ABSTRACT
The research on substance use disorders is ongoing in the quest to find anti-addiction vaccines to treat drug abuse. This article provides a systematic review of clinical trials that have been conducted on humans to evaluate the efficacy, safety, and abstinence rates of anti-addiction vaccines for different drugs, with useful results regarding cocaine and nicotine vaccines in particular; this study includes also a meta-analysis to establish the antibody-titer production following the nicotine vaccination, while a meta-analysis of cocaine vaccines was not performed due to the small number of included trials. The articles taken into consideration were published between 2002 and 2015, including searches through 2022. Overall, 13 articles were selected with 2,266 participants from different ethnic groups. The meta-analysis of nicotine vaccines showed that vaccinated groups were 50 times more likely to create specific antibodies compared to the non-vaccinated. These results demonstrated how the nicotine vaccine has good immunogenicity.
Introduction
The possibility of using preventive and/or therapeutic active immunization (vaccination) against substance use disorders is extremely interesting and topical, even if over the years research in this area has gone through alternating phases of progression and inactivity.
The concept of anti-drug vaccination is usually linked to combatting addiction. Addiction can be defined as a treatable, chronic medical disease involving complex interactions among brain circuits, genetics, the environment, and an individual’s life experiences.Citation1 People who suffer from addiction use substances that can be dangerous or engage in behaviors that become compulsive despite harmful consequences.Citation2
specifies the data of the analysis made by the United Nations Office on Drugs and Crime (UNODC), referring to June 2021. During this period, around 275 million people used drugs, up by 22% from 2010.Citation3
Table 1. Illicit drug use at the global level among people aged 15–64 years in 2021.
The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) has also reported rising levels of drug abuse;Citation4 as shown in . Given the alarming data, anti-drug vaccines represent an important development in the pharmacotherapy of chemical dependency.Citation5
Table 2. Statistical bulletin (2021) on the drug situation in Europe.
Table 3. Statistical bulletin 2021 on the use of heroin and other opioids in Europe.
What is the immunotherapeutic principle of the anti-addiction mechanism?
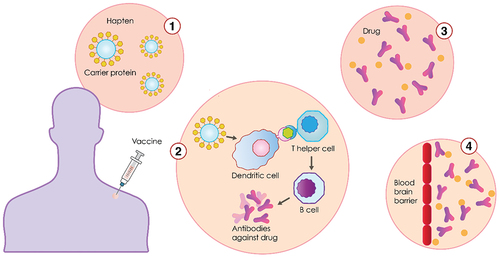
Vaccines against substance use disorders are made by a carrier protein, haptens, and adjuvants. Anti-drug vaccines are active immunizations, which means that they trigger an immunological response against the agent when administered.Citation6 This contrasts with passive immunization using polyclonal or monoclonal antibodies where no immunological memory is formed: when antibody levels fall, the antibodies themselves must be administered again. In the vaccine formulation, the active immune response is given by the presence of a chemical derivative of the drug (hapten), conjugated to an immunogenic carrier protein. For instance, the cocaine vaccine is comprised of succinylnorcocaine molecules covalently linked to a carrier protein derived from the cholera B toxin, which is adsorbed to an aluminum adjuvant. This vaccine can stimulate B-cells to produce antibodies to cocaine as well as its conjugate. Once the immune system has been primed by active vaccination, the later introduction of the vaccine complex should result in the production of an antigen-specific, immunoglobulin G (IgG)-mediated antibody response.Citation7 The effectiveness of the vaccine can then be measured by its ability to create antibodies with specificity and high binding affinity for the drug of abuse; the robustness of the antibody response, i.e., the concentration of antibody produced, is another measure of effectiveness.Citation8
Vaccines against addictions: state-of-the-art and functional principle
An anti-drug vaccine is typically administered along with an adjuvant which increases the strength of the antibody response. In clinical trials, mineral salts like alum, are the most commonly used adjuvant, followed by emulsions (MF59, AS03), Microparticles (Virus-Like Particles and Virosomes), and Immune potentiators (TLR1/2 Agonists).Citation9 An anti-drug vaccine will be considered clinically useful if the antibody response it provokes is sufficiently strong and long-lasting, and if it treats the disorder based on pre-specified criteria developed in collaboration with regulatory authorities. To prevent the drug from having psychoactive effects, the blood concentration of the antibody, or titer, must be high enough to intercept all or most of the target drug molecules before they enter the brain through the blood-brain barrier ().
Large epidemiological studies and clinical trials have identified a range of environmental factors which are contributors to the efficacy of the vaccine: age, sex, ethnicity, size (body-mass index), and health, including smoking status, of individuals as well as the dose, route of administration and quality of storage of the vaccine.Citation10 The main drugs under study for the development of specific vaccines are listed below.
Vaccination against substance use disorders shows efficacy in animal models. Several preclinical findings specifically identified molecules like InterleukinsCitation11 or Naïve B cells,Citation12 showing that they may provide screening tools to predict vaccine clinical efficacy against drugs of abuse or other small molecules, as well as use these molecules as pharmacological target and a potential biomarker of vaccine efficacy. This kind of preclinical trial and other studies may suggest that the vaccine efficacy for substance abuse can be also influenced by the genetics and the immune system of the individual. The host genetic polymorphisms, for example, can modulate the immune response in multiple ways on different scales.Citation13
Cocaine vaccines
The most successful cocaine vaccine to date is a cocaine hapten conjugated to inactivated cholera toxin B, known as TA-CD.Citation14 The TA-CD vaccine generates cocaine-specific antibodies that minimally bind to inactive cocaine metabolites such as benzoylecgonine, ecgonine methyl ester, and benzoic acid.Citation15 Antibody-bound cocaine molecules are then broken down by pseudocholinesterase in circulation, or by nonenzymatic hydrolysis which converts cocaine into inactive metabolites.Citation16 The latter no longer bind to the antibody and are consequently excreted. Since the metabolites do not bind antibodies, this frees up the antibody to bind to more cocaine, prolonging and enhancing its capacity for blocking more cocaine from reaching the brain.
Amphetamine vaccines
Several studies have been carried out to develop an effective vaccine for amphetamine, in particular methamphetamine (METH) addiction, but research so far has been unsuccessful. However, increasing attention has been paid to METH-conjugated vaccine developments through hapten design, and some studies in animal models have demonstrated promising results in preclinical studies which may be translated into clinical trials in the future, proving that METH-specific antibodies were produced.Citation17–19
Nicotine vaccines
Nicotine is the main addictive component of tobacco and plays an important role in the reward system in the brain.Citation20 Nicotine vaccines can be potentially as effective as cocaine vaccines because those who are vaccinated are typically highly motivated to quit smoking and do not share the common withdrawal ambivalence experienced by cocaine users and other addicts.Citation21
Anti-nicotine antibodies created by the vaccine bind to the nicotine molecules, making them too large to pass through the blood-brain barrier. If nicotine is prevented from reaching the brain, this prevents the increased production of the neurotransmitter dopamine, which is responsible for creating feelings of pleasure in the smoker.Citation22 Nic-Qb (NIC002), NicVax, Niccine, and TA-NIC are the most known vaccines to have been used in clinical trials.
Opioid vaccines
The principle is to stimulate the immune system to generate antibodies that attach themselves to the opioid, blocking its passage from the blood into the brain. In this specific case, the creation of a vaccine is particularly complex since, in addition to the obstacles common to all anti-addiction vaccine attempts, the metabolism of heroin generates active metabolites (acetyl morphine and morphine), thus necessitating the development of a vaccine capable of stimulating an immune response to several structurally related but distinct molecules.Citation23,Citation24 At present, no definitive results have been obtained, although it is worth mentioning a recent project at Columbia University concerning Phase 1a/1b clinical trials of multivalent opioid vaccine components.Citation25
The purpose of this meta-analysis and systematic review was to analyze the available studies to get a statistical figure on how many vaccines were able to stimulate an immune response and to know whether they represent an available option for treating substance use disorder. It is well known that the real factor determining the efficacy of the vaccine is the neutralization of the psychotropic substance leading to a decrease in its consumption. Regarding nicotine, several studies suggest that high antibody titers are related to smoking cessation.
Methods
Systematic review
Study design
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.Citation26 The study protocol was registered with PROSPERO (CRD42021256905).
Information sources
A systematic literature search of studies published up to 20 April 2022, was conducted on the Medline database (PubMed, Scopus, and ClinicalTrials.gov).
Search strategy
The generic free-text search terms were “addictive behavior” AND “vaccine” AND “nicotine” AND “heroin” AND “methamphetamine” AND “cocaine” AND “fentanyl.” The search of the PubMed database was carried out by applying both the medical subject headings “MeSH” and the “All field” tags to all the generic free-text terms. The exclusion criteria for the initial search involved all terms that could be associated with human clinical trials on vaccines but not related to the drug topic; the generic free-text search terms did NOT include: “animal models,” “virus,” “papilloma,” “air,” “ataxia,” “covid,” “hepatitis,” “HIV,” “aids,” “bacteria,” “environment,” “pulmonary,” “gene therapy,” “weight,” “bacterium,” “RSV,” “bacillus,” “rabies,” “influenza,” “firearms,” “fetal,” “streptococcus,” “economic,” “stress,” “antidote,” “cost,” “social,” “murine,” “rat,” “rabbit,” and “fever.”
Selection process
All records identified by our search strategy were exported to EndNote software. Duplicate articles were removed from the list (first author). One reviewer screened the titles and abstracts of the identified articles (second author). Duplicates were discarded and only the studies involving clinical trials performed on humans were taken forward. Searches by title and abstract yielded a total of 13 articles. All five authors of the study analyzed the text of each selected article, verifying whether it met the inclusion criteria set for the search. The search method was presented in a PRISMA flow chart showing the included studies and those that were excluded, with reasons for exclusion.
Meta-analysis
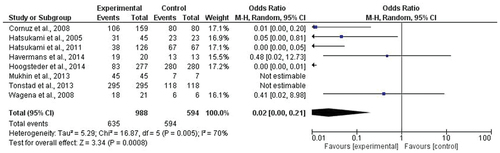
The meta-analysis was performed using all the clinical trials regarding nicotine vaccines found in the databases for the systematic review. The eight studies focus on the use of NicVAX from Nabi/GlaxoSmithKline and the Niccine vaccine from Independent Pharmaceutica in Phase II.
The statistical analysis was performed using RevMan software (version 5.4, The Cochrane Collaboration), taking into consideration frequencies of effectiveness and non-effectiveness of treatment as compared to control groups. The model of random effects and the Mantel-Haenszel statistical method were used, and the odds ratio was the measure of effect.
Heterogeneity among the results from the different studies was detected using the chi-square test, by calculating the I2 statistic. For all the analyses, a significance level of 5% was used.
Results
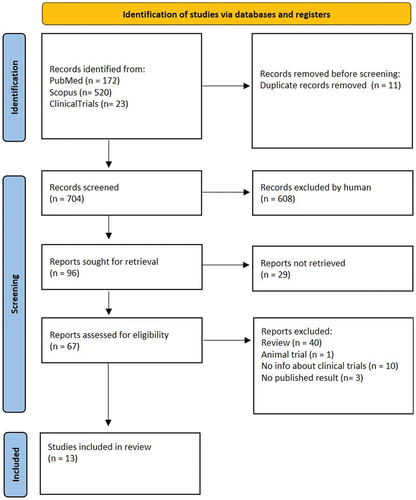
A PRISMA flow diagram highlights the information obtained at different phases of a systematic review. It maps out the number of records identified, included, and excluded, and the reasons for exclusions. The flow diagram used for this research is outlined in .
Figure 2. PRISMA 2020 flow diagram for new systematic reviews which include searches of databases and registers only.
Study selection
The primary search through the database identified 715 studies. One duplicated study was removed, and 704 studies were screened for titles and abstracts. A total of 608 studies were excluded, and 67 articles were assessed for eligibility. 54 studies were excluded because they were review articles or clinical trials performed on animals, or because they did not present information about clinical trials or published results. As a result, a total of 13 studies met the inclusion and exclusion criteria for the review and meta-analysis, as shown in . Eight of them involve the use of the nicotine vaccine and were used for the meta-analysis, and the other five were examined to evaluate cocaine vaccine immunogenicity, safety, and abstinence rates.
Study characteristics
The articles taken into consideration were published from 2002 to 2015. The studies involved roughly 2,266 participants from different ethnic groups (this type of data was shown in a few articles). For the five cocaine vaccine clinical trials, the mean age of the participants was 36.87 years, and the male distribution was wider, roughly 77.5%. Regarding the eight studies on the nicotine vaccine, the mean age was 42.8 years, and females constituted roughly 54.8% of the total population of the samples. The type of vaccines used in the selected clinical trials was: TA-CD to treat cocaine addiction,Citation6–14–Citation27–29 as shown in , and NicVax,Citation30–34 Niccine,Citation35 and Nic002, formerly NicQbeta,Citation36,Citation37 to treat nicotine addiction, as shown in .
Table 4. Comparison of cocaine vaccine clinical trials.
Table 5. Comparison of nicotine vaccine clinical trials.
The results of the meta-analysis demonstrate that vaccines are effective against nicotine abuse, with an increase in the probability of antibody formation of 50 times greater compared to the non-vaccinated group ().
Discussion
Cocaine vaccines
Most of the clinical trials selected from the literature search involved the participation of small numbers of subjects, as shown in . For this reason, it was not possible to perform a meta-analysis to establish statistically relevant levels of vaccine efficacy between vaccine and placebo groups, in terms of antibody formation. On the other hand, two interesting studies shed light on efficacy, safety, and abstinence rates. The clinical trials involved the use of TA-CD, an active vaccine developed by the Xenova Group and created by combining norcocaine with inactivated cholera toxin.Citation14 In 2009, Martell et al. conducted a 24-week, phase 2b, randomized, double-blind, placebo-controlled trial, involving 115 methadone-maintained subjects, randomized to vaccine or placebo. The 21 vaccinated subjects (38%) who attained serum IgG anti-cocaine antibody levels of 43 μg/mL or higher (i.e. high IgG level) had significantly more cocaine-free urine samples than those with levels less than 43 μg/mL (i.e. low IgG level) and the placebo-receiving subjects during weeks 9 to 16 (45% vs. 35% cocaine-free urine samples, respectively). The proportion of subjects showing a 50% reduction in cocaine use was significantly greater in the subjects with a high IgG level than in subjects with a low IgG level (53% of subjects vs. 23% of subjects, respectively) (P = .048). The most common adverse effects were injection site induration and tenderness. There were no treatment-related serious adverse events, withdrawals, or deaths. Attaining high (>or = 43 μg/mL) IgG anti-cocaine antibody levels was associated with significantly reduced cocaine use, but only 38% of the vaccinated subjects attained these IgG levels and they had only two months of adequate cocaine blockade.Citation29 The most influential study was conducted by Kosten et al. in 2014, a Phase III randomized double-blind placebo-controlled trial to evaluate the immunogenicity, efficacy, and safety of succinylnorcocaine conjugated to cholera toxin B protein as a vaccine for cocaine dependence. The 300 subjects had smoked cocaine on average for 13 days monthly at baseline. The authors hypothesized that retention might be better and positive urines lower for subjects with anti-cocaine IgG levels of ≥42 μg/mL (high IgG), which was attained by 67% of the 130 vaccine subjects receiving five vaccinations. Almost 3-times fewer high than low IgG subjects dropped out (7% vs. 20%). Although for the full 16 weeks cocaine-positive urine rates showed no significant difference between the three groups (placebo, high, low IgG), after week 8, more vaccinated than placebo subjects attained abstinence for at least two weeks of the trial (24% vs. 18%), and the high IgG group had the most cocaine-free urines for the last two weeks of treatment (OR = 3.02), but neither were significant. Injection site reactions of induration and tenderness differed between placebo and active vaccine, and the 29 serious adverse events did not lead to treatment-related withdrawals or deaths. Phase III clinical trials showed no significant difference between the placebo group and users given TA-CD. Patients in the high antibody group had a lower dropout rate and fewer cocaine-positive urine results in the last two weeks of the trial, but it was not significant compared to the low antibody or placebo group. However, at other stages in the study, high antibody users had more positive urine results. This is most likely due to users trying to overcome the antibodies by taking more excessive amounts of cocaine.Citation28
Nicotine vaccines
We found eight interesting clinical trials using a vaccine to treat nicotine addiction, as shown in . They were analyzed to perform a meta-analysis of immunogenicity between vaccinated and placebo groups, but they were also examined to gain insights into safety and abstinence rates. The clinical trials involved the use of different vaccines:
Niccine
Niccine was developed by Independent Pharmaceutica AB using tetanus toxoid. Niccine is a nicotine hapten tetanus-toxoid conjugate vaccine. In 2013, Tonstad et al. conducted a Phase II trial to evaluate the clinical efficacy of Niccine for tobacco smoking relapse prevention. In this study, 355 smokers aged 25–50 years were enrolled in a randomized, double-blind, parallel group for a trial lasting one year. Niccine 40 μg or placebo was administered on days 0, 28, 56, 90, 150, and 210. Niccine demonstrated no effect on smoking status at 6 or 9 months; exhaled carbon monoxide levels, time to relapse, abstinence, and withdrawal symptoms did not differ between Niccine and placebo groups. Nicotine antibody levels increased (mean = 1.34 μg/ml; SD = 2.84 μg/ml) in the Niccine group, but were not related to relapse.Citation35
Nic-Qb
This vaccine, also called NIC002, is a virus-like particles (VLPs) vaccine and it was developed by Cytos Biotechnology.Citation38 In 2008, Cornuz et al. conducted a study in which 229 subjects were randomized to receive five intramuscular injections of Nic-Qb and 112 to receive placebo at monthly intervals. All subjects received individual behavioral smoking cessation counseling. The vaccine was shown to be safe, generally well tolerated, and highly immunogenic, inducing a 100% antibody responder rate after the very first injection. Point prevalence of abstinence at month 2 showed a statistically significant difference between subjects treated with Nicotine-Qβ (47.2%) and placebo (35.1%) (P = .036), but continuous abstinence between months 2 and 6 was not significantly different. However, in a subgroup analysis of the per-protocol population, the third of the subjects with the highest antibody levels showed higher continuous abstinence from month 2 until month 6 (56.6%) than placebo-treated participants (31.3%) (OR 2.9; P = .004). Medium and low antibody levels did not increase abstinence rates. After 12 months, the difference in continuous abstinence rate between subjects on placebo and those with high antibody response was maintained (difference 20.2%, P = .012). Although Nicotine-Qβ did not significantly increase continuous abstinence rates in the intention-to-treat population, subgroup analyses of the per-protocol population suggested that such a vaccination against nicotine can significantly increase continuous abstinence rates in smokers when sufficiently high antibody levels are achieved.Citation36
Mukhin et al. conducted a clinical trial in 2013 to find out how vaccine-induced antibodies change the way the body processes nicotine from cigarettes. 55 subjects received four subcutaneous injections of 0.1 mg Nicotine-QB (NIC002) in an alum vaccine with a four-week interval between injections. Ten subjects received four subcutaneous injections of indistinguishable placebo (alum alone) with a four-week interval between injections. Four subcutaneous vaccinations were performed over three months, with four weeks between each vaccination. The administered volume of 0.65 mL of sterile water contained 100 μg of NIC002 and 0.46 mg aluminum hydroxide. Four placebo injections were administered subcutaneously over three months, with four weeks between each vaccination. The administered volume of 0.65 mL of sterile water contained 0.46 mg aluminum hydroxide. The vaccine was demonstrated to be safe and well tolerated. Vaccination resulted in a statistically significant but highly variable increase (CV = 88%) in serum binding capacity B/F = 0.18 ± 0.03 (mean ± SE, n = 29, p < .001). Anti-nicotine immunization can produce both a decrease and an increase in brain nicotine accumulation during smoking, depending on the quality (affinity) and quantity of the produced antibodies.Citation37
NicVax
This vaccine, also called 3’-AmNic-rEPA, is a nicotine conjugate vaccine developed by Nabi Pharmaceuticals, using Pseudomonas exoprotein A, and is currently being further evaluated for clinical use by Glaxo-SmithKline. Hatsukami et al. conducted two studies, the first of which (2005) aimed to assess the safety and immunogenicity of NicVAX and its effects on smoking behavior. Smokers (N = 68) were recruited for a non-cessation treatment study and assigned to one of three doses of the nicotine vaccine (50, 100, or 200 µg) or a placebo. They were injected on days 0, 28, 56, and 182 and monitored for 38 consecutive weeks. Results showed that the nicotine vaccine was safe and well tolerated. Vaccine immunogenicity was dose-related (P < .001), with the highest dose eliciting antibody concentrations within the anticipated range of efficacy. There was no evidence of compensatory smoking or precipitation of nicotine withdrawal with the nicotine vaccine. The 30-day abstinence rate was significantly different across the four doses (P = .02), with the highest rate of abstinence occurring at 200 µg32. The second study (2011) was a double-blinded, placebo-controlled multicentre clinical trial (N = 301 smokers); the researchers tested the results of 200 and 400 µg doses administered four or five times for 6 months, as compared with a placebo. 3‘AmNic-rEPA recipients with the highest serum antinicotine Ab response (top 30% by the area under the curve [AUC]) were significantly more likely than the placebo recipients (24.6% vs. 12.0%, P = .024, odds ratio (OR) = 2.69, 95% confidence interval (CI), 1.14–6.37) to attain eight weeks of continuous abstinence from weeks 19 to 26. The five-injection, 400 µg dose regimen elicited the strongest Ab response, with significantly higher abstinence rates than the placebo. This study demonstrated, as proof of concept, that 3 AmNic-rEPA elicits Abs to nicotine and is associated with higher continuous abstinence rates (CAR).Citation30 Havermans et al. (2014) designed a study to assess whether immunization attenuates nicotinic stimulation of the brain and whether it elucidates brain and behavioral responses during exposure to smoking cues and a working memory task. Forty-eight male smokers were randomized to receive five injections with either 400 μg/ml of the 3-aminomethylnicotine Pseudomonas aeruginosa r-Exoprotein-conjugated vaccine (NicVax) or placebo. Subjects were tested on two separate occasions, once after a nicotine challenge and once after a placebo challenge, and were asked to refrain from smoking ten hours before testing. In response to a nicotine challenge, no significant effects of immunization on brain activity were established. Therefore, this vaccine is not likely to be an effective aid in smoking cessation.Citation32 Hoogsteder et al. conducted a randomized placebo-controlled trial to examine the efficacy of adding NicVAX versus placebo to varenicline and behavioral support as an aid in smoking cessation and relapse prevention. A total of 558 smokers were assigned randomly to six injections with NicVAX (n = 278) or placebo (n = 280) both co-administered with open-label varenicline and behavioral support. There was no difference in abstinence rates between NicVAX and placebo from weeks 9 to 52 [27.7 vs. 30.0%, odds ratio (OR) = 0.89, 95% confidence interval (CI) = 0.62–1.29] or weeks 37 to 52 (33.8 vs. 33.2%, OR = 1.03, 95% CI = 0.73–1.46). The top 30% antibody responders, compared to the placebo group, showed a non-significant tendency toward higher abstinence rates from weeks 37 to 52 (42.2 vs. 33.2%, OR = 1.47, 95% CI = 0.89–2.42).Citation33 According to these findings, the nicotine vaccine, NicVAX, does not appear to improve a smoker’s chances of stopping when given in addition to varenicline and behavioral support.
Wagena et al. conducted a randomized, placebo-controlled phase 1/2 trial in 2008 to evaluate the safety and immunogenicity of four doses of a nicotine vaccine in smokers and nonsmokers. Subjects were 21 smokers and 9 nonsmokers in good physical and mental health. They received four spaced intramuscular injections of 100 µg of purified 3’-aminomethylnicotine conjugated to detoxified Pseudomonas aeruginosa r-exoprotein A or placebo, both adsorbed to 800 µg aluminum into the deltoid muscle of alternating arms. Intensive follow-up for 266 days revealed that the vaccine was well tolerated. The researchers found no significant differences in adverse events between the vaccine and placebo groups. Significant increases in the geometric mean titer (GMT) levels of nicotine-specific antibodies were observed from 7 days after the second vaccination (day 21), reaching nicotine-specific antibody levels of at least 8 µg/ml in half of the subjects (50%) at day 49. A fourth dose administered on day 182 significantly boosted waning antibody levels to a GMT of 10.8 µg/ml on day 217 (95% CI 6.0–19.3). Results showed that the immunogenicity of the vaccine was not impeded by the presence of nicotine. These observations provided evidence that the vaccine used may represent a feasible strategy for evoking type-specific antibodies against nicotine.Citation34
TA-NIC (Celtic Pharma): TA-NIC was developed using a recombinant cholera toxin-B subunit as a carrier protein for the nicotine vaccine,Citation39 based on a similar principle to the TA-CD vaccine against cocaine. No preclinical results have been published to date; hence the literature search did not find articles related to this kind of vaccine. However, it is documented that Xenova Group in the United Kingdom completed two Phase I/II studies with this vaccine candidate in 120 patients who were smokers, showing the efficacy and safety of the vaccine. Although the results of these preliminary studies herald that vaccination may facilitate tobacco withdrawal, the researchers agree that more in-depth investigation is needed to support positive conclusions.Citation40
Limitations
This study has some limitations. The relatively small number of included trials and their high heterogeneity must be considered when interpreting the results. Through a literature search, we found a relatively small number of studies performed on humans, with most of the preclinical studies being conducted on animal models, on which research has in recent years made significant progress with regard to nicotine and cocaine. On the other hand, we chose not to include this type of studies conducted on animals, to restrict the field to more specific information about the human organism;Citation41 and some of them demonstrated promising results, which may be translated into clinical trials in the future. Most of the human trials cited stopped at a certain point in time, and results were not always published for different reasons. There were not enough studies to perform a statistically relevant meta-analysis on vaccines against cocaine. All of the clinical trials with promising results that were selected for this systematic review were designed with different aims. For this reason, it was not possible to develop a complete classification of data considering various aspects such as ethnicity or abstinence rates, because these parameters were not considered in some of the studies.
Conclusions
In a single contribution and through a systematic review, this paper reports on all of the studies, both preliminary and complete, that have been conducted thus far concerning vaccination as a tool for drug addiction. According to the review, we can deduce that all of the vaccine formulations tested are safe, with mild to moderate side effects. Some individuals showed “flu-like” symptoms such as headache, fatigue, muscle ache, and mild skin reaction at the injection site. The meta-analysis of studies on nicotine vaccines confirms the property of the vaccine to create specific antibodies against the target molecule, with an increase in the probability of antibody formation of 50 times greater compared to the non-vaccinated. Phase II clinical trials of nicotine vaccines suggest only slight efficacy in aiding smoking cessation. The abstinence rates among vaccinated smokers were lower or equal to those in the placebo control groups. However, in some studies, the abstinence rates of individuals with high antibody levels were higher than in the placebo group. Through the literature search, we did not find any Phase III clinical trials for NicVAX, Niccine, or NIC002. However, according to press statements,Citation42 two Phase III NicVax trials have been performed and failed to show the efficacy of the vaccine between the experimental and control group. Regarding vaccines to treat cocaine addiction, TA-CD Phase III clinical trials showed no significant difference between experimental and placebo groups.Citation28 This type of vaccine never succeeded beyond a Phase III clinical trial, so we assume that there is no firm evidence of vaccine efficacy against substance use disorders. To establish the efficacy of these vaccines for drug use cessation, more trials involving the use of different vaccines should be performed. Soon, the search for new vaccines against nicotine and other drugs must continue with advanced studies tested on a large number of patients. To counter the phenomenon of drug addiction, it will also be necessary to invest in parallel the behaviors and attitudes that would motivate the consumer to abstain from drugs.
The results of the meta-analysis offer an important overview by suggesting that the best-known vaccines for the treatment of substance use disorders that have been tested so far are capable of creating specific antibodies; on the other hand, the efficacy of the vaccine is not based on immunogenicity but rather on the neutralization of the psychotropic substance which leads to a decrease in its consumption. The deficient effect on craving is not caused by the absence of specific antibodies but other reasons must be taken into consideration: exposure to triggers, stress, interpersonal problems, etc., represent concrete risk factors that contribute to drug desire and relapse. It is also not excluded that vaccination may best work with patients who are highly motivated to quit.Citation43 In the future it would be useful to propose a research study that takes into account the detection of the antibody titer on vaccinated subjects, and administering to them a specific questionnaire to test the stop smoking motivation and the presence of any relapses.
Acknowledgements
Thanks to Jemma Dunnill for proofreading the manuscript. Thanks also to Lorenzo Ranaldi for the graphic elaboration of the Figure n. 1.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kampman K, Jarvis M. American Society of AddictionMedicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):1–11. doi:10.1097/ADM.0000000000000166.
- Grant JE, Potenza MN, Weinstein A, Gorelick DA. Introduction to behavioral addictions. Am J Drug Alcohol Abuse. 2010;36(5):233–41. doi:10.3109/00952990.2010.491884.
- United Nations Office on Drugs and Crimes. UNODC world drug report 2021: pandemic effects ramp up drug risks, as youth underestimate cannabis dangers; 2021 [accessed 2022 Apr 10]. https://www.unodc.org/unodc/press/releases/2021/June/unodc-world-drug-report-2021_-pandemic-effects-ramp-up-drug-risks–as-youth-underestimate-cannabis-dangers.html.
- European Monitoring Centre for Drugs and Drug Addiction. Statistical Bulletin; 2021 [accessed 2022 Apr 10]. https://www.emcdda.europa.eu/data/stats2021_en.
- Shen X, Kosten TR. Immunotherapy for drug abuse. CNS Neurol Disord Drug Targets. 2011;10(8):876–79. doi:10.2174/187152711799219352.
- Kosten TR, Rosen M, Bond J, Settles M, Roberts JSC, Shields J, Jack L, Fox B. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20(7–8):1196–204. doi:10.1016/S0264-410X(01)00425-X.
- Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clin Pharmacol Ther. 2012;91(1):60–70. doi:10.1038/clpt.2011.281.
- Orson FM, Kinsey BM, Singh RA, Wu Y, Kosten TR. Vaccines for cocaine abuse. Hum Vaccin. 2009;5(4):194–99. doi:10.4161/hv.5.4.7457.
- Pulendran B, PS A, DT O. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;20(6):454–75. doi:10.1038/s41573-021-00163-y.
- Mentzer AJ, O’Connor D, Pollard AJ, Hill AVS. Searching for the human genetic factors standing in the way of universally effective vaccines. Philos Trans R Soc Lond B Biol Sci. 2015;370(1671):20140341. doi:10.1098/rstb.2014.0341.
- Crouse B, Robinson C, Huseby Kelcher A, Laudenbach M, Abrahante JE, Pravetoni M. Mechanisms of interleukin 4 mediated increase in efficacy of vaccines against opioid use disorders. NPJ Vaccines. 2020;5(1):99. doi:10.1038/s41541-020-00247-7.
- Taylor JJ, Laudenbach M, Tucker AM, Jenkins MK, Pravetoni M. Hapten-specific naïve B cells are biomarkers of vaccine efficacy against drugs of abuse. J Immunol Methods. 2014;405:74–86. doi:10.1016/j.jim.2014.01.010.
- Linnik JE, Egli A. Impact of host genetic polymorphisms on vaccine induced antibody response. Hum Vaccin Immunother. 2016;12(4):907–15. doi:10.1080/21645515.2015.1119345.
- Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58(2):158–64. doi:10.1016/j.biopsych.2005.04.032.
- Kinsey BM, Kosten TR, Orson FM. Anti-cocaine vaccine development. Expert Rev Vaccines. 2010;9(9):1109–14. doi:10.1586/erv.10.102.
- Deng SX, de Prada P, Landry DW. Anticocaine catalytic antibodies. J Immunol Methods. 2002;269(1–2):299–310. doi:10.1016/S0022-1759(02)00237-5.
- Hossain MK, Hassanzadeganroudsari M, Nurgali K, Apostolopoulos V. Vaccine development against methamphetamine drug addiction. Expert Rev Vaccines. 2020;19(12):1105–14. doi:10.1080/14760584.2020.1857738.
- Miller ML, Aarde SM, Moreno AY, Creehan KM, Janda KD, Taffe MA. Effects of active anti-methamphetamine vaccination on intravenous self-administration in rats. Drug Alcohol Depend. 2015;153:29–36. doi:10.1016/j.drugalcdep.2015.06.014.
- Kosten T, Domingo C, Orson F, Kinsey B. Vaccines against stimulants: cocaine and MA. Br J Clin Pharmacol. 2014;77(2):368–74. doi:10.1111/bcp.12115.
- Benowitz NL. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu Rev Pharmacol Toxicol. 2009;49(1):57–71. doi:10.1146/annurev.pharmtox.48.113006.094742.
- Maurer P, Bachmann MF. Vaccination against nicotine: an emerging therapy for tobacco dependence. Expert Opin Investig Drugs. 2007;16(11):1775–83. doi:10.1517/13543784.16.11.1775.
- Esterlis I, Hannestad JO, Perkins E, Bois F, D’Souza DC, Tyndale RF, Seibyl JP, Hatsukami DM, Cosgrove KP, O’Malley SS. Effect of a nicotine vaccine on nicotine binding to β2*-nicotinic acetylcholine receptors in vivo in human tobacco smokers. Am J Psychiatry. 2013;170(4):399–407. doi:10.1176/appi.ajp.2012.12060793.
- Neil Stowe G, Schlosburg JE, Vendruscolo LF, Edwards S, Misra K K, Schulteis G S, Zakhari J, Koob GF, Janda KD. Developing a vaccine against multiple psychoactive targets: a case study of heroin. 2011;10(8):865–75. doi:10.2174/187152711799219316.
- Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AAK, Stowe GN, Edwards S, Janda KD, Koob GF. Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci U S A. 2013;110(22):9036–41. doi:10.1073/pnas.1219159110.
- Columbia University Irving Medical Center. Experimental opioid vaccine being tested at Columbia; 2021 [accessed 2022 Apr 10]. https://www.cuimc.columbia.edu/news/experimental-opioid-vaccine-being-tested-columbia.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
- Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. Biol Psychiatry. 2010;67(1):59–65. doi:10.1016/j.biopsych.2009.08.031.
- Kosten TR, Domingo CB, Shorter D, Orson F, Green C, Somoza E, Sekerka R, Levin FR, Mariani JJ, Stitzer M, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42–47. doi:10.1016/j.drugalcdep.2014.04.003.
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116–23. doi:10.1001/archgenpsychiatry.2009.128.
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim REF, et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89(3):392–99. doi:10.1038/clpt.2010.317.
- Hatsukami DK, Rennard S, Jorenby D, FIORE M, Koopmeiners J, Devos A, Horwith G, Pentel P. Safety and immunogenicity of a nicotine conjugate vaccine in current smokers [published correction appears in Clin Pharmacol Ther. 2006 Apr; 79 (4):396]. Clin Pharmacol Ther 2005;78(5):456–67. doi:10.1016/j.clpt.2005.08.007.
- Havermans A, Vuurman EF, van den Hurk J, Hoogsteder P, van Schayck OCP. Treatment with a nicotine vaccine does not lead to changes in brain activity during smoking cue exposure or a working memory task. Addiction. 2014;109(8):1260–67. doi:10.1111/add.12577.
- Hoogsteder PH, Kotz D, van Spiegel PI, Viechtbauer W, van Schayck OCP. Efficacy of the nicotine vaccine 3′-AmNic-rEPA (NicVAX) co-administered with varenicline and counselling for smoking cessation: a randomized placebo-controlled trial. Addiction. 2014;109(8):1252–59. doi:10.1111/add.12573.
- Wagena EJ, de Vos A, Horwith G, van Schayck C. The immunogenicity and safety of a nicotine vaccine in smokers and nonsmokers: results of a randomized, placebo-controlled phase 1/2 trial. Nicotine Tob Res. 2008;10(1):213–18. doi:10.1080/14622200701704921.
- Tonstad S, Heggen E, Giljam H, Lagerback P-A, Tonnesen P, Wikingsson LD, Lindblom N, de Villiers S, Svensson TH, Fagerstrom K-O. Niccine®, a nicotine vaccine, for relapse prevention: a phase II, randomized, placebo-controlled, multicenter clinical trial. Nicotine Tob Res. 2013;15(9):1492–501. doi:10.1093/ntr/ntt003.
- Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Müller P, Willers J, et al. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3(6):e2547. doi:10.1371/journal.pone.0002547.
- Mukhin AG. Improving the efficacy of anti-nicotine immunotherapy (PETNic002). ClinicalTrials.gov identifier: NCT01280968; 2013 [accessed 2022 Apr 10]. https://clinicaltrials.gov/ct2/show/NCT01280968.
- Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, Renner WA, Müller P, Bachmann MF. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol. 2005;35(7):2031–40. doi:10.1002/eji.200526285.
- Goniewicz ML, Delijewski M. Nicotine vaccines to treat tobacco dependence. Hum Vaccin Immunother. 2013;9(1):13–25. doi:10.4161/hv.22060.
- Escobar-Chávez JJ, Domínguez-Delgado CL, Rodríguez-Cruz IM. Targeting nicotine addiction: the possibility of a therapeutic vaccine [published correction appears in Drug Des Devel Ther. 2011; 5:487]. Drug Des Devel Ther 2011;5:211–24. doi:10.2147/DDDT.S10033.
- Ohia-Nwoko O, Kosten TA, Haile CN. Animal models and the development of vaccines to treat substance use disorders. Int Rev Neurobiol. 2016;126:263–91.
- Nabi Biopharmaceuticals. News release: Nabi biopharmaceuticals announces results of second nicvax(r) phase iii clinical trial; 2011 [accessed 2022 Apr 10]. https://www.fiercebiotech.com/biotech/nabi-biopharmaceuticals-announces-results-of-second-nicvax-r-phase-iii-clinical-trial-0.
- Kantak KM. Vaccines against drugs of abuse: a viable treatment option? Drugs. 2003;63(4):341–52. doi:10.2165/00003495-200363040-00001.