ABSTRACT
The association between COVID-19 vaccines and vasovagal malaise (VVM) has recently been reported in the literature. Our study aimed to describe COVID-19 vaccines associated VVM cases and to identify risk factors of COVID-19 vaccines associated VVM. To this end, we performed a descriptive study of VVM reports associated with COVID-19 vaccines from two French mass COVID-19 vaccination centers. We also extracted reports of VVM associated with all-COVID-19 vaccines in VigiBase®, the World Health Organization (WHO) pharmacovigilance database to analyze demographic data. In the two French mass vaccination center database, 408 entries reported VVM after the standard administration of tozinameran - Pfizer® (1.63/1,000 vaccinated persons). Of these cases, 213 (52.2%) occurred in women, and 193 (47.3%) occurred in the 18–29 year-old (yo) age group. In 232 cases (56.8%), patients had a history of anxiety related to needles or medical visits, 213 (52.2%) reported a fear of COVID-19 vaccination in particular, and 233 (57.1%) had a history of VVM. In VigiBase®, 336,291 notifications of COVID-19 vaccines associated with VVM were identified in the adult population during the period of analysis. The most reported age class was 18–44 years (52.4%), and women represented 71.7% of the reports. Reporting widely differed depending on the country. This study, performed in real-life conditions, highlights that VVM is associated with all-COVID-19 vaccines. Young age and history of anxiety related in young adults could be a triggering factor of vaccines-associated VVM. Further studies are needed to confirm our results.
Introduction
Vasovagal malaise (VVM) has been reported with various medical procedures, including simple venipuncture,Citation1 the viewing of needles or blood,Citation2 invasive medical proceduresCitation2 and vaccination.Citation3 Incidents of VVM are generally not serious, but related to falls can cause injuries. Because VVM has been reported in response to the use of vaccines with different technologies and excipients, these reactions have been attributed to the vaccination process and not to the vaccines themselves.Citation4 In the actual context of the COVID-19 pandemic, messenger ribonucleic acid (mRNA) vaccines have been recommended for the first time and assessing the safety of COVID-19 vaccines is consequently important, including that of COVID-19 mRNA vaccines, which are widely used around the world.Citation5–7 Indeed, in the general population, the words “COVID,” “vaccines” and “mRNA” are a cause of fear, and we believe this fear could be a triggering factor of vaccine-associated VVM.
Therefore, we selected to describe COVID-19 vaccine-associated symptoms and identify risk factors for VVM by conducting a retrospective study of COVID-19 vaccines-associated VVM at two mass vaccination sites in France. Furthermore, we also performed a descriptive study in VigiBase®,Citation8–10 the WHO pharmacovigilance database, of the demographic data of VVM associated with all COVID-19 vaccines marketed before 26 January 2022. This analysis allowed us to study the reporting of VVM associated with all-COVID-19 vaccines to determine the precise clinical characteristics of patients suffered from VVM after COVID-19 vaccination.
Materials and methods
Retrospective study in two COVID-19 mass vaccination centers
For this study, all COVID-19 vaccine adverse reactions occurring at the two mass vaccination sites in Caen (Normandy, France) from 1 January to 15 October 2021 were coded into a local database in accordance with local guidelines. The COVID-19 vaccine systematically used was tozinameran – Pfizer®.
After receiving authorization for the current study by the Ethics Department of University of Caen Normandy (Comité local d’éthique de la recherche en santé – Authorization n°2709, July 27, 2021), we have retrospectively collected further information on the cases of VVM occurring during COVID-19 vaccination at these two mass vaccination sites.
We defined VVM [derived from WHO definitionCitation11 and literatureCitation1,Citation3,Citation12] as a sympathetic nervous system stimulation in a setting of fear or emotional distress, followed by sudden onset of hypotension that often results in syncope or presyncope. These symptoms could occur in a person during the 15-min post-vaccination observation period and may be associated with tachycardia (rapid heart rate), hyperventilation (rapid breathing), dyspnea (difficulty breathing), chest pain, paresthesia (numbness or tingling), light-headedness, hypotension (low blood pressure), headache, pallor, or syncope.
Subjects who received diphenhydramine or epinephrine at the vaccination visit or syncopal events that occurred off-site or ≥1 hour after vaccine administration were excluded because these events might have represented hypersensitivity reactions or other etiologies.
After a pilot study of one pharmacist, two physicians and five patients, a validated questionnaire was used for each patient with VVM (Supplemental Figure 1). All patients who experienced VVM were retrospectively contacted by telephone by the mass vaccination centers on site, and the following information was retrieved using the formalized questionnaire: history of anxiety related to needles or medical visit, history of VVM, and common signs and symptoms associated with VVM (chest pain, hypotension, light-headness or dizziness, nausea/vomiting, pallor or diaphoresis, seizure-like activity, tachycardia, syncope, brief transient loss of consciousness). We have also collected the oral informed consent from the participants. For each VVM, we have also collected administrative information (mass vaccination center, date of event, type of vaccine administration, first or second administration), and demographic patient data (gender, age).
Study in VigiBase
For this descriptive study, we used data from VigiBase®, the WHO pharmacovigilance database. Since 1978, the Uppsala Monitoring Center (UMC) in Sweden has had the technical and operational responsibility of the WHO program for International Drug Monitoring, including the maintenance of VigiBase®. VigiBase®, the WHO global individual case safety report database, was used to retrieve information.Citation13 It contains more than 29 million individual case safety report (ICSR) received from more than 120 country members worldwide. ICSRs include administrative information (country, type of report, and qualification of reporter), patient data (gender, age), date of onset, reaction and the nature of the outcome using Medical Dictionary for Regulatory Activities (MedDRA 24.1) terms, WHO assessment causality, and drug(s) involved (name, drug start and stop dates, time to onset, indication, dose, dechallenge, and rechallenge). Each report was characterized as “serious” or “nonserious” according to the WHO definition. Seriousness criteria included death, life-threatening situations, hospitalization, hospitalization prolongation, persistent incapacity or disability, clinically relevant situation judged by the physician reporting the case.Citation14
For our descriptive analyses reports of VVM were identified with the following MedDRA preferred terms: “Fall,” “Loss of consciousness,” “Malaise,” “Presyncope,” “Syncope” (version 24.1). Vaccines of interest were the following: mRNA vaccines (elasomeran – Moderna®, Tozinameran – Pfizer®), COVID-19 vaccines with more traditional technologies (ChAdOx1 – AstraZeneca®, NRVV Ad26 – Janssen®, NRVV Ad26 and NRVV Ad5 - Sputnik®, HB02 – Sinopharm®, CZ020 – Coronavac® and NIV-2020-770 – Covaxin®). For each vaccine, the following information was described: age class, sex, seriousness, death, and region. The number of report of VVM was compared to the number of report of other adverse reactions.
To explore the homogeneity of the reporting of COVID-19 vaccines-associated adverse effects in VigiBase®, we searched the number of cases reported for the 01/01/2021 to 01/26/2022 for the following eight countries: France, United States of America, the United Kingdoms, the Popular Republic of China, the Russian Federation, India, Philippines, and Morocco. Those countries were chosen to explore the geographical use of COVID-19 vaccines and the reporting among different pharmacovigilance systems.
Results
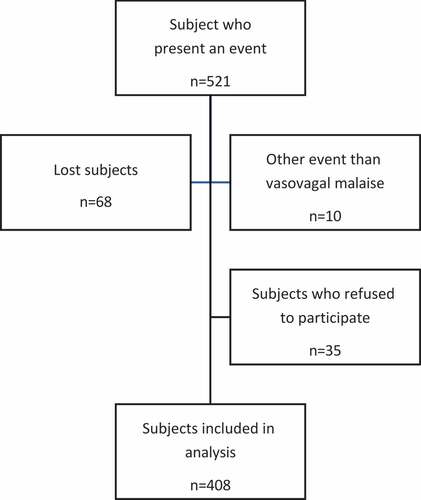
Descriptive study based on a French mass vaccination center database ()
From 01/18/2021 to 10/15/2021, 249,872 COVID-19 vaccine injections were administered at two mass COVID-19 vaccination centers. In total, 521 VVMs after COVID-19 vaccination were reported, and 408 subjects agreed to be included in the analysis (). Therefore, 1.63 ‰ of the vaccinations ended with a VVM. A total of 78.2% (319/408) were associated with the first dose and 52.2% (213/408) of the VVM were observed in women. VVM occurred mainly in younger patients (23.5% and 47.3% in 12–17 and 18–29 age ranges respectively). Age (p = .6), sex (p = .9) or outside temperature (p = .3) did not significantly differ between the 408 subjects included and the 35 subjects who refused to participate. The mean time to onset of the VVM after COVID-19 vaccination was 5.0 ± 4.2 minutes and mean time to recovery was 40.8 ± 236.7 minutes. VVM did not appear before vaccination. Of the patients who presented with COVID-19 vaccine-associated VVM, only six patients (1.5%) needed to be transported to the emergency department. None of the patients required hospitalization. Subjects who experienced a VVM after COVID-19 vaccination frequently reported anxiety with needles or medical examinations, especially with COVID-19 vaccination [56.8% (232/408) and 52.2% (213/408) respectively]. A history of VVM was reported in 57.1% of cases (233/408) ().
Table 1. Characteristics of vasovagal malaise events after receipt of COVID-19 vaccine (n = 408) at two mass vaccination sites (Caen, Normandy, France), January 18, 2021-October 15, 2021.
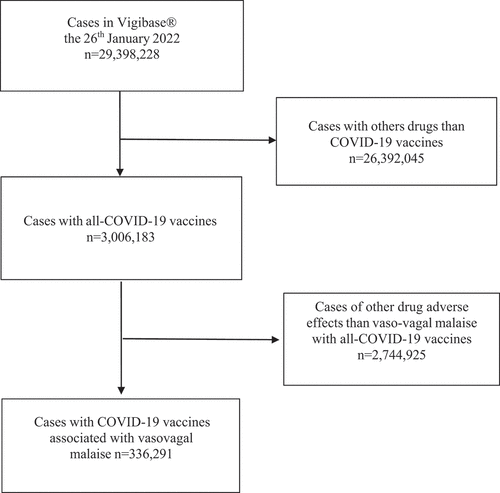
Demographic data in VigiBase®
Among the 29,398,228 ICSRs listed in Vigibase® on 26 January 2022, 3,006,183 were related to the nine COVID-19 vaccines. According to the selection criteria, 336,291 suffered from VVM after COVID-19 vaccination (11.2%) (). In descending order, 163,626 (48.7%) were associated to tozinameran – Pfizer®; 83,370 (24.8%) to ChAdOx1 – AstraZeneca®; 60,117 (17.9%) to elasomeran – Moderna®; 22,161 (6.6%) to NRVVAd26 – Janssen®; 3,678 (1.1%) to HB02 – Sinopharm®; 2,905 (0.9%) to CZ020 – Coronavac®; 153 (0.05%) to NRVV Ad5 – Sputnik® II; 146 (0.04%) to NRVV Ad26 – Sputnik® I and 135 (0.04%) to NIV-2020-770 – Covaxin®. Most COVID-19 vaccine-associated events were reported in individuals aged 18–44 years (52.4%), and women represented 71.7% of the reports. Adverse events were considered serious in 18.7% of the reports (). The number of reported cases markedly differed among the COVID-19 vaccines. Furthermore, the reporting pattern appeared very different among the eight countries assessed in the supplementary analysis ().
Table 2. Demographic data of COVID-19 vaccines associated with vasovagal malaise in VigiBase®.
Table 3. Number of reports of COVID-19 vaccines associated adverse effects per country shared to VigiBase® during the period of the study in a list of eight countries.
Discussion
This study was realized to investigate the link between VVM and COVID-19 vaccines under real-world conditions, which we believe could be enhanced by anxiogenic context. We highlighted that VVM is associated with all COVID-19 vaccines and consequently is likely not affected by the technology used. VVM events after COVID-19 vaccination occurred mainly in a young population, especially in people with a history of VVM associated with injections or needle aversion. The impact of sex is probable but requires further discussion.
Vasovagal malaise and vaccines
Syncope can occur immediately after vaccination with any vaccine.Citation15 Syncope can be heralded by presyncopal manifestations, such as lightheadedness, dizziness, diaphoresis, and visual changes, followed by a brief transient loss of consciousness. It occurs more commonly in women and adolescents or young adults than young in young children or elderly individuals.Citation16–18 In a review of 697 cases of syncope after different vaccinations (measles-mumps-rubella, different combinations of diphtheria-tetanus-pertussis, hepatitis B, influenza, Haemophilus influenzae type B, and typhoid), when the time to onset was known, 57% occurred within 5 minutes, 80% occurred within 15 minutes, and 88% occurred within 30 minutes after vaccination. Brief tonic-clonic movements were observed in 24% of these individuals. In this study, 10% of the individuals who experienced syncope after immunization required hospitalization for serious injuries, including skull fractures and cerebral hemorrhages.Citation12 In our study, incidents of VVM after COVID-19 vaccination were much less serious: only 1.5% of them needed to be transported to the emergency department and none of the patients required hospitalization. Many subjects who experienced VVM after COVID-19 vaccination reported anxiety with needles or medical visit, especially with COVID-19 vaccination [56.8% (232/408) and 52.2% (213/408) respectively]. A History of VVM were reported in 57.1% of cases (233/408). Finally, 1.63‰ of the vaccinations at the two COVID-19 mass centers considered in our study ended with VVM. Hause et al.19 previously reported a rate of syncope after NRVV Ad26 – Janssen® COVID-19 vaccination of 0.8‰ from March 2 to April 11 based on a review of Vaccine Adverse Events Reporting System (VAERS), the American vaccine safety monitoring program20. Although our definition markedly differed, we noted a similar incidence rate. Moreover, 60 reports of syncope after the influenza vaccination were identified during July 1, 2019 – June 30, 2020 (0.5‰ episodes of syncope after influenza vaccine) in VAERS.Citation19
Vasovagal malaise and COVID-19 vaccines
As mentionned above, Hause et al. have previously described anxiety-related adverse event clusters after NRVV Ad26 – Janssen® vaccination in the United-States of America.Citation19 Five mass vaccination sites reported 64 anxiety-related events, including 17 events of syncope (fainting) after administration of NRVV Ad26 – Janssen®, occurring during April 2021 to the VAERS19. Thirteen (20%) of the patients informed staff members of a history of VVM associated with injections or needle aversion. The prevalence of anxiety-related adverse events ranged from 5.2 to 13.5 per 1,000 vaccinated persons. Among the 64 total cases, a majority [39 cases (61%)] occurred in women with a median age of 36 yo (range = 18–77 yo). As the prevalence of anxiety during the COVID-19 pandemic was higher in women and younger individuals,Citation20 we believe preexisting anxiety could have played a role in the onset of vaccine-associated VVM. Most events resolved within 15 minutes with supportive care. In total, 20% of patients were transported to an emergency department for further medical evaluation, and all were released from medical care on the same day. In addition, a review of all VAERS reports containing the MedDRA terms “syncope” or “syncope vasovagal” after vaccination with NRVV Ad26 – Janssen® during March 2–April 11 2021, identified 653 eligible reports. During March and April 2021, the VAERS reporting rate of syncope after NRVV Ad26 – Janssen® was 8.2 per 100,000 doses among 7.98 million dose of NRVV Ad26 – Janssen® administered in the United States. Only 3% of the 653 syncope/presyncope reports (3%) were classified as serious. Moreover, 123 (19%) reports indicated that the recipient had a history of syncope associated with receiving injections or needle aversion, and 327 (50%) cases occurred in women with a median age of 30 years (range = 18–82 yo). The largest proportion of reported syncopal events after the administration of NRVV Ad26 – Janssen® occurred among persons aged 18–29 yo, and this rate inversely correlated with age.
Reports of syncope were approximately 164 times more common after NRVV Ad26 – Janssen® (8.2 per 100,000) than after influenza vaccination (0.1 per 100,000). The stress of an ongoing pandemic might also increase anxiety surrounding COVID-19 vaccination.Citation19 This Weber bias is well described and is defined as a variation in reporting over time that results in an increase in the number of reports immediately after a drug is marketed (due to incomplete safety profile and increasing exposure).Citation21
Physiopathological mechanisms
These syncopal events are thought to be caused by vasovagal or vasodepressor mechanisms.Citation16 This reaction is a well-described syndrome consisting of sympathetic nervous system stimulation, often in a setting of fear or emotional distress, followed by sudden onset of hypotension that often results in syncope or presyncope.Citation1,Citation12 Syncopal seizures unrelated to epilepsy also occur as a result of a relative cerebral anoxia,Citation22 and electrocardiographic monitoring of patients undergoing vasovagal syncope has shown that a period of at least 5 seconds of asystole is a common occurrence.Citation2 Vasovagal reactions are known to be elicited by a variety of stimuli, including simple venipuncture, or seeing of blood or invasive medical procedure, such as vaccination.Citation1,Citation2,Citation12
As VVM and hypersensitivity reactions occur soon after the administration of vaccines, observation for at least 15 minutes is recommended for individuals receiving vaccines in French mass COVID-19 vaccination centers before being discharged.Citation23 In this study, the time delay of VVM occurrence was 5.0 ± 4.2 minutes. Based on our data, this national protocol seems to be adapted to this phenomenon and agrees with the literature. Brown et al. described that syncope after immunization can occur in 88.8% of cases within 15 minutes.Citation12
Proposals to avoid vasovagal malaise after COVID-19 vaccination
To avoid VVM and its consequences after COVID-19, we suggest identifying subjects at risk with an in-depth interview prior to vaccination. The following risk factors could be identified: as past medical history of acute VVM after immunization and a fear of needles or medical context in general. Particular attention should be given to young persons. When a patient is identified with risk factors of VVM, intervention to reduce pain and prevent syncope should be provided. Many authors have previously proposed intervention in this matter.Citation24,Citation25 For example, soothing background music has been proposed by Kuntz et al.Citation24 Moreover, exposure-based psychological interventions and applied muscle tension have shown evidence of benefit in the reduction of needle fear in pediatric and adult population.Citation25 In contrast, drinking water does not seem to be associated with a decrease of postvaccination presyncope in particular in adolescents.Citation26 Finally, the site capacity and layout of the mass COVID-19 vaccination site must be adapted to permit the isolation of people feeling unwell and decrease the risk of multiple anxiety events.Citation27
Strengths and limitations
The present study has several strengths. The study of local data could provide details on COVID-19 vaccine-associated risk factors associated with the onset of VVM, with hundreds of patients included in the study, but only with mRNA-based COVID-19 vaccine Tozinameran – Pfizer®. Furthermore, the study in VigiBase® allowed us to describe the reporting of VVM with all-COVID-19 vaccines. However, as discussed above, the marketing of COVID-19 vaccines widely differs between countries, the pharmacovigilance system is poorly developed in a wide range of countries, and geopolitical interest might have played a role in the reporting of COVID-19 vaccine-associated adverse effects to VigiBase®. Thus, we could not conduct a disproportionality analysis that would have been biased. Moreover, our study was limited due to the use of a pharmacovigilance database. Underreporting is the most important limitation in pharmacovigilance, but this phenomenon did not appear to change the results and significance of a case/non-case study.Citation28 Missing data is another limitation in pharmacovigilance database extractions. Therefore, we selected a sensitive definition for VVM in VigiBase®. However, this definition may have lack specificity.
Furthermore, the seriousness of the adverse event was assessed based on pharmacovigilance guidelines, which might, in rare cases, differ from other seriousness scales, such as the Common Terminology Criteria for Adverse Events (CTCAE).Citation29,Citation30 Because COVID-19 vaccines are new drugs which are highly mediatized, a reporting bias might exist. The retrospective design could also provoke a memory bias. Moreover, comparing patients who experienced VVM at the two mass vaccination centers to those who did not present VVM was impossible, as the latter were not retrospectively contacted in accordance with the authorization granted for the study. Moreover, data regarding the history of generalized anxiety were not collected in our study. Indeed, adolescent girls are usually affected by VVM, and episodes are associated with anxiety or are a component of an anxiety disorder. Episodes often recur, and the diagnosis of anxiety disorder may be missed and ascribed to cardiac events or another life-threatening disorder.Citation11 The cumulative incidence of syncope in Framingham is approximately 50% in men and women 80 years-old, but VVM was only responsible for 21.2% of all episodes.Citation31,Citation32 These two COVID-19 mass vaccination centers were selected based on the convenience of investigators, but they also represent 2/3 of the activity of COVID-19 mass vaccination centers in the area of the study for the considered period. Moreover, this centers were opened at the start of the national vaccination campaign in January and March 2021, and before primary care vaccination (general practitioners, nurses and pharmacists) was available.
Conclusion
This study, performed in real-life conditions, described the clinical characteristics of VVM associated to all-COVID-19 vaccines. From a practical point of view, the present study suggests that patients with a history of VVM and/or needle aversion and in particular young people are at higher risk of VVM following COVID-19 vaccination. Moreover, the technology of the vaccine is likely not an important factor in the development of VVM. Prior to vaccine administration, we suggest the identification of patients at higher risk of VVM to adapt the medical procedure and reduce the risk of VVM. After the vaccination, all patients should be observed for 15 minutes. This protocol will help promote the vaccination and avoid serious injuries potentially caused by the VVM.
Abbreviations
ADR adverse drug reaction
aROR adjusted reporting odds ratio
ATC anatomical therapeutic chemical classification
CI confidence interval
CTCAE Common Terminology Criteria for Adverse Events
ICSR individual case safety report
MedDRA Medical dictionary for regulatory activities
mRNA messenger ribonucleic acid
ROR reporting odds ratio
UMC Uppsala monitoring center
VAERS Vaccine adverse events reporting system
VVM vaso-vagal malaise
WHO World Health Organization
yo years old
Supplemental Material
Download Zip (14 KB)Disclosure statement
The authors declare that they have no conflict of interest to disclose.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2135918
Additional information
Funding
References
- Graham DT, Kabler JD, Lunsford L. Vasovagal fainting: a diphasic response. Psychosom Med. 1961;23(6):1–8. doi:10.1097/00006842-196111000-00004.
- Ost LG, Sterner U, Lindahl IL. Physiological responses in blood phobics. Behav Res Ther. 1984;22:109–17. doi:10.1016/0005-7967(84)90099-8.
- Hedberg AG, Schlong A. Eliminating fainting by school children during mass inoculation clinics. Nurs Res. 1973;22:352–53. doi:10.1097/00006199-197307000-00013.
- Fainting after Vaccination. Vaccine Safety | CDC [Internet]. 2020 [cited 2022 Jan 7]. https://www.cdc.gov/vaccinesafety/concerns/fainting.html
- COVID-19 Vaccines. FDA [Internet]. [cited 2022 Jan 7]. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines
- COVID-19: latest updates. European medicines agency [Internet]. [cited 2022 Jan 7]. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/covid-19-latest-updates
- Tau N, Yahav D, Shepshelovich D. Vaccine safety - is the SARS-CoV-2 vaccine any different? Hum Vaccines Immunother. 2021;17:1322–25. doi:10.1080/21645515.2020.1829414.
- Centre UM. About VigiBase [Internet]. [cited 2022 Sep 23]. https://who-umc.org/vigibase/
- Chrétien B, Dolladille C, Hamel-Sénécal L, Sassier M, Faillie JL, Miremont-Salamé G, Lelong-Boulouard V, Le Boisselier R, Fedrizzi S, Alexandre J, et al. Comparative study of hypoglycaemia induced by opioids. Is it a class effect? Expert Opin Drug Saf. 2019;18:987–92. doi:10.1080/14740338.2019.1646246.
- Chrétien B, Lelong-Boulouard V, Chantepie S, Sassier M, Bertho M, Brazo P, Humbert X, Alexandre J, Fedrizzi S, Dolladille C. Haematologic malignancies associated with clozapine v. all other antipsychotic agents: a pharmacovigilance study in VigiBase ®. Psychol Med. 2021;51(9):1459–66. doi:10.1017/S0033291720000161.
- WHO. Immunization stress-related response: a manual for program managers and health professionals to prevent, identify and respond to stress-related responses following immunization [Internet]. [cited 2022 Jan 11]. https://www.who.int/publications-detail-redirect/10665330277
- Braun MM, Patriarca PA, Ellenberg SS. Syncope after immunization. Arch Pediatr Adolesc Med. 1997;151:255–59. doi:10.1001/archpedi.1997.02170400041007.
- Bate A, Lindquist M, Edwards IR. The application of knowledge discovery in databases to post-marketing drug safety: example of the WHO database. Fundam Clin Pharmacol. 2008;22:127–40. doi:10.1111/j.1472-8206.2007.00552.x.
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet Lond Engl. 2000;356:1255–59. doi:10.1016/S0140-6736(00)02799-9.
- Committee to Review Adverse Effects of Vaccines, Institute of Medicine. Adverse Effects of Vaccines: evidence and Causality [Internet]. Washington (DC): National Academies Press (US); 2011. cited 2022 Jan 11 Available from: http://www.ncbi.nlm.nih.gov/books/NBK190024/.
- Babl FE, Lewena S, Brown L. Vaccination-related adverse events. Pediatr Emerg Care. 2006;22:514–9;quiz 520–2. doi:10.1097/01.pec.0000227874.44878.40.
- Centers for Disease Control and Prevention (CDC). Syncope after vaccination–United States, January 2005-July 2007. MMWR Morb Mortal Wkly Rep 2008;57:457–60.
- Crawford NW, Clothier HJ, Elia S, Lazzaro T, Royle J, Buttery JP. Syncope and seizures following human papillomavirus vaccination: a retrospective case series. Med J Aust. 2011;194:16–18. doi:10.5694/j.1326-5377.2011.tb04138.x.
- Hause AM, Gee J, Johnson T, Jazwa A, Marquez P, Miller E, Su J, Shimabukuro TT, Shay DK. Anxiety-Related adverse event clusters after Janssen COVID-19 vaccination - Five U.S. mass vaccination sites, April 2021. MMWR Morb Mortal Wkly Rep. 2021;70:685–88. doi:10.15585/mmwr.mm7018e3.
- Santabárbara J, Lasheras I, Lipnicki DM, Bueno-Notivol J, Pérez-Moreno M, López-Antón R, De la Cámara C, Lobo A, Gracia-García P. Prevalence of anxiety in the COVID-19 pandemic: an updated meta-analysis of community-based studies. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110207. doi:10.1016/j.pnpbp.2020.110207.
- Faillie J-L. Case-non-case studies: principle, methods, bias and interpretation. Therapie. 2019;74:225–32. doi:10.1016/j.therap.2019.01.006.
- Lin JT, Ziegler DK, Lai CW, Bayer W. Convulsive syncope in blood donors. Ann Neurol. 1982;11:525–28. doi:10.1002/ana.410110513.
- Ministère de la santé. DGS-URGENT. [cited 2022 Feb 3]. https://solidarites-sante.gouv.fr/IMG/pdf/dgs_urgent_44_campagne_az.pdf
- Kuntz JL, Firemark A, Schneider J, Henninger M, Bok K, Naleway A. Development of an Intervention to reduce pain and prevent syncope related to adolescent vaccination. Perm J. 2019;23:17–136. doi:10.7812/TPP/17-136.
- McMurtry CM, Noel M, Taddio A, Antony MM, Asmundson GJG, Riddell RP, Chambers CT, Shah V, HELPinKids&Adults Team. Interventions for individuals with high levels of needle fear: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin J Pain. 2015;31:S109–123. doi:10.1097/AJP.0000000000000273.
- Kemper AR, Barnett ED, Walter EB, Hornik C, Pierre-Joseph N, Broder KR, Silverstein M, Harrington T. Drinking water to prevent postvaccination presyncope in adolescents: a randomized trial. Pediatrics. 2017;140:e20170508. doi:10.1542/peds.2017-0508.
- Loharikar A, Suragh TA, MacDonald NE, Balakrishnan MR, Benes O, Lamprianou S, Hyde TB, McNeil MM. Anxiety-related adverse events following immunization (AEFI): a systematic review of published clusters of illness. Vaccine. 2018;36:299–305. doi:10.1016/j.vaccine.2017.11.017.
- Montastruc J-L, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011;72:905–08. doi:10.1111/j.1365-2125.2011.04037.x.
- Common Terminology Criteria for Adverse Events (CTCAE). Protocol Development | CTEP [Internet]. [cited 2022 Sep 23]. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- EMA. Good pharmacovigilance practices [Internet]. Eur Med Agency. 2018. cited 2022 Sep 23. Available from: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices.
- Soteriades ES, Evans JC, Larson MG, Chen MH, Chen L, Benjamin EJ, Levy D. Incidence and prognosis of syncope. N Engl J Med. 2002;347:878–85. doi:10.1056/NEJMoa012407.
- Colman N, Nahm K, Ganzeboom KS, Shen WK, Reitsma J, Linzer M, Wieling W, Kaufmann H. Epidemiology of reflex syncope. Clin Auton Res Off J Clin Auton Res Soc. 2004;14(Suppl 1):9–17. doi:10.1007/s10286-004-1003-3.