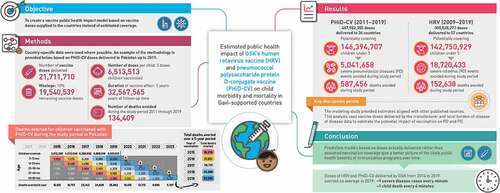
ABSTRACT
Vaccine impact models against rotavirus disease (RD) and pneumococcal disease (PD) in low- and middle-income countries assume vaccine coverage based on other vaccines. We propose to assess the impact on severe disease cases and deaths avoided based on vaccine doses delivered by one manufacturer to Gavi-supported countries. From the number of human rotavirus vaccine (HRV) and pneumococcal polysaccharide protein D-conjugate vaccine (PHiD-CV) doses delivered, we estimated the averted burden of disease 1) in a specific year and 2) for all children vaccinated during the study period followed-up until 5 years (y) of age. Uncertainty of the estimated impact was assessed in a probabilistic sensitivity analysis using Monte-Carlo simulations to provide 95% confidence intervals. From 2009 to 2019, approximately 143 million children received HRV in 57 Gavi-supported countries, avoiding an estimated 18.7 million severe RD cases and 153,000, deaths. From 2011 to 2019, approximately 146 million children received PHiD-CV in 36 countries, avoiding an estimated 5.0 million severe PD cases and 587,000 deaths. The number of severe cases and deaths averted for all children vaccinated during the study period until 5 years of age were about 23.2 million and 190,000, respectively, for HRV, and 6.6 million and 749,000, respectively, for PHiD-CV. Models based on doses delivered help to assess the impact of vaccination, plan vaccination programs and understand public health benefits. In 2019, HRV and PHiD-CV doses delivered over a 5-y period may have, on average, averted nine severe disease cases every minute and one child death every 4 min.
Plain Language Summary
What is the context?
The WHO added the pneumococcal conjugate vaccine and the rotavirus vaccine in the recommended vaccination schedule of all countries in 2007 and 2009, respectively.
Previous studies estimated the public health benefit of these vaccines by approximating the number of children who received them.
What is new?
We used an alternative approach to estimate the benefit based on actual number of doses of the vaccines, human rotavirus vaccine (HRV; Rotarix) and pneumococcal polysaccharide protein D-conjugate vaccine (PHiD-CV; Synflorix) delivered to each country considered.
The study analyzed data from children under 5 years of age in 60 Gavi-supported countries by identifying the number of vaccine doses delivered, estimating the number of children fully covered, applying the country-specific disease epidemiology, estimating the number of severe disease cases and deaths avoided.
From 2009 to 2019, approximately 143 million children were vaccinated with HRV avoiding an estimated 18.7 million severe rotavirus disease cases and 153,000 deaths.
From 2011 to 2019, about 146 million children were vaccinated with pneumococcal vaccine avoiding an estimated 5.0 million severe pneumococcal disease cases and 587,000 deaths.
What is the impact?
The benefit of HRV and PHiD-CV in Gavi-supported countries is often estimated based on assumptions of vaccine coverage rates.
A modeling approach based on doses delivered by the vaccine manufacturer can provide an additional view on the potential vaccine benefits and improve planning, contribution, and sustainability of the immunization programs at a country level.
In 2019, HRV and PHiD-CV together averted nine cases of severe disease each minute and one child death every 4 minutes.
Graphical Abstract
Introduction
Vaccination is estimated to save up to 3 million lives every year and is considered to be the most successful and most cost-efficient public health intervention.Citation1 Its implementation worldwide has been achieved through the Expanded Programme on Immunization (EPI) that was initiated by the World Health Organization (WHO) in 1974 and provided universal immunization of essential vaccines to young children. The program started with six classic infant vaccines against diphtheria, pertussis, tetanus, measles, poliomyelitis and tuberculosis. It now also includes vaccines against hepatitis B, rubella, rotavirus disease (RD) (the leading cause of diarrhea), pneumococcal disease (PD) and Haemophilus influenzae type b (Hib) (two major causes of bacterial meningitis, sepsis, pneumonia and acute otitis media).Citation2
The EPI is built upon the collaboration of a wide range of stakeholders including people on the ground delivering the vaccines, EPI managers, government officials, regulatory authorities, vaccine manufacturers, and international organizations such as the United Nations Children’s Fund (UNICEF) and Gavi, the Vaccine Alliance. Gavi and UNICEF are playing major roles in facilitating funding and the procurement of vaccines to EPI. Gavi was founded in 1999 as a public−private program with the aim of improving the immunization coverage of children living in the poorest countries of the world and to accelerate access to new vaccines.
Fifty-seven countries in the world in 2021 are eligible to apply for Gavi support based on their gross national income, which must be below the threshold of 1,630 US$/capita.Citation3 Approximately 80 million children (or 60% of the global annual birth cohort) are born every year in Gavi-supported countries.Citation4 In reality, Gavi supports 73 countries because some countries continue to receive Gavi funding based on previous eligibility. Once countries have graduated from Gavi support, they are responsible for fully self-financing vaccine costs, with the exception of pneumococcal vaccine (PCV) under the Advance Market Commitment and with access to the reduced Gavi price for some antigens. Research done by Gavi and by independent study groups has shown that the organization has helped countries to substantially improve their vaccine coverage, which resulted in reductions of mortality caused by targeted infectious diseases.Citation5–9 The health impact of childhood vaccination programs against 10 pathogens in 98 low- and middle-income countries has been evaluated using model-based disease burden estimates.Citation10 Increases in vaccine coverage and the introduction of new vaccines were estimated to result in a 45% reduction of deaths associated with the 10 pathogens from 2000 to 2019 compared with no vaccination.Citation10 Moreover, for the 2019 annual birth cohort, vaccination was estimated to have prevented 72% of such deaths.Citation10
The leading causes of death in children under 5 years (y) of age globally are pneumonia (15%), diarrhea (8%), and malaria (5%), most of which occur in low-income countries.Citation11 The WHO has estimated that pneumonia only killed more than 808,000 children under 5 y in 2017.Citation12 Estimates for RD deaths (the main cause of diarrhea for the same age group) were between 122,000 and 215,000 in 2013.Citation13,Citation14 Approximately half of all the PD and RD associated deaths occurred in just four countries: India, Nigeria, the Democratic Republic of the Congo and Pakistan.Citation13
Gavi and UNICEF have supported vaccine programs against RD since 2007 and against PD since 2009, but the vaccines preventing these two diseases are more costly than those using earlier antigens. By the end of 2019, Gavi had supported 48 countries to immunize more than 125 million children against RD and 60 countries to immunize more than 215 million children against PD.Citation4 One of the major supplier of vaccines to these countries via UNICEF, as part of the Gavi-supported country programs, is GSK that also has a large contribution of newer antigens such as the human rotavirus vaccine (HRV; Rotarix) against RD and the pneumococcal polysaccharide protein D-conjugate vaccine (PHiD-CV; Synflorix) against PD. Currently, in Gavi-supported countries, 88% of the demand for RD vaccine is covered by HRV, while approximately half of the demand for PD vaccines is covered by PHiD-CV.Citation15,Citation16 In this study, we estimated the public health benefit of HRV and PHiD-CV for children under 5 y of age in Gavi-supported countries. The impact reported in the literature has often been estimated based on assumed approximations of vaccine coverage rates. In the present analysis, we had access to the number of vaccine doses delivered by the manufacturer to each country. This provides additional information on coverage rates that might in turn result in more precise impact estimates. Our analysis and reporting are descriptive, but we compare our outcomes with other estimates published in the literature.
Materials and methods
Our analysis covered 60 countries that are currently supported by Gavi or have graduated from Gavi support and have received vaccines supplied by the manufacturer. A total of 57 countries were included in the RD analysis (Supplement Table S1) and 36 were included in the PD analysis (Supplement Table S2).
General
Two models were developed, one for HRV and one for PHiD-CV, looking at the direct impacts of disease on children up to 5 y of age. Each model first estimated the burden of the disease under study based on country-specific disease incidence rates before vaccine introduction and then the number of children who were able to receive the vaccine according to the doses delivered to each Gavi-supported country adjusted for wastage. In the second step, we estimated the benefit gained through vaccination for each disease, focusing on two main outcomes: disease-specific deaths and severe disease cases avoided. We estimated the benefit in children under 5 y of age for three time periods: 1) outcomes averted in a specific year, by weighting the doses delivered in the previous 5 y with the age-specific distribution of disease; 2) sum of the outcomes averted during the study period (2009 − 2019 for HRV and 2011 − 2019 for PHiD-CV); 3) total outcomes averted for children vaccinated during the study period, including cases averted beyond 2019 (). No community protection was assumed in the model to take a conservative approach.
Figure 1. Example calculation for PHiD-CV for Pakistan in 2019.
We also conducted a scenario analysis adjusting for country-specific all-cause mortality in children under 5 y of age, based on data from the World Bank.Citation17
The models employed Monte Carlo simulations to address parameter uncertainty, including vaccine efficacy as well as disease-specific severe case and death rates, based on minimum and maximum estimates for each country, on which an inverse beta distribution function was generated. The reported confidence ranges presented in the figures were based on 90% intervals of 10,000 bootstrap simulations.
Model inputs
Both disease models were developed in MS Excel. The study period considered was from 2009 for HRV (approved by Gavi in 2006) and from 2011 for PHiD-CV (approved by Gavi in 2011), up to the end of 2019. Vaccine doses were delivered to Gavi-supported countries prior to these dates for local clinical studies but were not included in this analysis. Both models follow the same analytical approach, applying specific inputs per vaccine and pathogen type for each country (). The total number of doses of each vaccine delivered to a country per year was extracted from the manufacturers’ internal databases.Citation18 Vaccine wastage was accounted for from the Gavi estimate of 5% for single-dose vials and 10% for multi-dose vials.
Table 1. Data retrieved from databases and literature for RD vaccination.
Rotavirus model
Disease burden
Three organizations have produced estimates of RD deaths in children under 5: Child Health Epidemiology Reference Group (CHERG), Global Burden of Disease (GBD) Study 2013, and the WHO/Centers for Disease Control and Prevention CDC (joint estimates).Citation13,Citation14,Citation19,Citation20 We calculated the country-specific RD-related mortality rate under 5 y of age as the average of the three sources divided by the at-risk population estimates from the CDC (Supplement Table S1).Citation13
Country-specific numbers of severe RD cases are not available in the literature. Therefore, we inferred the number of severe RD cases per country from the GBD 2015 report from overall diarrhea episodes in children under 5 y of age, using the following three steps.Citation21 First, the number of diarrhea events per diarrhea-related death was retrieved by country from the GBD Study 2015.Citation21 Secondly, based on the same study, it was assumed that 14% of diarrhea events were severe. Thirdly, we applied a country-specific estimate for the proportion of severe diarrhea cases attributable to rotavirus infections, ranging from 38% to 44%, based on the countries World Bank income group (2000–2004).Citation22 Supplement Figure S1 shows the numbers used in the model of country-specific severe RD cases corresponding to one RD death.
The age distributions of RD were then obtained by adjusting the epidemiological estimates according to the age-specific distributions of diarrhea reported by Kosek et al.:Citation25 8.28% for children 4 y of age; 8.88% for 3 y; 15.38% for 2 y; 23.08% for 1 y; and 44.38% for 0 − 11 months. We assumed that the same proportions would be applicable to diarrhea events caused by RD.
HRV vaccine effectiveness
Vaccine effectiveness was based on the review by Jonesteller et al.Citation25 of estimates from the first 10 y post-licensure of HRV vaccine effectiveness across a range of low-, medium- and high-mortality settings. Low-mortality countries were considered to be those in the lowest quartile of mortality rates for children under 5 y, medium-mortality as those in the second quartile, and high-mortality as those in the two highest quartiles, based on figures from UNICEF.Citation26 Vaccine effectiveness was considered to be 84% in low-mortality countries, 75% in medium-mortality countries and 57% in high-mortality countries.
Pneumococcal model
Disease burden
Only a limited number of sources were found that reported PD incidences in Gavi-eligible countries. Two publications provided country-specific PD morbidity and mortality estimates for children under 5 y. Data for community acquired pneumonia were obtained from a 2010 report by Rudan et al.Citation27 on the epidemiology and etiology of childhood pneumonia in 192 countries. Data for PD meningitis and non-pneumonia non-meningitis invasive PD (NPNM IPD) were reported according to WHO region by O’Brien et al.Citation28 Based on those reports, we estimated the country-specific numbers of severe acute lower respiratory infections (ALRI) and IPD cases (meningitis and NPNM IPD) and the number of deaths in the absence of vaccination (Supplement Table S2). Disease-specific age distributions for PD (community-acquired pneumonia [CAP], PD meningitis, and NPNM IPD) were based on a global review by age and region, reported by Russell et al.Citation29 We did not consider acute otitis media.
PHiD-CV vaccine efficacy
We applied disease-specific vaccine efficacy from the COMPAS trial as the level of direct protection against each disease outcome: 23.4% (95% confidence interval CI: 8.8 − 35.7) for severe pneumonia and 66.7% (95% CI: 21.8 − 85.9) for any IPD (regardless of the serotype).Citation30
Estimation of the disease burden avoided by vaccination
An example of how the methodology was applied is provided below, based on PHiD-CV doses delivered to Pakistan during 2019 (). An example based on HRV doses delivered to Ghana is provided in the Supplement.
1. From the manufacturer’s database, we retrieved the amount of PHiD-CV doses supplied to Pakistan in 2019: 21,711,710.
2. Accounting for wastage (10%), we assumed 19,540,539 doses remained.
3. Considering the full schedule of three doses per child, we estimated that 6,513,513 children could have been fully covered ().
4. We assumed a vaccine effect of 5 y per vaccinated child. The total follow-up period during which the vaccine has an effect was estimated to be 32,567,565 y (6,513,513 * 5).
5. The number of disease-specific deaths expected during the follow-up period without vaccination was calculated from the annual disease-specific death rate in children under 5 y in Pakistan multiplied by the total follow-up period.Citation27,Citation28 The number of deaths expected without vaccination up to the age of 5 y was 125,626 (= years of children fully protected * [annual death rates for pneumonia/meningitis/NPNM IPD]).
6. Vaccine efficacy applied for PHiD-CV was 23.4% for severe pneumonia and 66.7% for any pneumococcal IPD, based on the COMPAS trial.Citation30 The number of deaths avoided was calculated by multiplying the disease-specific vaccine efficacy by the number of deaths expected in the cohort without vaccination. This gave 31,230 deaths averted in the 2019 vaccinated cohort by the age of 5 y (= number of children up to the age of 5 y dying from pneumonia/meningitis/NPNM IPD * vaccine efficacy [severe pneumonia/IPD]). Note that not all of those deaths are avoided in the year of vaccination; rather, they are spread over a 5-y follow-up period accounting for the age-specific distribution of the disease (, illustrated by colored highlighting).
7. The total number of deaths avoided during one specific year (2019) was calculated by looking at the number of children vaccinated over the previous 5 y (including 2019), weighting the deaths averted by the age-wise distribution of disease and summing the deaths averted in children aged 1 through 5 y in 2019 (25,882; ).
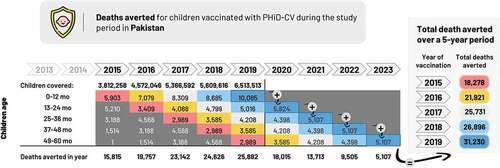
8. The number of deaths avoided during the study period was calculated by summing the number of deaths avoided in 2011 through 2019 (134,409; ).
9. The number of deaths averted in all children vaccinated during the study period and followed up until 5 y of age was calculated by summing the number of deaths avoided from 2011 through 2023 (180,748; ).
Results
Doses distributed
Across the Gavi-supported countries, 301 million doses of HRV were delivered between 2009 and 2019 to 57 countries and 488 million doses of PHiD-CV from 2011 to 2019 to 36 countries.
HRV
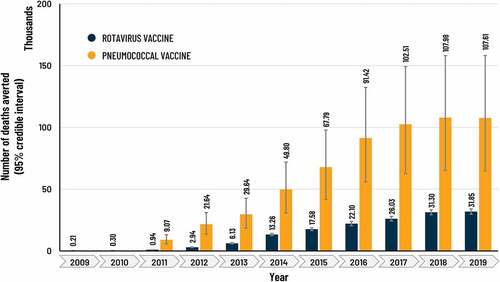
The estimated number of children vaccinated with HRV rose from approximately 821,000 in 2009 to 23.0 million in 2019 (), with an accumulated total of 143 million. The yearly number of severe RD cases and deaths averted rose over time as the number of vaccinated children increased, from approximately 61,000 severe cases and 215 deaths averted in 2009, to about 3.8 million severe cases and 32,000 deaths averted in 2019 (). The sum of severe RD cases and deaths averted during the study period (2009 − 2019) in vaccinated children was estimated at 18,7 million (95% CI: 17,3 − 20,3) and 153,000 (95% CI: 142,000 − 164,000), respectively.
Figure 2. Number of children estimated to be vaccinated with HRV and PHiD-CV across Gavi-supported countries from 2009–2019.
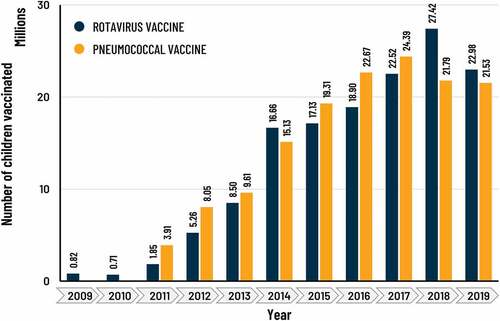
Figure 3. Estimate of number of severe RD and PD disease cases averted by HRV and PHiD-CV vaccination per year in Gavi-supported countries.
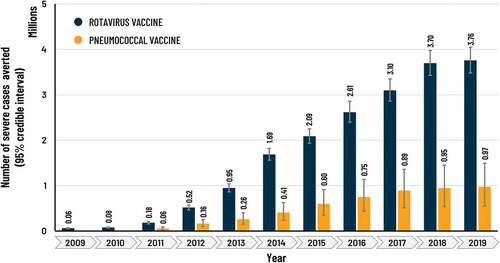
Figure 4. Estimate of number of RD and PD deaths averted by HRV and PHiD-CV vaccination per year in Gavi-supported countries.
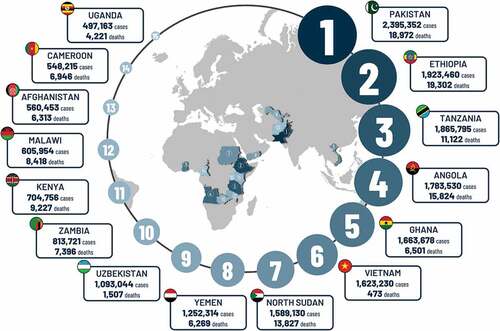
The total number of severe cases and deaths averted for all children vaccinated during the study period and followed up until 5 y of age (i.e. including cases averted beyond 2019) was 23,2 million (95% CI: 21,2 − 25,2) and 190,000 (95% CI: 176,000 − 205,000), respectively. The three countries with the highest estimates of severe RD cases averted for this time period were Pakistan (2,395,352), Ethiopia (1,923,460) and Tanzania (1,865,795) (), while the highest numbers of expected RD-specific deaths averted were seen in Ethiopia (19,302), Pakistan (18,972) and Angola (15,824) (). These numbers are influenced by the size of the birth cohort, the date of vaccine implementation, the uptake of the vaccine per year and the local burden of disease.
Figure 5. Total estimated severe RD cases and deaths averted for all children vaccinated during study period and followed up until 5 y of age in selected countries.
In the scenario analysis adjusting for country-specific all-cause mortality in children under 5 y of age, the sum of severe RD cases and deaths averted between 2009 and 2019 was estimated at 22.2 million and 182,000, respectively.
PHiD-CV vaccine
The estimated number of children vaccinated from 2011 to 2019 was 146 million, increasing from approximately 3.9 million in 2011 to 21.5 million in 2019 (). The number of severe PD cases averted per year rose over time from about 57,000 in 2011 to 973,000 cases in 2019 () and the number of deaths averted rose from 9,000 to 108,000 (). For the study period 2011 − 2019, the sum of severe PD cases and deaths averted in vaccinated children was estimated at 5,0 million (95% CI: 2,9 − 7,7) and 587,000 (95% CI: 359,000 − 855,000), respectively.
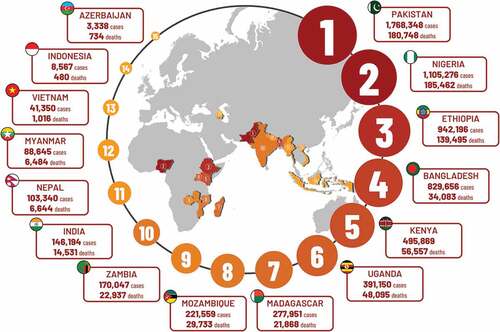
The total number of severe cases and deaths averted for all children vaccinated during the study period and followed up until 5 y of age was estimated at 6,6 million (95% CI: 3,7 − 10,2) and 749,000 (95% CI: 452,000 − 1,101,000), respectively. Over the same period, Pakistan had the highest estimate of severe PD cases averted (1,768,348), followed by Nigeria (1,105,276) and Ethiopia (942,196) (). The highest numbers of PD deaths averted were estimated in the same countries with most in Nigeria (185,462) followed by Pakistan (180,748) and Ethiopia (139,495) ().
Figure 6. Total estimated severe PD cases and deaths averted for all children vaccinated during study period and followed up until 5 y of age in selected countries.
The scenario analysis adjusting for all-cause mortality in children under 5 y estimated that the sum of severe PD cases averted between 2011 and 2019 was approximately 6.3 million and the number of deaths averted was 719,000.
Discussion
The present analysis evaluated the impact of HRV and PHiD-CV from 2009 to 2019 in Gavi-supported countries. Based on the total number of vaccine doses supplied by one manufacturer to Gavi-supported countries from 2009 to 2019 (approximately 300 million), the RD model estimated that by 2019, an accumulated 143 million children were vaccinated in 57 Gavi-supported countries. During that period, HRV vaccination was estimated to avert more than 18.7 million cases of severe RD gastroenteritis and more than 150,000 RD-specific deaths. From 2011 to 2019 approximately 490 million doses of the PHiD-CV vaccine were delivered, vaccinating an accumulated 146 million children in 36 Gavi-supported countries. In the same period, the vaccine averted more than 5 million severe cases of PD and almost 590,000 deaths. Looking at 2019 alone, the doses of both vaccines delivered over the last 5 y would have directly averted nine cases of severe disease each minute and one child death every 4 min on average. It is noteworthy that the HRV has a much greater impact on avoidance of severe RD events than PHiD-CV, while the PHiD-CV has a greater impact on avoidance of disease-specific mortality, due to the higher incidence of RD and the higher case-fatality rate of PD, especially due to pneumonia.
While spectacular results from vaccination might have been accomplished over the past 10 y, we are still a long way from achieving full coverage of every child in Gavi-supported countries. Based on GAVI estimates, only to cover the 74 supported countries with RD and PD vaccines, all vaccine producers together would need to deliver ~176 million doses of RD vaccines and ~219 million doses of the PD vaccines each year.Citation31 This goal could only be achievable if manufacturers are well coordinated, if there is a clear plan of progressive coverage and if long-term contracts are set that allow manufacturers to make the up-front investments needed to secure sustainable supply and capacity building. This would ultimately result in direct health benefits in the target population, as well as wider local, regional and global public health benefits.
The comparison of our data with other published sources is a challenge. Only one published study has estimated the specific contribution of rotavirus vaccine on RD mortality reduction. In 2016, Troeger et al.Citation32 reported an estimated reduction of 28,800 specific deaths worldwide and 24,200 specific deaths in sub-Saharan Africa, where most Gavi-supported countries are located. The estimated number of deaths avoided in sub-Saharan Africa reported by Troeger et al.Citation32 is close to our estimate of 22,096 RD deaths avoided in Gavi-eligible countries in 2016. It therefore appears that using the number of doses delivered as a starting point for estimating vaccine impact provides similar estimates of deaths avoided to those produced by other models. Studies looking at the impact of rotavirus vaccination on severe RD cases in Africa reported an estimated 47% reduction in hospitalizations, whereas we used a 57% reduction in severe cases.Citation33 This suggests that our estimate of severe RD events avoided could be an overestimation, although it could be argued that not all severe cases are hospitalized in Gavi-supported countries due to reduced access to care; thus, reported hospital cases could be an underestimation of the true severe cases impacted by the vaccine. A recent publication from Nigeria confirmed that 50% of severe gastroenteritis cases are managed at home.Citation34 Therefore, our numbers might be close to reality.
In estimating vaccine efficacy against PD, there was a paucity of studies evaluating PHiD-CV in Gavi eligible countries. A report evaluating PHiD-CV showed a 28% (range: 6–45%) efficacy against clinical pneumonia in Finland.Citation35 The same report highlighted changes in incidence of clinical pneumonia in Fiji and Kenya, suggesting reductions of approximately 13–32% post-PHiD-CV introduction.Citation35 These ranges support our efficacy estimates of 23.4% against PD as based on the COMPAS trial data.Citation30
The focus of our analysis was on direct vaccine effects, and we made no attempt to look at indirect effects. It would be challenging to investigate the presence and amount of indirect vaccine effects in low- and middle-income countries, where primary and secondary sources of infections are more often present than in high-income countries. If there is some community protection induced by vaccination, our reported benefit estimates would rather be an underestimation. However, it remains a challenge to obtain credible community protection estimates when robust detailed estimates of the disease burden prior to vaccination, current coverage and local effectiveness data are not available.
How important is it to measure vaccine impact based on doses delivered, instead of estimating a vaccine coverage rate as done in other modeling exercises? Estimates based on vaccine doses are helpful to better understand the evolution of vaccine impact over time and show when and if substantial vaccine coverage is achieved. For example, in Pakistan, a sufficient number of HRV doses to cover approximately 70% of the birth cohort was only delivered from 2017 − 18. The impact of vaccination on an outcome measure, such as RD-specific deaths avoided in a specific year, can only be reliably estimated from 2022 − 23 onwards. It can therefore be helpful to use the number of vaccine doses delivered per year and per country to better understand how and when certain goals can be reached. The ultimate aim is to achieve Universal Health Coverage, which seeks to ensure that all people have access to quality, effective and affordable health services and is one of the WHO Sustainable Development Goals.Citation36 Immunization not only provides benefit at the individual level but is also a public health intervention that provides global societal benefits. That societal gain will only be captured if a high enough coverage of the target population is achieved around the world. Therefore, real vaccine doses delivered could allow for a better assessment of the time when these societal benefits could be expected.Citation37–39 Unlike other vaccine impact models, our study utilizes actual vaccine doses delivered to Gavi-supported countries by one major vaccine manufacturer, to provide an alternate method to evaluate and reinforce the impact of vaccination.
A limitation of this study was that it did not consider potential confounding or influencing factors on the effect of vaccine doses once administered. This was due to the difficulty of estimating the impact of such factors on the effect of vaccines. For instance, events such as Ebola or COVID-19 outbreaks could have a significant impact on vaccine coverage, but it is assumed that once a vaccine dose is administered, the impact of the outbreak on the vaccine effect is limited. Additionally, our study did not account for increased vaccine wastage that could have been the result of such events. Another limitation is that vaccine doses supplied by other vaccine manufacturers were not considered, because the purpose of this study was to estimate the public health contribution a vaccine manufacturer can have by supplying vaccines through UNICEF. As a consequence, the overall vaccine coverage may have been underestimated. Moreover, it cannot be excluded that local policies prioritized the introduction of vaccines in high-risk areas first. This could potentially have resulted in a higher vaccine impact but was not considered in this study. Potential herd effects of the vaccines have also not been taken into account, which could have resulted in an underestimation of the vaccine effects.
Conclusion
Gavi, the Vaccine Alliance, started supporting rotavirus and pneumococcal vaccination in 2007 and 2009, respectively, for 73 countries. The potential impact of those vaccines on avoidance of severe disease cases and deaths has so far been estimated using extended modeling techniques. We have taken here an alternative approach of basing our models on the actual number of doses delivered by a manufacturer who is a major provider of vaccines to Gavi-supported countries and UNICEF. Our simple analysis resulted in estimated benefits that are in line with those calculated by using more sophisticated techniques. This dose calculation method allows for a more precise indication of where and when public health benefits from vaccination can be expected. It also illustrates the contribution that a global vaccine manufacturer can provide to support global public health interventions.
Highlights
Vaccine benefit is often estimated on assumptions of vaccine coverage
Another approach is to use the number of vaccine doses supplied by manufacturers
This provides a simple method to ascertain gaps in planning and implementation
HRV averted an estimated 18 million severe RD cases and 150,000 deaths (2009−2019)
PHiD-CV averted estimated 5 million severe PD cases and 600,000 deaths (2011−2019)
HRV and PHiD-CV together averted 9 severe cases/min and 1 death every 4 min (2019)
Article in a tweet
Vaccine impact against rotavirus disease and pneumococcal disease is estimated in low- and middle-income countries with an approach based on vaccine doses delivered through manufacturers, GAVI and UNICEF, by which the impact on severe disease cases and deaths avoided was assessed.
Additional information
Trademark
Rotarix and Synflorix are trademarks owned by or licensed to GSK.
Contributorship
AM, BS, DVO, LS, MO, PI, PP and SM were involved in the design of the study. AM, BS, PI, PP and SM collected or generated the data. All authors analyzed and/or interpreted the data and participated to the development of this manuscript and in its critical review with important intellectual contributions. All authors had full access to the data and gave approval of the final manuscript before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with ICMJE recommendations for conduct, reporting, editing and publications publishing of scholarly work in medical journals. The corresponding author had the final responsibility to submit for publication.
Statements of ethical approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Supplemental Material
Download MS Word (134.2 KB)Acknowledgments
The authors would like to thank Tathyana Giannotti Cousseau for her input regarding the initiation, collection of the actual sales volume per year and assessment of various methodologies adopted by Supranational agencies like Gavi and John Hopkins for “Lives Saved” model. The authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK, and TVF communications platform for the design support for the digital illustration, on behalf of GSK. Mary Greenacre (An Sgriobhadair Ltd) provided writing support for this literature review.
Disclosure statement
AM, DVO, LS, MO, PI, PP, and SM are employed by/hold shares in GSK. BS was employed by and held shares in GSK at the moment of the study. All authors declare no other financial or non-financial relationships or activities that could have influenced professional judgment.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2135916
Additional information
Funding
References
- World Health Organization. Fact sheet -Immunization coverage. Geneva: Newsroom; 2021 Jul 15 [accessed 2020 Sept 28]. https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
- World Health Assembly. The expanded programme on immunization: the 1974 resolution by the world health assembly. Assignment Child 1985;69-72:87–11.
- Gavi the Vaccine Alliance. Eligibility. 2020 Aug 26 [accessed 2021 March 02]. https://www.gavi.org/types-support/sustainability/eligibility.
- Gavi The Vaccine Alliance. Facts and figures. 2022 Feb 01 [accessed 2021 Mar 02]. https://www.gavi.org/programmes-impact/our-impact/facts-and-figures.
- Gavi The Vaccine Alliance. Evaluation studies. 2021 Dec 21 [accessed 2021 Mar 03]. https://www.gavi.org/programmes-impact/our-impact/evaluation-studies.
- Bustreo F, Okwo-Bele J-M, Kamara L. World health organization perspectives on the contribution of the global alliance for vaccines and immunization on reducing child mortality. Arch Dis Child. 2015;100(Suppl 1):S34–7. doi:10.1136/archdischild-2013-305693.
- Lee LA, Franzel L, Atwell J, Datta SD, Friberg IK, Goldie SJ, Reef SE, Schwalbe N, Simons E, Strebel PM, et al. The estimated mortality impact of vaccinations forecast to be administered during 2011-2020 in 73 countries supported by the GAVI Alliance. Vaccine. 2013;31(Suppl 2):B61–72. doi:10.1016/j.vaccine.2012.11.035.
- Jaupart P, Dipple L, Dercon S. Has Gavi lived up to its promise? Quasi-experimental evidence on country immunisation rates and child mortality. BMJ Glob Health. 2019;4(6):e001789. doi:10.1136/bmjgh-2019-001789.
- Ozawa S, Clark S, Portnoy A, Grewal S, Stack ML, Sinha A, Mirelman A, Franklin H, Friberg IK, Tam Y, et al. Estimated economic impact of vaccinations in 73 low- and middle-income countries, 2001-2020. Bull World Health Organ. 2017;95(9):629–38. doi:10.2471/BLT.16.178475.
- Li X, Mukandavire C, Cucunubá ZM, Echeverria Londono S, Abbas K, Clapham HE, Jit M, Johnson HL, Papadopoulos T, Vynnycky E, et al. Estimating the health impact of vaccination against ten pathogens in 98 low-income and middle-income countries from 2000 to 2030: a modelling study. Lancet (London, England). 2021;397(10272):398–408. doi:10.1016/S0140-6736(20)32657-X.
- United Nations Inter-agency Group for Child Mortality Estimation. Stillbirth and child mortality estimates. 2021 Dec 20 [accessed 2020 Sept 28]. https://childmortality.org/.
- World Health Organization. Health topics. Pneumonia. 2022 [accessed 2021 Jul 26]. https://www.who.int/health-topics/pneumonia#tab=tab_1.
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000-2013. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America. 2016;62(Suppl 2):S96–s105. doi:10.1093/cid/civ1013.
- Clark A, Black R, Tate J, Roose A, Kotloff K, Lam D, Blackwelder W, Parashar U, Lanata C, Kang G, et al. Estimating global, regional and national rotavirus deaths in children aged <5 years: current approaches, new analyses and proposed improvements. PloS one. 2017;12:e0183392. doi:10.1371/journal.pone.0183392.
- UNICEF. Rotavirus vaccine: supply and demand update. Geneva: UNICEF Supply Division; 2020 Feb 01 [accessed 2021 Dec 03]. https://www.unicef.org/supply/sites/unicef.org.supply/files/2020-03/rotavirus-vaccine-supply-and-demand-update_0.pdf.
- UNICEF. Pneumococcal conjugate vaccine: supply and Demand Update. Geneva: UNICEF Supply Division; 2016 Aug 01 [accessed 2021 Dec 03]. https://www.unicef.org/supply/media/4936/file/%20PCV-supply-update-August-2016.pdf.
- The World Bank. Mortality rate, under-5 (per 1,000 live births). Washington DC: UN Inter-agency Group for Child Mortality Estimation; 2022 [accessed 2021 Mar 17]. https://data.worldbank.org/indicator/SH.DYN.MORT.
- UNICEF. Gavi shipments and product menu. UNICEF; [accessed 2022 Jul 25]. https://www.unicef.org/supply/gavi-shipments-and-product-menu.
- GBD Diarrhoeal Diseases Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;385:117–71. doi:10.1016/S0140-6736(14)61682-2.
- Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PloS one. 2013;8:e72788. doi:10.1371/journal.pone.0072788.
- GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the global burden of disease study 2015. Lancet Infect Dis. 2017;17(9):909–48. doi:10.1016/S1473-3099(17)30276-1.
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12(2):304–06. doi:10.3201/eid1202.050006.
- Parmar D, Baruwa EM, Zuber P, Kone S. Impact of wastage on single and multi-dose vaccine vials: implications for introducing pneumococcal vaccines in developing countries. Hum Vaccin. 2010;6(3):270–78. doi:10.4161/hv.6.3.10397.
- Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global postlicensure data, 2006-2016. Clinical Infectious Diseases. 2017;65(5):840–50. doi:10.1093/cid/cix369.
- Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81(3):197–204. [Accessed 2022 Nov 30]. https://apps.who.int/iris/bitstream/handle/10665/268903/PMC2572419.pdf?sequence=1&isAllowed=y
- UNICEF. Child mortality data, December 2021.2021 Dec 01 [accessed 2021 Sept 28]. https://data.unicef.org/resources/dataset/child-mortality/.
- Rudan I, O’Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, Lukšić I, Fischer Walker CL, Black RE, Campbell H. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3(1):010401. doi:10.7189/jogh.03.010401.
- O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet (London, England). 2009;374(9693):893–902. doi:10.1016/S0140-6736(09)61204-6.
- Russell F, Sanderson C, Temple B, Mulholland K. Global review of the distribution of pneumococcal disease by age and region. 2011 Oct 01 [accessed 2020 Nov 21]. https://blogs.lshtm.ac.uk/vaccineschedules/files/6.-Russel-review-age-specific-epidemiology-PCV-schedules-session-nov11.pdf.
- Tregnaghi MW, Sáez-Llorens X, López P, Abate H, Smith E, Pósleman A, Calvo A, Wong D, Cortes-Barbosa C, Ceballos A, et al. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med. 2014;11(6):e1001657. doi:10.1371/journal.pmed.1001657.
- Gavi The Vaccine Alliance. Base demand forecast. gavi the vaccine alliance. 2019. [accessed 2022 Aug 04]. https://www.gavi.org/sites/default/files/2021-12/Gavi-BDF-v19-Public-Summary.pdf.
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatr. 2018;172(10):958–65. doi:10.1001/jamapediatrics.2018.1960.
- Shah MP, Tate JE, Mwenda JM, Steele AD, Parashar UD. Estimated reductions in hospitalizations and deaths from childhood diarrhea following implementation of rotavirus vaccination in Africa. Expert Rev Vaccines. 2017;16(10):987–95. doi:10.1080/14760584.2017.1371595.
- Okafor CE. Introducing rotavirus vaccination in Nigeria: economic evaluation and implications. PharmacoEco. Open. 2021;5(3):545–57. doi:10.1007/s41669-020-00251-6.
- International Vaccine Access Center. Pneumococcal Conjugate Vaccine (PCV) product assessment. Baltimore (MD): Johns Hopkins Bloomberg School of Public Health; [accessed 2022 Jul 25]. https://www.jhsph.edu/ivac/wp-content/uploads/2018/05/pcv-product-assessment-april-25-2017.pdf.
- World Health Organization. Sustainable Development Goals (SDGs). 2022 [accessed 2021 Jan 06]. https://www.who.int/health-topics/sustainable-development-goals#tab=tab_2.
- Bärnighausen T, Berkley S, Bhutta ZA, Bishai DM, Black MM, Bloom DE, Constenla D, Driessen J, Edmunds J, Evans D, et al. Reassessing the value of vaccines. Lancet Global Health. 2014;2(5):e251–e2. doi:10.1016/S2214-109X(13)70170-0.
- Bärnighausen T, Bloom DE, Canning D, O’Brien J. Accounting for the full benefits of childhood vaccination in South Africa. S Afr Med J. 2008;98:844–46. [Accessed 2022 Nov 30]. https://cdn1.sph.harvard.edu/wp-content/uploads/sites/1288/2013/10/PGDA_WP_39.pdf
- Jit M, Hutubessy R, Png ME, Sundaram N, Audimulam J, Salim S, Yoong J. The broader economic impact of vaccination: reviewing and appraising the strength of evidence. BMC Med. 2015;13(1):209. doi:10.1186/s12916-015-0446-9.
