R, Ester PV, Álvaro-Meca A, San Martín Rodŕiguez M, De Miguel ÁG. The burden of hospitalizations for head and neck neoplasm in Spain (1997-2008): an epidemiologic study. Hum Vaccines Immunother. 2012;8:788–98.
- Castellsagué X, Rémy V, Puig-Tintoré L, de la Cuesta R, Gonzalez-Rojas N, Cohet C. Epidemiology and costs of screening and management of precancerous lesions of the cervix in Spain. J Low Genit Tract Dis [Internet]. 2009;13:38–45. [accessed 2021 Oct 8]. https://pubmed.ncbi.nlm.nih.gov/19098605/.
- Larrañaga I, Millas J, Soto-Gordoa M, Arrospide A, San Vicente R, Irizar M, Lanzeta I, Mar J. The impact of patient identification on an integrated program of palliative care in Basque Country. Aten Primaria. 2019;51:80–90.
- Oblikue Consulting S. Base de datos de costes sanitarios y ratios coste-efectividad españoles: eSalud [Internet]. [accessed 2020 Dec 1]. http://www.oblikue.com/bddcostes/.
- Vallejo-Torres L, García-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ [Internet]. 2018;27(4):746–61. doi:10.1002/hec.3633.
- Marur S, Souza GD’, Westra WH, Forastiere AA, Professor A, Hopkins J. HPV-associated head and neck cancer: a virus-related cancer epidemic – a review of epidemiology, biology, virus detection and issues in management. Lancet Oncol. 2010;11:781–89.
- Daley EM, Vamos CA, Thompson EL, Zimet GD, Rosberger Z, Merrell L, Kline NS. The feminization of HPV: how science, politics, economics and gender norms shaped U.S. HPV vaccine implementation. Papillomavirus Res. 2017;3:142–48.
- Joint Commitee on Vaccination and Immunisation. JCVI interim statement on extending HPV vaccination to adolescent boys. London: The Joint Committee on Vaccination and Immunisation; 2017. p. 1–21.
- Joint Commitee on Vaccination and Immunisation. Statement on HPV vaccination joint committee on vaccination and immunisation. London: The Joint Committee on Vaccination and Immunisation; 2018.
- Datta S, Pink J, Medley GF, Petrou S, Staniszewska S, Underwood M, Sonnenberg P, Keeling MJ. Assessing the cost-effectiveness of HPV vaccination strategies for adolescent girls and boys in the UK. BMC Infect Dis. 2019;19(1):552. doi:10.1186/s12879-019-4108-y.
- O’Mahony JF, Paulden M. The joint committee on vaccination and immunisation’s advice on extending human papillomavirus vaccination to boys: were cost-effectiveness analysis guidelines bent to achieve a politically acceptable decision? Value Heal. 2019;22:1227–30.
- Qendri V, Bogaards JA, Berkhof J. Pricing of HPV vaccines in European tender-based settings. Eur J Heal Econ. 2019;20:271–80.
- Kjaer SK, Nygård M, Sundström K, Dillner J, Tryggvadottir L, Munk C, Berger S, Enerly E, Hortlund M, Ágústsson ÁI, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClin Med. 2020;23:100401.
- Mennini FS, Bonanni P, Bianic F, de Waure C, Baio G, Plazzotta G, Uhart M, Rinaldi A, Largeron N. Cost-effectiveness analysis of the nine-valent HPV vaccine in Italy. Cost Eff Resour Alloc. 2017;15(1):11. doi:10.1186/s12962-017-0073-8.
- Largeron N, Petry KU, Jacob J, Bianic F, Anger D, Uhart M. An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. Expert Rev Pharmacoecon Outcomes Res. 2017;17(1):85–98. doi:10.1080/14737167.2016.1208087.
- Sankaranarayanan R, Joshi S, Muwonge R, Esmy PO, Basu P, Prabhu P, Bhatla N, Nene BM, Shaw J, Poli URR, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early Find Ind Stud Vaccine. 2018;36:4783–91.
- Basu P, Malvi SG, Joshi S, Bhatla N, Muwonge R, Lucas E, Verma Y, Esmy PO, Poli URR, Shah A, et al. Vaccine efficacy against persistent human papillomavirus (HPV) 16/18 infection at 10 years after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre, prospective, cohort study. Lancet Oncol [Internet]. 2021;22(11):1518–29. [accessed 2021 Nov 18]. https://pubmed.ncbi.nlm.nih.gov/34634254/.