ABSTRACT
The very first influenza virus exposure in a human during infancy is known to imprint the host immune system. However, it is unclear how the memory B cells that first target virus epitopes affect antibody response to the stalk of hemagglutinin (HA) domain of influenza virus. Our study is designed to measure the cross-reactivity of antibodies induced by inactivated H7N9 virus using isolated human peripheral blood B cells. Most of the participants displayed higher levels of plasma IgG against the seasonal strains A/Vic11 and A/Cali09 than those binding to historical outbreak A/HK68 and A/PR8. H3 stalk-binding antibodies were detected in plasma at a 1:5000 dilution in 12 of 13 donors, H1 stalk-binding antibodies in all donors, indicating the existence of H3 and H1 stalk-reactive memory B cells. A moderate to high level of broadly cross-reactive antibodies was induced in memory B cells from all donors after in vitro stimulation of B cells with H7N9 virus. H3 stalk-binding antibodies were also detected in most subjects, with cross-reactivity to H1 and H7 stalk domains. The stalk-reactive antibodies bound to five H3 strains spanning 45 years and different H1, H2, H3, H5, H6, H7, H9 and B strains. Interestingly, H1- and H3-reactive IgG were much higher than H7-binding antibodies after 6 days of H7N9 stimulation. Our results demonstrate that HA stalk-reactive antibodies induced by H7N9 viruses more efficiently bound to yearly circulating both H3N2 and H1N1 strains than the boosting strain, indicating that HA stalk immunological imprint can be extended across currently circulating strains or vaccines.
Introduction
Since pandemic H1N1 in 1918, influenza A virus is a persistent challenge to global human health. Influenza A as well as influenza B viruses, each with its own subtypes and clades, currently circulate in humans, which result in about 3 to 5 million cases of severe illness and about 290 000 to 650 000 respiratory deaths.Citation1 Antibody responses or B-cell memory mainly target the variable head domains of influenza hemagglutinin (HA),Citation2 which leads to reduction in vaccine effectiveness due to mismatches between circulating viruses and vaccine strains from antigenic drift within the head domain of influenza A subtype HA.Citation3,Citation4 In 1968, H3N2 viruses caused one of the three major influenza pandemics of the 20th century, and since then, this virus subtype became a seasonal pathogen of respiratory disease.Citation5,Citation6 Antigenic drift in the head of the H3N2 HA protein leads to decreased vaccine effectiveness. For example, during the 2014–2015 influenza season, the circulating H3N2 strain was found to be antigenically distinct from the A/Texas/50/2012 strain contained within both the trivalent and quadrivalent influenza vaccine formulations that year, leading to extremely low vaccine effectiveness in the northern hemisphere.Citation7,Citation8
In contrast to the antigenically variable head domain, the stalk region is more conserved, particularly within the same viral group.Citation9–11 Stalk-specific antibodies can cross-reactively bind to other viral subtypes, which share conserved stalk structures, and protect from infection by different strains of influenza A, or even B viruses.Citation12–16 Influenza A viruses have been clustered on the basis of the HA sequence into two phylogenetic groups: group 1 (H1, H2, H5, H6, H8, H9, H11, H12, H13, H16, H17 and H18 subtypes) and group 2 (H3, H4, H7, H10, H14 and H15 subtypes).Citation2,Citation17 H7N9 avian influenza viruses, falling phylogenetically within group 2, share conserved stalk epitopes with other members, such as H3N2, of this group. Individuals who were exposed to seasonal or pandemic H3N2 subtypes would likely have H3 stalk-reactive memory B cells, which could rapidly respond to viruses containing identical stalk epitopes. A recent study demonstrated that an H3 stalk-based chimeric HA vaccine induced high antibody titers against H7 HA and protected mice from H7N9 viral challenge.Citation15 Gostic et alCitation18 reported that the people who were born after 1968 when only H3N2 circulating had lower morbidity and mortality associated with H7N9 infection than other populations.
Humans are routinely infected with antigenically drifted viral strains throughout life. Such infections have been found to boost antibody responses against the influenza strains encountered in childhood. In 1960, Tom Francis, Jr. introduced the concept of “original antigenic sin (OAS)” that antibody responses to initial childhood influenza virus infections are preferentially recalled later in life upon exposure to antigenically drifted viral strains.Citation19 In the intervening decades, and especially the last twenty years, evidence has accumulated that exposure early in life is a crucial factor shaping immune responses to influenza viruses both within and between subtypes. In 2012, Lessler and his colleaguesCitation20 collected sera from 151 residents of Guangdong Province, China, 7 to 81 years of age. They found that serum antibody titers were highest for H3N2 strains circulating in an individual’s first decade of life. The term “antigenic seniority” was proposed, as there were elevated responses toward not only the first strain circulating in an individual’s lifetime but also a list of earlier encountered strains.
Most studies of OAS or antigenic seniority have analyzed antibody responses elicited by sequential exposures with heterosubtypic influenza virus or drifted strains of the same influenza virus subtype.Citation21–24 More recently, Arevalo et al.Citation25 found that antibodies against H1 or H3 stalk are surprisingly boosted upon subsequent infections with antigenically distinct influenza A virus subtypes, H3N2 or H1N1, but these heterosubtypic-boosted HA stalk antibodies do not bind efficiently to the boosting influenza virus strain. Their results implied that an individual’s HA stalk antibody response is dependent on the specific subtype of influenza virus that they encounter early in life. However, it is not clear whether the stalk of H3N2 HA immunologically imprint antibody responses to heterosubtypic strain H7N9. In this study, we investigated whether preexisting H3 stalk-reactive memory B cells could respond to inactivated H7N9 viruses in vitro and their reactivity to H7N9 virus and other group 1, group 2 and B strains. Our data demonstrate that cross-reactive anti-H3 stalk antibodies were induced in memory B cells upon in vitro stimulation with H7N9 viruses and less efficiently binding to the boosting H7N9 virus than those seasonally circulating subtypes.
Methods
Study subjects
This study was approved by the Institutional Review Board at the University of Rochester Medical Center (RSRB protocol RSRB00066522). A total of 13 adults with an age range of 26 to 63 years (mean 43.7 years) were recruited at the University of Rochester through local advertisement in 2016, four males and 9 females. Informed consent was signed by all participants prior to phlebotomy for the study. Of them, one (S12) was born before 1957, three (S2, S9 and S13) between 1957 and 1967, three (S1, S4 and S7) between 1968 and 1976, and six (S3, S5, S6, S8, S10 and S11) after 1977. Twelve of 13 subjects stated a history of being previously vaccinated with seasonal influenza vaccines, while one subject (S4) indicated that she had never received any influenza vaccine. All subjects never had primary immunodeficiency or other causes of immunosuppression. None of the participants had been confirmed influenza disease or had been immunized with a vaccine <3 months prior to study enrollment. The details of the participant characteristics are shown in . Peripheral blood was obtained from all subjects as part of the study for B cell stimulation and analysis of baseline influenza-specific antibodies.
Table 1. Subject characteristics.
Isolation of human B cells
Human peripheral blood was collected by routine phlebotomy in heparinized vacutainers (BD Biosciences, San Diego, CA) and diluted 1:2 with PBS containing 2% FBS. Diluted blood was then layered over Ficoll-Paque PLUS (GE Healthcare, Chicago, IL) for density gradient centrifugation. The plasma layer was collected for the detection and quantitation of anti-influenza antibodies. The lymphocyte layer was then washed twice with PBS (Invitrogen, Carlsbad, CA). B cells were then enriched by negative selection using the EasySep Human Pan-B cell Enrichment Kits according to the manufacturer's recommendations (Stemcell Technologies, Vancouver, BC, Canada).
In vitro activation of B cells
Primary human B cells were treated with inactivated H7N9 viruses, as previously described.Citation26 In brief, B cells were resuspended in complete RPMI medium at 5 × 105/well, 1 mL/well, and were then treated with 6 µg/mL CpG (Integrated DNA Technologies, SanDiego, CA), 50 ng/mL IL-15 (BD Pharmingen, San Diego, CA) and 10 µg/mL of betapropiolactone (BPL) inactivated A/Shanghai/1/2013 (A/SH13, H7N9, IRR catalog No: FR-1281) or A/Victoria/361/2011 (A/Vic11, H3N2, IRR catalog No. :FR-1041) viruses at 37°C and 5% CO2. Six days after incubation, supernatants were harvested for detection of anti-influenza IgG.
HA-reactive IgG analysis
HA-reactive IgG levels were measured in plasma and B cell supernatants using mPLex-Flu assay, as previously described.Citation27 The assay panels included whole HA, heads of HA and chimeric HA, as listed in . Briefly, 25 µL of plasma dilution (1:5000) or undiluted B cell culture supernatants were incubated with 25 µL of HA-coupled beads at room temperature for 2 hours on a rotary shaker in the dark. Phycoerythrin (PE) conjugated goat anti-human IgG (Southern Biotech, Birmingham, Al) was then added (150 µL) and incubated for another 2 hours. The levels of anti-reactive IgG were analyzed on a Magpix Multiplex Reader (Luminex, Austin, TX).
Table 2. mPLEX-Flu hemagglutinin panel.
Statistical analysis
Statistical analysis was performed using SPSS11.5 statistical software. The Mann-Whitney two-tailed test was used to identify statistically significant differences between stimulation groups. P < .05 was accepted as significant.
Results
Prevalence of HA-specific and stalk-reactive IgG
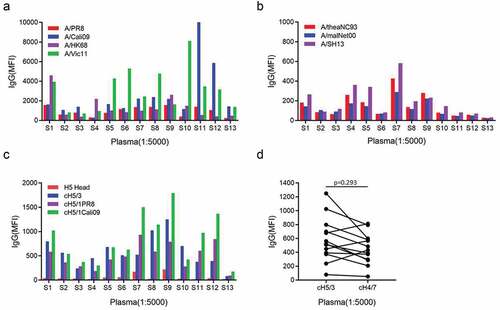
Swine origin influenza virus subtypes H1N1 and H3N2 co-exist in human beings since H1N1 virus reappeared in 1977. B cells produce antibodies against influenza viruses after exposure to influenza viruses through infection or vaccination. To investigate the presence of anti-influenza antibodies reactive with various influenza strains, we analyzed the HA-specific IgG in plasma isolated from 13 healthy subjects using our mPLex-Flu assay. First, antibodies against four influenza strains, including the historic outbreak subtypes, A/Puerto Rico/8/1934 (A/PR8) and A/Hong Kong/1/1968 (A/HK68), and the recent seasonal subtypes A/California/07/2009 (A/Cali09) and A/Victoria/361/2011 (A/Vic11), were detected in all subjects. Interestingly, eight of 13 subjects displayed higher IgG levels against the seasonal strain A/Vic11 than historical outbreak A/HK68, and nine donors had more seasonal anti-A/Cali09 IgG than antibodies against historical A/PR8 strain. Three subjects, S1, S4 and S7 born in the years A/HK68 circulating, displayed three patterns for antibodies to H3N2 virus, similar level of anti-A/HK68 and anti-A/Vic11, higher A/HK68 and higher A/Vic11, respectively (). In addition to H1N1 and H3N2, low H7 subtype-reactive IgG was detected at a 1:5000 dilution in most subjects, including A/rhea/North Carolina/39482/1993 (A/rheaNC93) (H7N1), A/mallard/Netherlands/12/2000 (A/malNet00) (H7N3) and A/Shanghai/1/2013 (A/SH13) (H7N9), indicating a cross-reactivity (). Low or moderate H3 and H1 stalk-reactive IgG, including against cH5/3, cH5/1PR8 and cH5/1Calio9 head/stalk chimeras, was also detected in 12 and 13 of 13 participants, respectively, using either a chimeric protein (cH5/3) containing an H5 head and H3 stalk, or a chimeric protein (cH5/1) containing an H5 head and H1 stalk (). Low H7 stalk-binding antibodies were also observed in 12 subjects. No difference in stalk antibody levels was found between H3 and H7 (p>0.05) (). These results indicate that all subjects displayed peripheral antibodies reactive with H1 and H3 subtype influenza viruses, including H3 stalk reactive antibodies, suggesting that H1 and H3 imprint in all subjects.
Figure 1. Stalk-reactive IgG in human plasma. Plasma was obtained from 13 donors, 12 of which had been previously vaccinated within the past 5 years with seasonal influenza vaccines. Plasma baseline anti-influenza IgG was assessed by mPlex-Flu assay (n = 13). (a) levels of IgG against historic outbreak and recently circulating H3N2 and H1N1 strains. Each column depicts median fluorescence intensity (MFI), representing an individual donor. (b) levels of H7N1-, H7N3- and H7N9-reactive IgG. (c) H1 and H3 stalk-reactive IgG. (d) IgG-binding to cH5/3 and cH4/7 proteins. Each symbol and line represents one donor. Mann-Whitney two-tailed test was used to evaluate the difference among different groups. P < 0.05 was considered statistically significant. This analysis was performed at a 1:5,000 dilution.
Antibodies induced by H7N9 viruses in vitro react with different H7 subtypes
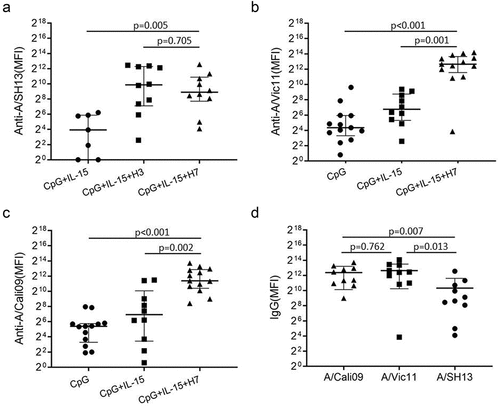
To evaluate memory B cell responses to H7N9 viruses, purified B cells were stimulated with BPL inactivated H7N9 virus, CpG2006ODN and IL-15 for 6 days. H7 subtype-reactive IgG was measured using HA antigen from three strains, H7N1 virus A/rheaNC93, H7N3 virus A/malNet00 and H7N9 strain A/SH13. B cells from all participants displayed rapid responses to inactivated A/SH13 H7N9 viruses. As shown in , the anti-A/SH13 IgG was observed after H7 stimulation and higher in the H7+CpG+IL-15 group than those in CpG alone and CpG+IL-15 groups (p < .05). The same phenomenon was observed when anti-A/rheaNC93 were measured (). Since most individuals were likely exposed to H3N2 viruses not H7N9, the levels of anti-A/SH13 IgG induced by H7N9 were compared with those by A/Vic11 H3N2 viruses. As shown in , we found no difference in the levels of antibodies against A/SH13 between the H3 and H7 groups. Moreover, the increases in recent seasonal strains A/Vic11 and A/Cali09-reactive IgG were observed after H7N9 stimulation (). The levels of antibodies against A/Vic11 and A/Cali09 were higher than those binding to A/SH13 (p < 0/05) ().
Figure 2. Anti-H7 IgG secretion stimulated by inactivated A/SH13 viruses. Purified B cells from 13 donors were obtained by negative selection and stimulated with CpG2006ODN alone or together with IL-15 and A/SH13 (H7) BPL inactivated virus for 6 days in vitro. Supernatants were collected and the IgG-binding to A/rheaNC93 H7N1 (a), A/malNet00 H7N3 (b) and A/SH13 H7N9 (c) HA were measured. Each symbol and line represents one donor. Mann-Whitney two-tailed test was used to evaluate the difference among different groups. P < 0.05 was considered statistically significant.
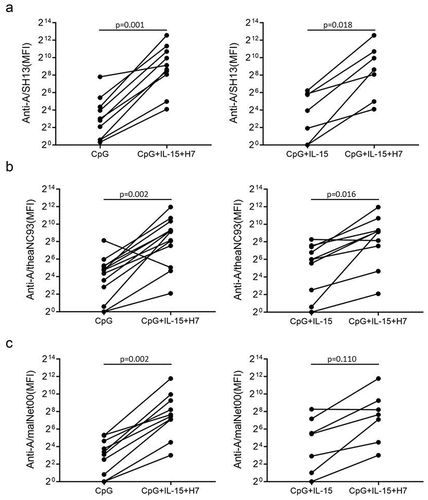
Figure 3. IgG induced by H7N9 viruses in vitro cross-react with A/Vic11 and A/Cali09 HA. Purified B cells were obtained by negative selection and stimulated with CpG2006ODN alone or together with IL-15 and A/SH13 inactivated virus for 6 days. Supernatants were collected, and H1 and H3 HA-reactive IgG were detected using mPlex-Flu assay. (a) levels of IgG against A/SH13 HA. H7-binding antibodies in supernatants of activated B cells were monitored using beads bound with HA from H7N9 strains (n = 13), and compared with CpG+IL-15+H3 group (n = 10). (b) A/Vic11- and (c) A/Cali09-reactive IgG levels in CpG (n = 13), CpG+IL-15 (n = 10) and CpG+IL-15+H7 (n = 10) groups. (d) The levels of H7N9-induced H1, H3 and H7 antibodies were compared. Each symbol represents an individual donor. Mann-Whitney two-tailed test was used to evaluate the difference among different groups. P < 0.05 was considered statistically significant.
Anamnestic antibodies more efficiently bound to seasonal strains
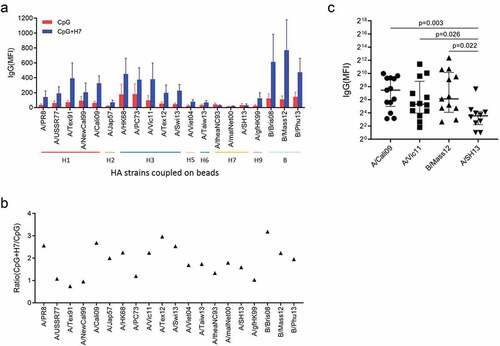
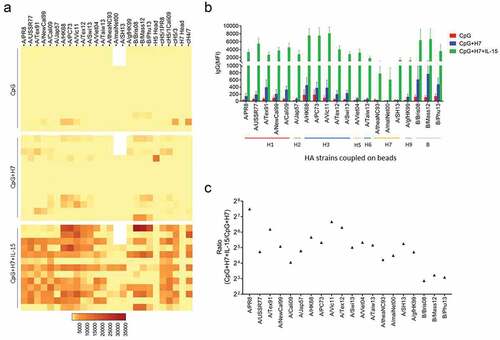
To evaluate the cross-reactivity of antibodies, we measured IgG binding to recombinant HAs from various influenza A strains, including H1, H2, H3, H5, H6 and H9, and B strains, elicited by in vitro stimulation of B cells with inactivated A/SH13 viruses. Five subtypes, H1, H2, H5, H6 and H9 belong to group 1, while H3 belongs to group 2. For H1, five strains covering 75 years of antigen drift of H1N1 viruses from 1934 to 2009 were selected, including A/PR8, A/USSR/90/1977 (A/USSR77), A/Texas/36/1991 (A/Tex91), A/New Caledonia/20/1999 (A/NewCal99) and A/Cali09. Five heterovariant H3N2 strains spanning 45 years (1968–2013), A/HK68, A/Port Chalmers/1/1973 (A/PC73), A/Vic11, A/Texas/50/2012 (A/Tex12) and A/Switzerland/9715293/2013 (A/Swi13) were used to evaluate anti-H3 antibodies. As shown in , antibodies binding to group 1, group 2 and B strains were detected in supernatants of B cells after stimulation with inactivated H7N9 viruses. Twelve of 13 donors displayed an increase in IgG binding to group 1 strains A/PR8 and A/Cali09, with median (range) fold changes of 2.56 (0.24, 36.84) and 2.68 (0.83, 24.69), respectively. For group 2 strains H3N2, increases were found in levels of IgG binding to strains A/HK68 (2.25 (0.09, 42.12)), A/Vic11 (2.0 (0.10, 43.56)), A/Tex12 (3.13 (0.10, 26.16)) and A/Swi13 (2.54 (0, 43.91)) in 10, 11, 10 and 7 donors, respectively. For the H7 subtypes, 8, 8, and 7 of the 13 donors had elevated levels of antibodies, with fold changes of A/theaNC93 (1.33 (0, 1.76)), A/maiNet00 (1.79 (0.24, 3.30), respectively)) and A/SH13 (1.59 (0.10, 12.66)). An increase was also observed in IgG binding to B/Brisbane/60/2008 (B/Bris08) (3.19 (0.25, 84.82)) and B/Mass/02/2012 (B/Mas12) (3.15 (0, 97.71)) (). Interestingly, antibodies binding to seasonal influenza A virus subtypes, H1N1 strain A/Cali09 and H3N2 strain A/Vic11, even B strain B/Mass12, were higher than the boosting H7N9 strain A/SH13 ().
Figure 4. Cross-reactive IgG induced by A/SH13 and CpG2006ODN. B cells were obtained by negative selection, and then stimulated with CpG2006ODN alone or together with A/SH13 for 6 days. Cross-reactive antibodies binding to H1, H2, H5, H6 and H7 subtypes induced in the B cell cultures were measured by mPlex-Flu assay. (a) CpG2006ODN with H7 antigen induces a broad recall response to different influenza strains (n = 13). (b) fold change in cross-reactive antibodies. All values of IgG levels (MFI) were subtracted from the culture medium alone values before calculating fold change (n = 13). Each symbol represents the median of fold change in IgG levels. (c) Comparison between seasonal subtypes and the boosting strain.
IL-15 enhanced the cross-reactive antibodies induced by H7N9 influenza viruses in cell culture supernatants
IL-15 is known to enhance the antibody recall response to H3 stalk by H3N2 viruses.Citation26 In this study, we used IL-15 to promote the secretion of antibodies induced by inactivated H7N9 viruses. As shown in , cells from most donors displayed strong antibody responses when treated with H7N9 viruses, CpG2006ODN and IL-15 (CpG+H7+IL-15), compared with CpG+H7 and CpG alone groups. The largest response was enhancement of antibodies against H3N2 strains A/HK68, A/PC73 and A/Vic11 (). B cells from all 13 donors displayed increases in IgG binding to all H3N2 strains, with fold changes of A/HK68 (51.06 (1.52, 841.56)), A/PC73 (40.44 (1.70, 462.06)), A/Vic11 (102.23 (2.32, 517.86)), A/Tex12 (79.17 (2.40, 514.07)) and A/Swi13 (32.41 (3.14, 488.83)). Increases in IgG reactive to H1 strains A/PR8 (180.50), A/USSR77 (26.72), A/Tex91 (72.70), A/NewCal99 (34.01) and A/Cali09 (16.63) were detected in supernatants derived from all donors. Increases in reactive IgG secretion also occurred to other group 1 strains, A/Jap57 (27.83), A/Viet04 (40.60), A/Taiw13 (35.97) and A/gfHK99 (26.38) and B strains B/Brisbane/60/2008 (B/Bris08) (7.32), B/Mass/02/2012 (B/Mas12) (9.40) and B/Phuket/2013 (B/Phu13) (8.51). IgG binding to the H7 subtypes was increased in all donors, with fold changes of A/theaNC93 (18.66 (0.62, 560.5)), A/maiNet00 (22.53 (4.47, 375.89)) and A/SH13 (38.34 (2.03, 1096.32)) ().
Figure 5. IL-15 enhanced the cross-reactive IgG induced by A/SH13. (a) B cells from 13 donors were stimulated with CpG2006ODN, inactivated A/SH13 viruses and IL-15. Strain-specific and HA stalk-reactive IgG in supernatants of activated B cells were measured after 6 days (n = 13). (b) correlation between influenza specific antibodies and HA antigenic sequence (n = 13). (c) fold change in cross-reactive antibodies (n = 13). IL-15 increased the concentration of anti-HA reactive IgG.
Stalk-reactive antibodies were induced by heterosubtypic H7N9 viruses
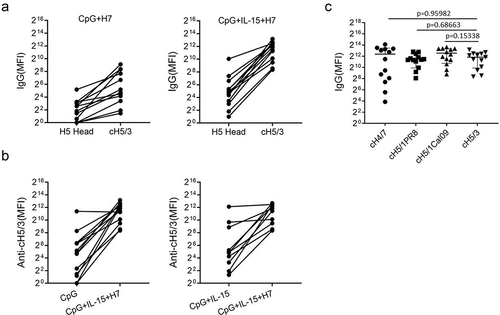
Avian influenza H7N9 viruses have conserved stalks domains shared with swine original H3N2 viruses, which belong to group 2 of influenza A. To detect stalk-reactive antibodies, we constructed a chimeric HA protein consisting of an H5 head domain and an H3 stalk (cH5/3). As most individuals are unlikely to have existing H5 immunity, this reagent allows for the assessment of H3 stalk domain reactive antibodies. Indeed, anti-cH5/3 IgG was detected in B cells from all 13 donors after the heterosubtypic H7N9 in vitro stimulation and much higher than H5 head-specific IgG (), indicating stalk-reactive antibody secretion. The antibody responses to conversed stalk were strongly upregulated by IL-15 in B cells from most of the donors (11 of 13). As shown in , high levels of anti-cH5/3 IgG were monitored in the CpG+IL-15+H7 group, with MFI ranging from 323 to 9134, whereas only 1 and 3 donors showed anti-cH5/3 levels of more than 300 when stimulated with CpG and CpG+IL-15, respectively. To assess the reactivity of anti-H7 and anti-H1 stalk IgG, chimeric HA proteins cH4/7 and cH5/1 were used. H1 stalk-reactive antibodies were detected using two chimeric HAs, cH5/1PR8 and cH5/1Cali09, representing the stalks of historical and recent seasonal H1 strains, respectively. No difference in stalk-reactive IgG levels was found between anti-cH5/3 and anti-cH4/7, anti-cH5/1PR8 and cH5/1Cali09 after stimulation with H7N9 virus at the presence of CpG and IL-15 ().
Figure 6. Stalk-reactive IgG induced by A/SH13 viruses. B cells from healthy donors were stimulated with CpG2006ODN alone or together with inactivated A/SH13 (H7N9) viruses and IL-15 in vitro. The levels of IgG against H5 head and cH5/3 were shown for individual subjects. 12 of 13 subjects displayed increases in stalk-reactive IgG after A/SH13 stimulation. (a) levels of IgG binding to H5 head and cH5/3. (b) levels of cH5/3-reactive IgG after stimulation with CpG2006ODN alone or together with inactivated A/SH13 (H7N9) viruses. (c) Comparation between H3- and H1-, H7-reactive antibodies.
Discussion
In this study, we analyzed preexisting H1 and H3 strain-specific and stalk-reactive antibodies in 13 donors using our mPLex-Flu assay and found high levels of specific IgG against H1 and H3 historical outbreak strains and recent seasonal stains in most donors. H3 stalk-reactive antibodies were detected in all participants, however, at much lower levels than H3-specific antibodies. The presence of low levels of stalk-reactive IgG suggests that immune-subdominant H3 stalk-specific memory B cells exist within the memory B cell pool. These findings are consistent with imprinting, where prior exposure to influenza results in most adults having preexisting antibodies against influenza viruses,Citation28–31 primarily directed against the immunodominant globular head of influenza virus HA. Antibodies binding to the conserved, immuno-subdominant HA stalk domain are generally undetectable by the hemagglutination inhibition (HI) test or detectable at low levels using hypersensitive Luminex assay following natural infection or influenza vaccination, despite being broadly protective against multiple influenza strains and subtypes.Citation12,Citation26,Citation32,Citation33
Most humans are first infected with influenza viruses in childhood and subsequently infected with antigenically distinct strains every 5 to 10 years. Influenza virus exposure early in life can leave long-lived immunological imprint. Since H1N1 pandemic breakout in 1918, older individuals were likely exposed to H1N1 in childhood as only H1N1 viruses circulated until 1957 when H2N2 emerging. After H3N2 pandemic breakout in 1968, individuals born after 1968 were more likely to be initially exposed to H3N2 virus. J. M. Fonville reported that an individual born in 1970, infected in 2009, had a substantial response back to the A/HK68 strain circulating in his childhood, even though the older viruses had not circulated for decades.Citation21 In this study, all 13 participants were born between 1953 and 1990, immunized with influenza vaccines except for subject S4. Three of them, S1, S4 and S7, were born between 1968 and 1977 when the H3N2 subtype was circulating. We found that most subjects displayed higher IgG levels against the seasonal H1N1 and H3N2 strains than historical outbreak strains. Only subject S4 who was never immunized with an influenza vaccine had a higher level of antibodies to historical H3N2 strain A/HK68 than recent season strain A/Vic11 and displayed low antibodies against the seasonal A/PR8 and historical outbreak A/Cali09 H1N1 strains, even lower than the anti-stalk IgG, indicating that vaccines may affect the immunological imprint established in childhood. There is limited information available on the infection and vaccination history, such as viral strains, vaccination time and vaccination frequency. This is a limitation for us to better understand the effect of previous exposure on the formation of immunological imprint.
In 2013, a novel avian virus (H7N9) emerging in humans, has a mortality rate of 39% and represents another major threat to global health.Citation34,Citation35 H7N9 antibodies had been detected by the HI test following natural infection and vaccination and exhibited cross-reactivity to other H7 subtype influenza virus,Citation36,Citation37 whereas none were seropositive among 1480 general population.Citation38 In this study, we also measured the plasma IgG binding to H7N9, H7N1 and H7N3 subtypes using the hypersensitive mPLex-Flu assay. Extremely low H7 subtype-reactive IgG was detected in 10 subjects, with MFI <600, much lower than the antibodies against H1N1 and H3N2 subtypes, which suggests the cross-reactivity of the HA stalk antibodies.
Our findings are consistent with IgG antibodies against influenza viruses reflecting influenza-specific memory B cells due to prior infection in most individuals. Memory B cells will rapidly proliferate and differentiate to produce antibodies if preexisting neutralizing antibodies are insufficient to bind and clear the newly invading pathogen.Citation39 The adult antibody response to influenza vaccine is driven by activation of preexisting memory B cells.Citation40,Citation41 In this study, we focused on the memory B cell recall response to inactivated H7N9 viruses following six days of H7 stimulation in vitro. Since only B cells were added in the in vitro stimulation without T cell help, CpG and IL-15 were used to promote the proliferation and Ig production of B cells, and the CpG+IL-15 groups were performed as controls. H7-, including A/rhea/NC93 H7N1, A/malNet00 H7N3 and A/SH13 H7N9 subtypes, reactive IgG was detected in B cell cultures derived from 13 donors, albeit at relatively low levels, indicating that H3 stalk memory B cells were activated and secreted cross-reactive antibodies binding to heterosubtypes. H7N9 HA falls phylogenetically within the group 2 influenza virus HA and shares conserved epitopes in the stalk domain with other members of this group (H3, H4, H10, H14 and H15). The stalk of HA composed of part of HA1 and HA2 displays a much higher level of conservation across influenza strains and even some identical central residues across all subtypes.Citation42,Citation43 The H3 stalk-based chimeric HA influenza virus vaccine induces cross-protection against H7N9 challenge.Citation15,Citation44 In this study, we found that the H3 stalk-reactive IgG was induced by heterosubtypic H7N9 viruses and bound to H1 and H7 stalk.
To investigate the cross-reactivity of antibody induced by H7N9 to seasonally circulating strains, five H1N1 and five H3N2 strains were used to evaluate the anti-H1 and anti-H3 antibodies. Consistent with the antibody levels in plasma, memory B cells secreted high levels of antibodies binding to not only recent seasonal and historical outbreak H1N1 and H3N2 strains but also other members of group 2 (H6) and most members of the group 1(H1, H2, H5, and H9) and B strains. Interestingly, although recall antibody responses were induced by the heterosubtypic H7N9, these antibodies more efficiently bound to seasonal circulating strains H3N2 as well as H1N1 than the boosting strain. Our results are consistent with the OAS and antigenic seniority theory. The term OAS has been used to describe how infections later in life can give an elevated recall response to the initial childhood priming strain.Citation19 The antigenic seniority theory proposed recently has been used to describe the phenomenon of how the priming strain has a more ‘‘senior” role in antibody response to subsequent infections.Citation20 In our study, the donors displayed elevated recall responses to H1N1 or H3N2 strains circulating early in their lifetime. Moreover, like the low levels of H7-binding IgG, the stalk-reactive antibodies were much lower than the antibodies against H1N1 and H3N2 subtypes, However, the immunological imprint for influenza may be more complicated due to re-exposure to multiple different influenza strains and immunization with influenza vaccines. There are two factors considered to be critical for memory B-cell to influenza viruses. First, anamnestic responses during all subsequent infections, cross-reactive neutralizing antibodies and cytotoxic T cells, will delete the total antigen available to naive B cells reactive to the new epitopes on the secondary strain. Second, affinity-matured memory B cells have more opportunities to enter a germinal center and get help from T follicular helper (TFH) cells for their differentiation into plasma cells.Citation45,Citation46 Memory B-cell responses focus on secondary strain epitopes shared with the first strain when encountered with antigenically distinct subtypes.Citation41,Citation47,Citation48 Re-exposure to antigenically drifted strains leads to immunological imprint to subdominant HA stalk. New epitopes can be activated by the vaccines composed of multiple strains when the numbers of memory B targeting on the old immunodominant epitopes decrease to a very low level. Arevalo et al.Citation25 reported that although H1 stalk-reactive antibodies in children with prior H1N1 exposures were boosted >10-fold following heterosubtypic H3N2 infection, H1 stalk-reactive antibodies that were boosted by H3N2 infection were absorbed by only H1 antigen not the boosting H3 antigen. Three protective epitopes located on the HA2 domain of influenza A virus have been identified, with varying levels of cross-reactivity among group 1, group 2 and B strains.Citation42–50 HA stalk memory B cells may be activated by heterosubtypic H7N9 influenza viruses through multiple low-affinity interactions with thousands of identical BCRs, but produce soluble antibodies that bind poorly to the boosting antigen. This may be one reason why the antibodies induced by H7N9 more effectively binding to H3 even H1 stalk due to the same or similar epitope shared within influenza A subtypes. However, some antibodies bound with low affinities were able to effectively protect against an antigenically drifted viral strain following passive transfer in vivo.Citation51
In conclusion, low levels of broadly stalk-reactive antibodies and memory B cells exist in individuals previously exposed to influenza viruses and/or influenza vaccines. We found that most subjects displayed higher levels of plasma IgG against the recent seasonal H1N1 and H3N2 strains than historical outbreak strains. The memory B cells targeting the epitopes on the stalk of HA can be activated by heterosubtypic H7N9 strains and produced antibodies binding to not only seasonal circulating strains but also boosting strain. Establishment of long-lived memory against the HA stalk of distinct influenza virus subtypes could provide universal immunity toward influenza virus later in life.
Author contributions
Conceptualization: JH, MSZ, and SPH; Investigation: JH and JG; Data analysis: YC and JH; Writing—original draft: JH; Writing—review and editing: MSZ, SPH, and JW; Project administration: JH, MSZ; Funding acquisition: JH.
Acknowledgement
This research was supported by the China Scholarship Council and the Zunyi Medical University Visiting Scholar Grant (201408525075; JH) and the National Natural Science Foundation of China (grant 81860376). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO. Influenza (seasonal). [ accessed 2022 March 25]. https://www.who.int/en/news-room/fact-sheets/detail/influenza-seasonal
- Sautto GA, Kirchenbaum GA, Ross TM. Towards a universal influenza vaccine: different approaches for one goal. Virol J. 2018;15(1):1. doi:10.1186/s12985-017-0918-y.
- Tewawong N, Prachayangprecha S, Vichiwattana P, Korkong S, Klinfueng S, Vongpunsawad S, Thongmee T, Theamboonlers A, Poovorawan Y, Krammer F. Assessing antigenic drift of seasonal influenza A(H3N2) and A(H1N1)pdm09 viruses. PLoS One. 2015;10(10):e0139958. doi:10.1371/journal.pone.0139958.
- Bedford T, Riley S, Barr IG, Broor S, Chadha M, Cox NJ, Daniels RS, Gunasekaran CP, Hurt AC, Kelso A, et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature. 2015;523(7559):217–11. doi:10.1038/nature14460.
- Allen JD, Ross TM. H3N2 influenza viruses in humans: viral mechanisms, evolution, and evaluation. Hum Vaccin Immunother. 2018;14(8):184047. doi:10.1080/21645515.2018.1462639.
- Hinojosa M, Shepard SS, Chung JR, King JP, McLean HQ, Flannery B, Belongia EA, Levine MZ. Impact of immune priming, vaccination, and infection on influenza A(H3N2) antibody landscapes in children. J Infect Dis. 2021;224(3):469–80. doi:10.1093/infdis/jiaa665.
- Chambers BS, Parkhouse K, Ross TM, Alby K, Hensley SE. Identification of hemagglutinin residues responsible for H3N2 antigenic drift during the 2014–2015 influenza season. Cell Rep. 2015;12(1):1–6. doi:10.1016/j.celrep.2015.06.005.
- Zimmerman RK, Nowalk MP, Chung J, Jackson ML, Jackson LA, Petrie JG, Monto AS, McLean HQ, Belongia EA, Gaglani M, et al. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis. 2016;63(12):1564–73. doi:10.1093/cid/ciw635.
- Neu KE, Henry Dunand CJ, Wilson PC. Heads, stalks and everything else: how can antibodies eradicate influenza as a human disease? Curr Opin Immunol. 2016;42:48–55. doi:10.1016/j.coi.2016.05.012.
- Fu Y, Zhang Z, Sheehan J, Avnir Y, Ridenour C, Sachnik T, Sun J, Hossain MJ, Chen LM, Zhu Q, et al. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat Commun. 2016;7:12780. doi:10.1038/ncomms12780.
- Christensen SR, Toulmin SA, Griesman T, Lamerato LE, Petrie JG, Martin ET, Monto AS, Hensley SE, Heise MT. Assessing the protective potential of H1N1 influenza virus hemagglutinin head and stalk antibodies in humans. J Virol. 2019;93(8): e02134-18. doi:10.1128/JVI.02134–18.
- Dhar N, Kwatra G, Nunes MC, Cutland C, Izu A, Nachbagauer R, Krammer F, Madhi SA. Hemagglutinin stalk antibody responses following trivalent inactivated influenza vaccine immunization of pregnant women and association with protection from influenza virus illness. Clin Infect Dis. 2020;71(4):1072–79. doi:10.1093/cid/ciz927.
- Ohshima N, Iba Y, Kubota-Koketsu R, Asano Y, Okuno Y, Kurosawa Y. Naturally occurring antibodies in humans can neutralize a variety of influenza virus strains, including H3, H1, H2, and H5. J Virol. 2011;85(21):11048–57. doi:10.1128/JVI.05397-11.
- Pan Y, Sasaki T, Kubota-Koketsu R, Inoue Y, Yasugi M, Yamashita A, Ramadhany R, Arai Y, Du A, Boonsathorn N, et al. Human monoclonal antibodies derived from a patient infected with 2009 pandemic influenza a virus broadly cross-neutralize group 1 influenza viruses. Biochem Biophys Res Commun. 2014;450(1):42–48. doi:10.1016/j.bbrc.2014.05.060.
- Krammer F, Margine I, Hai R, Flood A, Hirsh A, Tsvetnitsky V, Chen D, Palese P. H3 stalk-based chimeric hemagglutinin influenza virus constructs protect mice from H7N9 challenge. J Virol. 2014;88(4):2340–43. doi:10.1128/JVI.03183-13.
- Tan GS, Lee PS, Hoffman RM, Mazel-Sanchez B, Krammer F, Leon PE, Ward AB, Wilson IA, Palese P, Dermody TS. Characterization of a broadly neutralizing monoclonal antibody that targets the fusion domain of group 2 influenza a virus hemagglutinin. J Virol. 2014;88(23):13580–92. doi:10.1128/JVI.02289-14.
- Estrada LD, Schultz-Cherry S. Development of a universal influenza vaccine. J Immunol. 2019;202(2):392–98. doi:10.4049/jimmunol.1801054.
- Gostic KM, Ambrose M, Worobey M, Lloyd-Smith JO. Potent protection against H5N1 and H7N9 influenza via childhood hemagglutinin imprinting. Sci. 2016;354(6313):722–26. doi:10.1126/science.aag1322.
- Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104:572–78.
- Lessler J, Riley S, Read JM, Wang S, Zhu H, Smith GJ, Guan Y, Jiang CQ, Cummings DA, Basler CF. Evidence for antigenic seniority in influenza a (H3N2) antibody responses in southern China. PLoS Pathog. 2012;8(7):e1002802. doi:10.1371/journal.ppat.1002802.
- Fonville JM, Wilks SH, James SL, Fox A, Ventresca M, Aban M, Xue L, Jones TC, Le NMH, Pham QT, et al. Antibody landscapes after influenza virus infection or vaccination. Sci. 2014;346(6212):996–1000. doi:10.1126/science.1256427.
- Miller MS, Gardner TJ, Krammer F, Aguado LC, Tortorella D, Basler CF, Palese P. Neutralizing antibodies against previously encountered influenza virus strains increase over time: a longitudinal analysis. Sci Transl Med. 2013;5(198):198ra107. doi:10.1126/scitranslmed.3006637.
- Li Y, Myers JL, Bostick DL, Sullivan CB, Madara J, Linderman SL, Liu Q, Carter DM, Wrammert J, Esposito S, et al. Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J Exp Med. 2013;210(8):1493–500. doi:10.1084/jem.20130212.
- Worobey M, Plotkin S, Hensley SE. Influenza vaccines delivered in early childhood could turn antigenic sin into antigenic blessings. Cold Spring Harb Perspect Med. 2020;10(10):a038471. doi:10.1101/cshperspect.a038471.
- Arevalo CP, Le Sage V, Bolton MJ, Eilola T, Jones JE, Kormuth KA, Nturibi E, Balmaseda A, Gordon A, Lakdawala SS, et al. Original antigenic sin priming of influenza virus hemagglutinin stalk antibodies. Proc Natl Acad Sci U S A. 2020;117(29):17221–27. doi:10.1073/pnas.1920321117.
- Huang J, Hilchey SP, Wang J, Gerigan J, Zand MS. IL-15 enhances cross-reactive antibody recall responses to seasonal H3 influenza viruses in vitro. F1000res. 2017;6(2015). doi:10.12688/f1000research.12999.1.
- Wang J, Hilchey SP, Hyrien O, Huertas N, Perry S, Ramanunninair M, Bucher D, Zand MS, Krammer F. Multi-dimensional measurement of antibody-mediated heterosubtypic immunity to influenza. PLoS One. 2015;10(6):e0129858. doi:10.1371/journal.pone.0129858.
- Sasaki S, Jaimes MC, Holmes TH, Dekker CL, Mahmood K, Kemble GW, Arvin AM, Greenberg HB. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol. 2007;81(1):215–28. doi:10.1128/JVI.01957-06.
- Moody MA, Zhang R, Walter EB, Woods CW, Ginsburg GS, McClain MT, Denny TN, Chen X, Munshaw S, Marshall DJ, et al. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One. 2011;6(10):e25797. doi:10.1371/journal.pone.0025797.
- Wang J, Li D, Perry S, Hilchey SP, Wiltse A, Treanor JJ, Sangster MY, Zand MS, Rappuoli R. Broadly reactive IgG responses to heterologous H5 prime-boost influenza vaccination are shaped by antigenic relatedness to priming strains. mBio. 2021;12(4):e0044921. doi:10.1128/mBio.00449-21.
- Qin L, Wang D, Li D, Zhao Y, Peng Y, Wellington D, Dai Y, Sun H, Sun J, Liu G, et al. High level antibody response to pandemic influenza H1N1/09 virus is associated with interferon-induced transmembrane protein-3 rs12252-CC in young adults. Front Cell Infect Microbiol. 2018;8:134. doi:10.3389/fcimb.2018.00134.
- Islam S, Mohn KG, Krammer F, Sanne M, Bredholt G, Jul-Larsen Å, Tete SM, Zhou F, Brokstad KA, Cox RJ. Influenza a haemagglutinin specific IgG responses in children and adults after seasonal trivalent live attenuated influenza vaccination. Vaccine. 2017;35(42):5666–73. doi:10.1016/j.vaccine.2017.08.044.
- Tesini BL, Kanagaiah P, Wang J, Hahn M, Halliley JL, Chaves FA, Nguyen PQT, Nogales A, DeDiego ML, Anderson CS, et al. Broad hemagglutinin-specific memory B cell expansion by seasonal influenza virus infection reflects early-life imprinting and adaptation to the infecting virus. J Virol. 2019;93(8). doi:10.1128/JVI.00169-19
- Koopmans M, de Jong MD. Avian influenza a H7N9 in Zhejiang, China. Lancet (London, England). 2013;381(9881):1882–83. doi:10.1016/S0140-6736(13)60936-8.
- WHO. Monthly risk assessment summary. [accessed 2022 June 15]. https://www.who.int/teams/global-influenza-programme/avian-influenza/monthly-risk-assessment-summary
- Ma MJ, Wang XX, Wu MN, Wang XJ, Bao CJ, Zhang HJ, Yang Y, Xu K, Wang GL, Zhao M, et al. Characterization of antibody and memory T-cell response in H7N9 survivors: a cross-sectional analysis. Clin Microbiol Infect. 2020;26(2):247–54. doi:10.1016/j.cmi.2019.06.013.
- Gou X, Wu X, Shi Y, Zhang K, Huang J. A systematic review and meta-analysis of cross-reactivity of antibodies induced by H7 influenza vaccine. Human Vaccines Immun. 2020;16(2):286–94. doi:10.1080/21645515.2019.1649551.
- Xiang N, Bai T, Kang K, Yuan H, Zhou S, Ren R, Li X, Wu J, Deng L, Zeng G, et al. Sero-epidemiologic study of influenza A(H7N9) infection among exposed populations, China 2013-2014. Influenza Other Respi Viruses. 2017;11(2):170–76. doi:10.1111/irv.12435.
- Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol. 2015;15(3):149–59. doi:10.1038/nri3802.
- Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, Zheng NY, Mays I, Garman L, Helms C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–71. doi:10.1038/nature06890.
- Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109(23):9047–52. doi:10.1073/pnas.1118979109.
- Krystal M, Elliott RM, Benz EW Jr, Young JF, Palese P. Evolution of influenza a and B viruses: conservation of structural features in the hemagglutinin genes. Proc Natl Acad Sci U S A. 1982;79(15):4800–04. doi:10.1073/pnas.79.15.4800.
- Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Sci. 2009;324(5924):246–51. doi:10.1126/science.1171491.
- Asthagiri Arunkumar G, McMahon M, Pavot V, Aramouni M, Ioannou A, Lambe T, Gilbert S, Krammer F. Vaccination with viral vectors expressing NP, M1 and chimeric hemagglutinin induces broad protection against influenza virus challenge in mice. Vaccine. 2019;37(37):5567–77. doi:10.1016/j.vaccine.2019.07.095.
- Mesin L, Ersching J, Victora GD. Germinal center B cell dynamics. Immun. 2016;45(3):471–82. doi:10.1016/j.immuni.2016.09.001.
- McGuire HM, Vogelzang A, Warren J, Loetsch C, Natividad KD, Chan TD, Brink R, Batten M, King C. IL-21 and IL-4 collaborate to shape T-dependent antibody responses. J Immunol. 2015;195(11):5123–35. doi:10.4049/jimmunol.1501463.
- Huang KY, Rijal P, Schimanski L, Powell TJ, Lin TY, McCauley JW, Daniels RS, Townsend AR. Focused antibody response to influenza linked to antigenic drift. J Clin Invest. 2015;125(7):2631–45. doi:10.1172/JCI81104.
- Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, et al. A highly conserved neutralizing epitope on group 2 influenza a viruses. Sci. 2011;333(6044):843–50. doi:10.1126/science.1204839.
- Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza a hemagglutinins. Sci. 2011;333(6044):850–56. doi:10.1126/science.1205669.
- Wang TT, Tan GS, Hai R, Pica N, Petersen E, Moran TM, Palese PM, Fouchier RA. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 2010;6(2):e1000796. doi:10.1371/journal.ppat.1000796.
- Linderman SL, Hensley SE, Sant AJ. Antibodies with ‘original antigenic sin’ properties are valuable components of secondary immune responses to influenza viruses. PLoS Pathog. 2016;12(8):e1005806. doi:10.1371/journal.ppat.1005806.
