ABSTRACT
Human papillomavirus (HPV) vaccination is recommended for U.S. adolescents at ages 11–12 requiring two or three doses depending on if the vaccine series started before age 15. The objective was to compare HPV vaccination rates among medically underserved, economically disadvantaged, students in rural middle school districts (Rio Grande Valley [RGV], Texas) by age of initiation (≤ age 11 years vs. age 12 years and older). This quasi-experimental study included 1,766 students (884 females; 882 males) who received at least one HPV vaccine dose through our school-based vaccination program between 08/2016-06/2022. Summary statistics were stratified by age at initiation and gender. The overall HPV up-to-date (UTD) rate was 59.7% (95% Confidence Interval: 57.4–62.0%). The median age at HPV UTD (range) was 12 years (9–19) and median interval between HPV vaccine doses (range) was 316 days (150–2,855). Most students received the HPV vaccine bundled with other vaccinations (72.4%, 1,279/1,766). There was a higher HPV UTD rate among students who initiated the HPV vaccine on or before age 11 than those who initiated on or after age 12 (73.6% versus 45.1%, respectively). The median age of HPV UTD was age 12 for those initiating on or before 11 years versus age 13 for those initiating on or after 12 years of age. Initiating the HPV vaccine at age ≤11 years increased completion of the HPV vaccine series. Improving HPV vaccine coverage and introduction of pan-gender vaccination programs will significantly decrease HPV-related diseases in the RGV.
Introduction
HPV vaccination rates remain suboptimalCitation1 despite the evidence supporting the human papillomavirus (HPV) vaccine as a safe and effective strategy for reducing the morbidity and mortality of HPV-associated diseases at the population-level.Citation2–12 Routine HPV vaccination in the United States (US) has been recommended for females since 2006 and 2011 for males aged 9–26 years. Although HPV vaccination is recommended for adolescents aged 11–12, it can be initiated as early as 9 years of age.Citation13 If HPV vaccination rates rose to 80%, 53,000 more cervical cancer cases would be prevented over the lifetime of those aged ≤12 years.Citation14,Citation15
Texas continues to have a 10% lower HPV vaccine uptake than the rest of the nation. Texas ranks 47th in terms of HPV UTD vaccinations out of 50 states and the District of Columbia.Citation16 Due to HPV-associated cancers and lower HPV vaccination rates,Citation10–Citation17–20 offering the HPV vaccine at no cost is important in rural, medically underserved settings in Texas, such the Rio Grande Valley (RGV). The RGV consists of four counties bordering Mexico (Cameron, Hidalgo, Starr and Willacy Counties) with some of the worst health and economic disparities in the nation.Citation21 Hispanics are at higher risk for HPV-associated cancers. Culturally appropriate interventions and survey methods are needed to increase HPV uptake and improve efforts in engaging with this population.Citation22–25
Introduction of the HPV vaccine in a school-based setting provides a rare opportunity to build and strengthen adolescent health.Citation12 Research has shown that parents play a pivotal role in HPV vaccine uptakeCitation26 and healthcare providers play crucial roles in ensuring its administration.Citation27–34 The target age group (ages 11–12) presents particular challenges including more scrutiny of HPV vaccines than traditional childhood vaccines, e.g., age, sexual activity, and safety concerns.Citation12 The study objective is to evaluate how a community-based education and school-based HPV vaccination program increased HPV vaccination rates among medically underserved, economically disadvantaged, students in rural middle school districts (Rio Grande Valley [RGV], Texas) by age of initiation (≤ age 11 years vs. age 12 years and older).
Methods
This quasi-experimental study is part of a larger funded project evaluating an intervention program to increase HPV vaccine uptake in the RGV (Texas) to meet the 2016 National Immunization Survey – Teen HPV vaccination rates (initiation: 49.3%; completion: 32.9%)Citation35–37 To be included in this study, students had to receive at least one HPV vaccine dose from our school-based vaccination program (vaccination events). The study outcomes included HPV vaccine initiation and HPV up-to-date (UTD) status. HPV vaccine initiation was defined as receipt of the first dose of the HPV vaccine series. HPV UTD was defined as receipt of ≥ 3 doses if initiated after age 15 years or had immunocompromising conditions or receipt of 2 doses if initiated before age 15 years, with the minimum interval of 5 months between the first and second dose.Citation38
As described previously, the intervention combined community-based HPV education with school-based vaccinations Citation35–37 at middle schools to increase HPV vaccine uptake in the RGV. The intervention addressed factors affecting HPV vaccine uptake (e.g., social norms, knowledge, health provider recommendations and risk perception, accessibility, schedule, costs, bundling vaccines).Citation18,Citation26,Citation27,Citation31,Citation32,Citation36,Citation39,Citation40,Citation41 The physician-led educational events started in August 2016 in Cameron, Hidalgo, and Starr counties (located in a 15-mile radius encompassing the original intervention site, the Rio Grande City Independent School District [RGCISD] in Starr County) while the PSJA ISD school-based vaccination program (Hidalgo County) began in June 2019 with total enrollment of 6,481 students.Citation36 Approval for this program was obtained from the University of Texas Medical Branch’s Institutional Review Board, and RGCCISD, PSJA ISD, Roma Independent School District (Roma ISD) and Zapata County Independent School District (Zapata County ISD) School Boards. Informed consent was required for middle school students to be vaccinated.
The study period was from 1 September 2016 to 30 June 2022. Between June 2019 and June 2022, the school-based vaccination was implemented in PSJA ISD (starting with largest student enrollment schools in closest proximity to RGCCISD: August 2019 for Phase 1 [3 middle schools]; August 2020 for Phase 2 [3 middle schools]; and February 2021 for Phase 3 [2 middle schools]).Citation36 In August 2020, the school-based vaccination program was implemented in 2 middle schools in Roma ISD (Starr County) and 1 middle school in Zapata County ISD. Students were recruited through the four school districts. We collaborated with community and public health organizations to actively promote the school-based HPV vaccination program through stakeholder/PTA/school board meetings, social media, and radio. Although the target population included RGCCISD, PSJA ISD, Roma ISD, and Zapata County ISD middle school students, students who came to vaccination events that met the age criteria received HPV vaccinations. HPV vaccine series were initiated and completed during the school year at back-to-school events, progress report nights, and preview events. Up to 5 reminder letters, texts, and phone calls for subsequent doses were sent to the parents/guardians of children who initiated HPV vaccination. To ensure on-time vaccination and adherence to the dosing schedule, catch-up vaccination was scheduled through nearby clinics when requested by parents and subsequent events for missed doses.
We continued to strengthen our implementation strategies by having physicians addressing the audience, targeting female and male middle school students at the recommended ages (aged 11–12 years of age), bundling the HPV vaccine with recommended vaccines (e.g., flu, Meningococcal, Meningitis B, Tetanus, Diphtheria [TD], or Tetanus, Diphtheria, and Pertussis [TDAP] and Hepatitis A vaccines), addressing previously identified barriers, and extending the study area for school-based vaccinations from RGCCISD to PSJA ISD, Roma ISD, and Zapata County ISD.Citation26,Citation27,Citation31,Citation32,Citation35–42 Prior to coronavirus disease 2019 (COVID-19), school-based vaccination events were held in the nurses’ offices, conference rooms, nearby clinics at parents’ requests, and community events. When the COVID-19 pandemic hit in the middle of the first year of the school-based vaccination program and caused school closures, adaptions were made. We held outside events with social distancing, limited in-person activities, increased online activities, and provided more frequent stakeholder engagement through teleconference, navigational services, and mobile van vaccinations.Citation36
The HPV vaccination data was refreshed quarterly. It was collected from the vaccine vendor and school immunization records and reconciled with Immtrac2, Texas Immunization Registry. The registry is secure and confidential, and safely consolidates and stores immunization records from multiple sources in one centralized system. Summary statistics were computed and stratified by gender, age of initiation (9, 10, 11, 12, 13, 14+), and vaccination year. Assuming a small effect size (W = 0.1) and equal sample size in the two groups (Age of Initiation: ≤11 years vs. 12+ years) using Power Analysis and Sample Size (PASS 2022), a sample size of 786 achieves 80% power to detect a small effect size (W = 0.1) using Chi-Square Test with a significance level (alpha) of 0.05. SAS version 9.4 (SAS Institute Inc, Cary, NC) was used in conducting all analyses. Statistical significance was set at α|=|.05 (two-sided).
Results
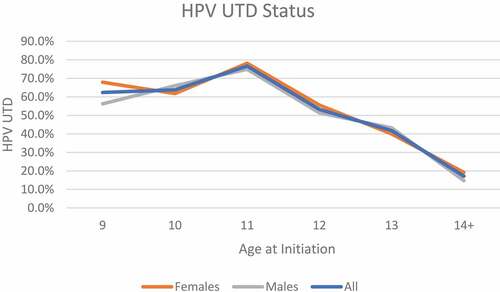
displays the demographic characteristics of the study population and HPV vaccination rates by gender. At baseline, 6,481 students were enrolled at PSJA ISD. Among 1,766 middle school students (females n = 884; males n = 882) who received at least one HPV vaccine dose through our school-based vaccination program, most were from RGCCISD (47.8%; 844/1,766) and PSJA ISD (46.8%; 827/1,766). Most middle school students initiated the HPV vaccine at age 11 (39.5%, 698/1,766) or age 12 (30.5%, 539/1,766) (). The overall HPV UTD rate was 59.7% (95% Confidence Interval: 57.4–62.0%). Overall, the median age at HPV UTD (range) was 12 years (9–19). The median days between HPV vaccine doses (range) was 316 days (150–2,855). Among the 1,766 middle school students who received the HPV vaccine, 72.4% (1,279/1,766) had received the HPV vaccine bundled with other recommended vaccinations (). The percentage of students who received their HPV vaccine with other recommended vaccinations were similar across female and male students (72.3% vs. 72.6%).
Table 1. Students’ Characteristics and HPV Vaccination Rates by Gender.
Most students initiated the HPV vaccine at ≤ age 11 years (n = 904) (). Regardless of gender, a higher percentage of students who initiated the HPV vaccine at age 9, age 10 or age 11 were HPV UTD compared to those who initiated at age 12 or older (). Almost 74% (665/904) of those who initiated the HPV vaccine at ≤ age 11 completed the HPV vaccine (results not shown). As shown in , HPV UTD was highest among middle school students who initiated the HPV vaccine at age 11 years (39.5%). A higher percentage of females were HPV UTD compared to males (30.9% versus 28.8%, respectively). A total of 195 students had received ≥3 HPV vaccine doses (11.0%; n = 195). Overall, 12.9% (114/884) of females who initiated the HPV vaccine received ≥3 HPV vaccine doses, while 9.2% (81/882) of males who initiated the HPV vaccine received ≥3 HPV vaccine doses.
Table 2. Summary of HPV Vaccine Initiation and HPV UTD by Gender.
shows students who initiated the HPV vaccine on or before age 11 had a higher HPV UTD (completion) rate than those who initiated the HPV vaccine on or after age 12 (73.6% versus 45.1%, respectively). The median age of HPV UTD or completion was age 12 for those initiating on or before 11 years old, and age 13 for those initiating on or after 12 years of age. The median (range) days between HPV vaccine doses was longer among those who initiated the HPV vaccine on or before age 11 years (358 days, 158–2,855) compared to those who initiated on or after age 12 years (268 days, 153, 2,016).
Table 3. HPV Vaccine Completion by Age at Initiation.
Discussion
Areas with higher incidence and mortality of HPV-related diseases and cancers, lower rates of Pap test screening and lower levels of HPV vaccination make these areas up at risk for continued higher rates of HPV-related cancers and diseases. This study provides information on how community-based HPV education and school-based vaccination program can influence HPV vaccine initiation and completion rates. Hence, potentially decrease future HPV-related disease prevalence in the area.
A total of 1,776 middle school students received at least one dose through our vaccination events. Almost 74% (665/904) of those who initiated the HPV vaccine at ≤ age 11 completed the HPV vaccine. We had students completing their HPV vaccination series at the recommended ages. Overall, the HPV UTD rate was 59.7% (95% Confidence Interval: 57.4–62.0%).
The results provide evidence supporting how initiation of the HPV vaccine series prior to age 11 improves population-level HPV vaccination coverage and timely completion of the series within a school year. The median age at HPV UTD (range) was 12 years (9–19). Most students initiated the HPV vaccine at ≤ age 11 years (n = 904). If they initiated the HPV vaccine series at younger ages (age 9, 10, or 11), they had higher rates of HPV UTD. The interval between vaccine doses were also in line in HPV vaccine guidelines and bundling with other vaccines increased HPV vaccine uptake. The median interval between HPV vaccine doses (range) was 316 days (150–2,855). Among students who received the HPV vaccine through our intervention, 72.4% (1,279/1,766) had received the HPV vaccine bundled with other recommended vaccinations.
Improving the timeliness of HPV vaccination is critical for protecting adolescents prior to HPV exposure. Adherence to the recommended dosing schedule for HPV vaccine is also important for adequate immune response and expected protection from HPV-associated diseases.Citation43 Since younger adolescents have a better immunologic response to the HPV vaccine, this may translate into improved effectiveness.Citation44 Emphasizing routine administration of the HPV vaccination at age 11–12 has shown to increase parents’ preference for on-time vaccination and improves adherence to the dose scheduling.Citation43,Citation45 Previous research has shown that framing recommendations involving fewer doses before age 15 may discourage on-time HPV vaccination and suggest to parents that routine HPV vaccine administration extends to this age.Citation45
Our study supports the importance of simplifying the messaging for HPV vaccination and recommending HPV vaccine initiation at younger ages.Citation43,Citation45 We emphasized HPV vaccination as cancer prevention and discuss the improved effectiveness of HPV vaccination at younger ages. Our study contributes to previous research by examining HPV vaccination rates on and before age 11 and 12 years and older through a school-based education and vaccination program in the RGV. Our findings suggest that adherence to the recommended dosing schedule remains relatively low among older adolescents. Therefore, findings from this research are important for the improvement in removing barriers to on-time vaccination and adherence to the HPV vaccine schedule.Citation46,Citation47
Our results must be considered in light of certain limitations. Limited information was collected on students and parents, such as students’ race and ethnicity and other socioeconomic status (SES), parents’ education and income levels, country of birth or knowledge and confidence in the HPV vaccines. Therefore, examination of rates by these important characteristics cannot be undertaken. We do not have complete information on students’ insurance, which may be important for examining access to care. This population is transient with some students changing schools during our study. For simplification, we followed our baseline cohort. Some students may have received the HPV vaccine outside the school settings through their local providers. If parents failed to report the HPV vaccine status to the schools, we would be unable to account for those in our study. The vendor and schools shared updated information, but it may not capture all vaccines received.Citation47 In addition, we suspect that the increased HPV vaccine uptake at the intervention school may be due to more motivation to share updated records because of onsite events, more exposure to study personnel, and better access to vaccinations. Future studies should explore issues, such as inadequate school-based health centers and vaccine billing as barriers for school-based HPV programs.Citation48
Conclusions
Initiating the HPV vaccine at age ≤11 years increased completion of the HPV vaccine series. Our community-based education and school-based intervention program provides the HPV vaccine in an alternative setting (schools), increases access and support through education and outreach, and encourages on-time HPV vaccination and completion. Improving HPV vaccine coverage and introduction of pan-gender vaccination programs will significantly decrease HPV-related diseases in the RGV. Given the positive influence of healthcare providers on parental decisions to vaccinate, future studies should target under recommended groups, men and Hispanics, to increase knowledge and awareness about HPV, the HPV vaccine, and HPV-associated cancers and promote greater HPV vaccine uptake and reduce parental hesitancy.
List of abbreviations
| ACIP | = | Advisory Committee on Immunization Practices |
| CDC | = | Centers for Disease Control and Prevention |
| COVID-19 | = | Coronavirus Disease 2019 |
| HPV | = | Human Papillomavirus |
| PSJA ISD | = | Pharr-San Juan-Alamo Independent School District |
| RGV | = | Rio Grande Valley |
| RGCCISD | = | Rio Grande City Consolidated Independent School District |
| Roma ISD | = | Roma Independent School District |
| TDAP | = | Tetanus, Diphtheria (TD), or Tetanus, Diphtheria, and Pertussis |
| U.S. | = | United States |
| UTD | = | Up-to-date |
| VFC | = | Vaccines for Children |
| Zapata County ISD | = | Zapata County Independent School District |
Author contributions
All authors contributed to the conceptualization of this manuscript. AMR and TQD wrote the first draft of the manuscript, and all authors contributed to the editing and finalization of the manuscript.
Acknowledgments
The authors are incredibly grateful for the support and assistance from the School Superintendents and School Boards for RGCCISD, PSJA ISD, Roma ISD, and Zapata County ISD, faculty/staff, school nurses, parents, and the RGV community (Cameron. Hidalgo, and Starr Counties) in implementing this project. We also thank Iris L. Tijerina, Iris I. Rivera, Nadia Garces, Maria F. Lincoln, and Jesus Moralez from the University of Texas Medical Branch for their work and involvement in this project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adjei Boakye E, Tobo BB, Rojek RP, Mohammed KA, Geneus CJ, Osazuwa-Peters N. Approaching a decade since HPV vaccine licensure: racial and gender disparities in knowledge and awareness of HPV and HPV vaccine. Hum Vaccin Immunother. 2017 11;13(11):1–7. doi:10.1080/21645515.2017.1363133.
- Dochez C, Bogers JJ, Verhelst R, Rees H. HPV vaccines to prevent cervical cancer and genital warts: an update. Vaccine. 2014 Mar 20;32(14):1595–601. doi:10.1016/j.vaccine.2013.10.081.
- Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. 2011 Mar 2;103(5):368–83. doi:10.1093/jnci/djq562.
- Thomas TL, Strickland O, Diclemente R, Higgins M. An opportunity for cancer prevention during preadolescence and adolescence: stopping human papillomavirus (HPV)-related cancer through HPV vaccination. J Adolesc Health. 2013 May;52(5, Supplement):S60–68. doi:10.1016/j.jadohealth.2012.08.011.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999 Sep;189(1):12–19. doi:10.1002/(sici)1096-9896(199909)189:1<12:aid-path431>3.0.co;2-f.
- Emiko Petrosky M, Bocchini JA Jr, Hariri S, Chesson H, Curtis CR, Saraiya M. Basic information about HPV-associated cancers. Centers for disease control and prevention. [accessed 2014 Aug 24]. https://www.cdc.gov/cancer/hpv/basic_info/
- Centers for Disease Control and Prevention. HPV-Associated cancers statistics; 2015. http://www.cdc.gov/cancer/hpv/statistics/index.htm
- Centers for Disease Control and Prevention Petrosky M, Bocchini JA Jr, MD, Hariri S PhD, Chesson H PhD, Curtis CR MD, Saraiya M MD, Unger ER MD, Markowitz LE MD. Use of 9-Valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–04. 25811679.
- Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini Jr. JA, Unger ER. Centers for Disease Control and Prevention . Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations Rep. 2014 Aug 29;63(RR–05):1–30.
- Shah PD, Gilkey MB, Pepper JK, Gottlieb SL, Brewer NT. Promising alternative settings for HPV vaccination of US adolescents. Expert Rev Vaccines. 2014 Feb;13(2):235–46. doi:10.1586/14760584.2013.871204.
- Lee LY, Garland SM. Human papillomavirus vaccination: the population impact. F1000res. 2017;6:866. doi:10.12688/f1000research.10691.1.
- Bloem P, Ogbuanu I. Vaccination to prevent human papillomavirus infections: from promise to practice. PLoS Med. 2017 Jun;14(6):e1002325. doi:10.1371/journal.pmed.1002325.
- Meites E , Kempe A , Markowitz LE. Use of a 2-dose schedule for human papillomavirus vaccination - Updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–08.
- Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013 - United States. MMWR Morb Mortal Wkly Rep. 2013 Jul 26;62(29):591–95.
- President’s Cancer Panel Annual Report. 2012–2013. Accelerating HPV vaccine uptake: urgency for action to prevent cancer. [accessed 2018 Aug 29] http://deainfo.nci.nih.gov/advisory/pcp/annualReports/HPV/ExecutiveSummary.htm#sthash.PcA0EnNk.dpbs
- Walker TY, Elam-Evans LD, Singleton JA, Yankey D, Markowitz LE, Fredua B, Williams CL, Meyer SA, Stokley S. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2016. MMWR Morb Mortal Wkly Rep. 2017 Aug 25;66(33):874–82. doi:10.15585/mmwr.mm6633a2.
- Vanderpool RC, Stradtman LR, Brandt HM. Policy opportunities to increase HPV vaccination in rural communities. Hum Vaccines Immunotherapeutics. 2019;15(7–8):1527–32. doi:10.1080/21645515.2018.1553475.
- Brandt HM, Vanderpool RC, Pilar M, Zubizarreta M, Stradtman LR. A narrative review of HPV vaccination interventions in rural U.S. communities. Prev Med. 2021 Apr;145:106407. doi:10.1016/j.ypmed.2020.106407.
- Sanderson M, Coker AL, Eggleston KS, Fernandez ME, Arrastia CD, Fadden MK. HPV vaccine acceptance among Latina mothers by HPV status. J Women’s Health. 2009;18(11):1793–99. doi:10.1089/jwh.2008.1266.
- Center for Reproductive Rights. Nuestro Voz, Nuestro Salud, Nuestro Texas: The Fight for Women’s Reproductive Health in the Rio Grande Valley; 2015. http://www.nuestrotexas.org/pdf/NT-spread.pdf
- AIDS Education and Training Centers National Coordinating Resource Center (AETC NCRC) Overview of HIV/AIDS in the Texas-Mexico Border Region. 2015. http://aidsetc.org/border/profile-texas
- Brown A. The unique challenges of surveying U.S. Latinos. 2015. http://assets.pewresearch.org/wp-content/uploads/sites/12/2015/11/2015-11-12_surveying-us-latinos.pdf
- Evans B, Quiroz RS, Athey L, McMichael J, Albright V, O‘Hegarty M, Caraballo RS . Customizing survey methods to the target population - Innovative approaches to improving; 2008. https://www.rti.org/sites/default/files/resources/evans_aapor08_paper.pdf
- O’Hegarty M, Pederson LL, Thorne SL, Caraballo RS, Evans B, Athey L, McMichael J. Customizing survey instruments and data collection to reach Hispanic/Latino adults in border communities in Texas. Am J Public Health. 2010 Aug 25;100(Suppl S1):S159–64. doi:10.2105/AJPH.2009.167338.
- CDC. HPV-associated cancers rates by race and ethnicity. Centers for disease control and prevention; 2018 [updated 2017 July 17]; [accessed 2018 June 12]. https://www.cdc.gov/cancer/hpv/statistics/race.htm
- Newman PA, Logie CH, Lacombe-Duncan A, Baiden P, Tepjan S, Rubincam C, Doukas N, Asey F. Parents’ uptake of human papillomavirus vaccines for their children: a systematic review and meta-analysis of observational studies. BMJ Open. 2018 Apr 20;8(4):e019206. doi:10.1136/bmjopen-2017-019206.
- Anderson A, Taylor Z, Georges R, Carlson-Cosentino M, Nguyen L, Salas M, Vice A, Bernal N, Bhaloo T . Primary care physicians’ role in parental decision to vaccinate with HPV vaccine: learnings from a South Texas Hispanic patient population. J Immigr Minor Health. 2018 Oct;20(5):1236–42. doi:10.1007/s10903-017-0646-9.
- Furgurson KF, Sandberg JC, Hsu FC, Mora DC, Quandt SA, Arcury TA. HPV knowledge and vaccine initiation among Mexican-Born farmworkers in North Carolina. Health Promot Pract. 2018 Mar 1;20(3):1524839918764671. doi:10.1177/1524839918764671.
- Fleming WS, Sznajder KK, Nepps M, Boktor SW. Barriers and facilitators of HPV vaccination in the VFC program. J Community Health. 2018 Jun;43(3):448–54. doi:10.1007/s10900-017-0457-x.
- Sherman SM, Nailer E. Attitudes towards and knowledge about Human Papillomavirus (HPV) and the HPV vaccination in parents of teenage boys in the UK. PLoS One. 2018;13(4):e0195801. doi:10.1371/journal.pone.0195801.
- Donahue KL, Hendrix KS, Sturm LA, Zimet GD. Human papillomavirus vaccine initiation among 9-13-year-olds in the United States. Prev Med Rep. 2015 Jan 01;2:892–98. doi:10.1016/j.pmedr.2015.10.003.
- Brown B, Gabra MI, Pellman H. Reasons for acceptance or refusal of Human Papillomavirus Vaccine in a California pediatric practice. Papillomavirus Res. 2017 Jun;3:42–45. doi:10.1016/j.pvr.2017.01.002.
- Clark SJ, Cowan AE, Filipp SL, Fisher AM, Stokley S. Understanding non-completion of the human papillomavirus vaccine series: parent-reported reasons for why adolescents might not receive additional doses, United States, 2012. Public Health Rep. 2016 May-Jun;131(3):390–95. doi:10.1177/003335491613100304.
- Rutten LJ, St Sauver JL, Beebe TJ, Wilson PM, Jacobson DJ, Fan C, Breitkopf CR, Vadaparampil ST, Jacobson RM. Clinician knowledge, clinician barriers, and perceived parental barriers regarding human papillomavirus vaccination: association with initiation and completion rates. Vaccine. 2017 Jan 3;35(1):164–69. doi:10.1016/j.vaccine.2016.11.012.
- Rodriguez AM, Do TQN, Jibaja-Weiss ML, Chen L, Schmeler KM, Montealnegre JR, KuoYK . Human papillomavirus vaccinations during the COVID-19 pandemic in middle schools in the Rio Grande Valley of Texas. Am J Public Health. 2022;112(9):1269–1272. doi:10.2105/ajph.2022.306970.
- Kaul S, Do TQN, Hsu E, Schmeler KM, Montealegre JR, Rodriguez AM. School-based human papillomavirus vaccination program for increasing vaccine uptake in an underserved area in Texas. Papillomavirus Res. 2019;8(12):100189. doi:10.1016/j.pvr.2019.100189.
- Victory M, Do TQN, Kuo Y-F, Rodriguez AM. Parental knowledge gaps and barriers for children receiving human papillomavirus vaccine in the Rio Grande Valley of Texas. Hum Vaccines Immunotherapeutics. 2019;15(7–8):1678–87. doi:10.1080/21645515.2019.1628551.
- Meites ESP, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. Morbidity Mortality Weekly Rep. 2019;68(32):698–702. Centers for Disease Control and Prevention. external icon. http://dx.doi.org/10.15585/mmwr.mm6832a3
- Henry KA, Swiecki-Sikora AL, Stroup AM, Warner EL, Kepka D. Area-based socioeconomic factors and human papillomavirus (HPV) vaccination among teen boys in the United States. BMC Public Health. 2017 07;18(1):19. doi:10.1186/s12889-017-4567-2.
- Kornides ML, McRee AL, Gilkey MB. Parents who decline HPV vaccination: who later accepts and Why? Acad Pediatr. 2018 Mar;18(2S):S37–43. doi:10.1016/j.acap.2017.06.008.
- Daley MF, Kempe A, Pyrzanowski J, Vogt TM, Dickinson LM, Kile D, Fang H, Rinehart DJ, Shlay JC. School-located vaccination of adolescents with insurance billing: cost, reimbursement, and vaccination outcomes. J Adolesc Health. 2014 Mar;54(3):282–88. doi:10.1016/j.jadohealth.2013.12.011.
- Szilagyi PG, Humiston SG, Gallivan S, Albertin C, Sandler M, Blumkin A. Effectiveness of a citywide patient immunization navigator program on improving adolescent immunizations and preventive care visit rates. Arch Pediatr Adolesc Med. 2011 Jun;165(6):547–53. doi:10.1001/archpediatrics.2011.73.
- Ejezie CL, Osaghae I, Ayieko S, Cuccaro P. Adherence to the recommended HPV vaccine dosing schedule among adolescents aged 13 to 17 years: findings from the national immunization survey-teen, 2019–2020. Vaccines (Basel). 2022 Apr 8;10(4):577. doi:10.3390/vaccines10040577.
- Iversen OE, Miranda MJ, Ulied A, Sortel T, Lazarus E, Chokephaibulkit K, Block SL, Skrivanek A, Azurah AGN, Fong SM . Immunogenicity of the 9-Valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. Comparative study multicenter study randomized controlled trial research support, Non-U.S. Gov’t. JAMA. 2016;316(22):2411–21. doi:10.1001/jama.2016.17615.
- Margolis MA, Brewer NT, Shah PD, Calo WA, Alton Dailey S, Gilkey MB. Talking about recommended age or fewer doses: what motivates HPV vaccination timeliness? Hum Vaccin Immunother. 2021 Sep 2;17(9):3077–80. doi:10.1080/21645515.2021.1912550.
- Rimer B, Harper H, Witte O. Accelerating HPV vaccine uptake: urgency for action to prevent cancer; a report to the president of the United States from the president’s cancer panel. Bethesda, MD: National Cancer Institute; 2014.
- Ashrawi D, Javaid M, Stevens L, Bello R, Ramondetta L. HPV vaccine uptake in Texas pediatric care settings: 2014-2015. 2015. (Houston, TX: MD Anderson Cancer Center). https://www.texascancer.info/pdfs/hpvenvironmentalscanreport.pdf
- Kempe A, Allison MA, Daley MF. Can school-located vaccination have a major impact on human papillomavirus vaccination rates in the United States? Acad Pediatr. 2018 Mar;18(2s):S101–s105. doi:10.1016/j.acap.2017.08.010.