ABSTRACT
This observational retrospective study was conducted on patients with epilepsy (PWE) in China who had at least one dose of COVID-19 vaccine and it investigated the safety of vaccination by analyzing changes in epileptic seizures and their influencing factors. Consecutive PWE who were followed up in the epilepsy clinic between June 2021 and May 2022 were enrolled. Data on vaccine type, demographic information, clinical characteristics of epilepsy, and treatment were collected through a questionnaire survey and retrospectively analyzed. PWE were divided into a stable seizure group and a worsening seizure group based on seizure episodes at least 90 days after the first vaccine dose. A total of 79 PWE were included. After vaccination, 14 patients (17.7%) had worsening seizures, 92.9% of whom had an increased seizure frequency. Compared with patients in the stable seizure group, patients in the worsening seizure group had significant differences in baseline monthly seizure frequency (P = .012), improper antiseizure medication (ASM) administration (P = .003) and a disrupted sleep routine (P = .016). Multivariate logistic regression analysis showed that improper ASM administration (OR 6.186, 95% confidence interval [CI] 1.312–29.170; p = .021) and a disrupted sleep routine (OR 6.326, 95% CI 1.326–30.174; p = .021) were significantly associated with seizure worsening. In short, COVID-19 vaccination is safe for PWE, and only those with poor seizure control have the possibility of seizure exacerbation after COVID-19 vaccination. The vaccination per se does not represent a major influencing factor, but the improper use of ASMs and a disrupted sleep routine may be correlated with seizure aggravation after vaccination.
Introduction
Coronavirus disease 2019 (COVID-19) is still prevalent worldwide. The COVID-19 vaccine is the first vaccine to be administered on a large scale globally and has wide coverage. According to World Health Organization (WHO) statistics, as of May 2022, the number of COVID-19 vaccine doses has exceeded 115 trillion worldwide.Citation1 A number of studies have shown that approved vaccines still have immunoprotective effects on mutant strains.Citation2–4 Sequential vaccination can generate a stronger neutralizing antibody response and still effectively prevent the occurrence of severe cases.Citation5
Epilepsy is one of the most common neurological diseases, and is characterized by an enduring predisposition to generate epileptic seizures. A Spanish study in March 2020 showed that patients with epilepsy (PWE) had greater disease severity and higher mortality rates from COVID-19 than patients without epilepsy.Citation6 In an analysis of Hungarian national mortality data from March 2020 to January 2021, the proportion of PWE among young people who died of COVID-19 was relatively high.Citation7 The finding of a recent meta-analysis established that PWEs are at risk of having poor COVID-19 outcomes.Citation8 A number of questionnaire surveys on the attitudes of PWE regarding COVID-19 vaccination have shown that PWE have concerns about vaccine safety and increased seizures after COVID-19 vaccination.Citation9–11 Low COVID-19 vaccination coverage (70.3%) and willingness (58%) in PWE have been reported by a recent meta-analysis.Citation12
In recent years, studies from Germany, Kuwait, TurkeyCitation13–15 and Sichuan, ChinaCitation16 showed no significant difference in adverse reactions between PWE and the general population after vaccination. A few studies also suggested that epilepsy-related problems such as an increase in seizure frequency and status epilepticus after COVID-19 vaccination were uncommon;Citation12 however, factors contributing to worsening seizures after vaccination have rarely been explored.
The International League Against Epilepsy (ILAE) indicated that there is currently no evidence to suggest that having epilepsy is specifically associated with a higher risk of side effects of the COVID-19 vaccine.Citation17 However, the recommendations for COVID-19 vaccination in PWE in various countries are still inconsistent. The United Kingdom and many European countries have listed PWE as a priority population for COVID-19 vaccination,Citation18,Citation19 while countries such as ChinaCitation20 have listed active epilepsy as a contraindication for vaccination.
In view of the fact that real-world studies on PWE and the safety profile of COVID-19 vaccination are underrepresented in the current literature, this study examined the safety and tolerability of vaccinations against COVID-19 in PWE by analyzing changes in seizures after vaccination and possible related factors, especially seizure exacerbation after vaccination and possible influencing factors. This study aimed to provide a practical basis for the development of COVID-19 prevention and control strategies for PWE.
Research subjects and methods
Setting and subjects
The study was an observational retrospective study conducted across the COVID-19 vaccination campaign. We consecutively recruited patients with a diagnosis of epilepsy according to the International League Against Epilepsy (ILAE) criteriaCitation21 who were admitted to the Outpatient Clinics from the epilepsy center of the First Affiliated Hospital of Guangxi Medical University between June 2021 and May 2022. In this study, we included patients who gave informed consent to participate, and had received at least one dose of a COVID-19 vaccine, and had their seizure frequency evaluated at least 90 days after the first dose.
The enrolled patients were further divided into a stable seizure group and a worsening seizure group. The related clinical information, including gender, age, medical history, etiology, seizure type, medication, seizure frequency before and after vaccination and predisposing factors of seizures, were retrospectively analyzed and compared between the two groups.
Stable seizures were defined as no change in seizures or seizures reduced by less than 50% compared with the baseline frequency before vaccination. Worsening seizures were defined as any one or more of the following three conditions:Citation22,Citation23 1. The monthly frequency of seizures after vaccination increased by more than 50% compared with the baseline frequency before vaccination; 2. The occurrence of new types of seizures after vaccination; or 3. The duration of epileptic seizures was increased (the duration of seizures was more than 50% longer than the average duration of seizures in the same patient with the same type of seizure), and even status epilepticus occurred.
The exclusion criteria were as follows: patients with intellectual disability, patients or caregivers who were unable to provide accurate information about their diseases and clinical status, patients who refused to provide informed consent, and patients with epilepsy without a COVID-19 vaccination.
Three inactivated virus vaccines (Vero Cells) were used, including Sinopharm/BIBP (Beijing Bio-Institute of Biological Products Co. Ltd), Sinopharm/WIBP (Wuhan Institute of Biological Products Co., Ltd) and CoranaVac (Beijing Kexing Zhongwei Biotech Co., Ltd.)
The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and was reviewed by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University(NO.2022-KY-E-(274)).
Data acquisition
Clinical data on epilepsy and COVID-19 vaccinations were obtained via a structured questionnaire and interview. The standardized questionnaire included information as follows: patient demographic characteristics (age and sex), medical history, epilepsy characteristics (age of disease onset, epilepsy duration, etiology, seizure and epilepsy type, 1-year prevaccination seizure frequency pattern, 90 days prevaccination baseline seizure frequency, and trigger factors of seizures), treatment with antiseizure medications (ASMs) (types of ASMs before vaccination, ASM variations, and adverse drug reactions), vaccination information, including the date of vaccination and the type of vaccine, postvaccine seizure relapse (occurrence and timing of seizures after the scheduled vaccine doses and type of seizure), other side effects due to vaccination (local or systemic reactions), and previous SARS-CoV-2 infection. The questionnaires were completed by trained epilepsy specialists through face-to-face interviews and/or telephone interviews.
Epilepsy, epileptic seizures, and etiology were classified according to the guidelines and consensus of the ILAE in 2010 and 2017,Citation24,Citation25 The one year prevaccination seizure frequency was divided into five patterns (i.e., average seizure of less than once per year, more than once per year, more than once every 3 months, more than once per month, and more than once per week). Seizure freedom is defined as freedom from seizures for ≥ thrice the longest inter-seizure interval or 12 months.Citation26 Predisposing factors of seizures included fatigue, disrupted sleep routine, excessive use of electronic products, and the consumption of alcohol or beverages containing caffeine and other excitatory substances.
The primary outcome in the present study was seizure changes after COVID-19 vaccination in PWE. Secondary outcomes included seizure worsening and influencing factors for the seizure worsening.
Statistical methods
Descriptive statistics were used to describe the demographic and clinical features of the sample. The Kolmogorov-Smirnov test was used for numerical variables with a normal distribution, which are expressed as the mean and standard deviation, and a t-test was used for comparisons between groups. Variables that did not meet the Kolmogorov-Smirnov test are expressed as the median (interquartile range), and the Mann-Whitney U rank sum test was used for comparisons between two groups. Categorical variables are presented as absolute numbers (n) and percentages, and were analyzed using the chi-square test or Fisher’s exact probability method.
Variables compared in the univariate analysis were entered into a multivariate logistic regression analysis to determine adjusted odds ratios (ORs). The model for multivariate analysis was made by choosing variables with the significance in the univariate comparison and for clinical relevance.
All statistics were performed using Statistical Package for Social Science (SPSS) software version 26 (SPSS, Inc.), P < .05 was considered statistically significant.
Results
Vaccination status of the enrolled patients
A total of 79 patients met the inclusion criteria and were ultimately enrolled in the study (Supplemental Figure). The median duration of follow-up was 118 (27.5) days. None of the enrolled patients were lost to follow-up.
All patients received vaccine doses according to the scheduled interval for each vaccine type. Seventy-six patients (96.2%) received an inactivated virus vaccine (Vero Cell), while three patients received a recombinant novel coronavirus vaccine (CHO cells) (3.8%). Seventy-three patients (92.4%) completed the entire course of two doses (inactivated virus vaccine) or three doses (recombinant novel coronavirus vaccine), and six patients (7.6%) received only the first dose (inactivated virus vaccine). The median interval between the first and the second dose was 26 (15.5) days. None of the PWE reported a history of COVID-19 infection.
Demographic and clinical characteristics of the PWE
In this cohort, 45 patients were males (56.0%) and 34 were females (44%), with a median age of 27 (13) years. Ten (12.7%) of the 79 patients were aged 12–18 years, while the other 69 patients (87.3%) were aged 18 years or older. The median epilepsy duration was 7.0 (8.0) years; 33 patients (41.8%) had an epilepsy duration ≤5 years, 17 patients (21.5%) had a duration of 5–10 years, and 29 patients (36.7%) had a duration of more than 10 years. Seven PWE (8.9%) had a history of febrile seizures (FS). Forty-five patients (57.0%) had a structural etiology, five patients had an infectious etiology (6.3%), four patients had a genetic etiology (5.1%), and the etiology in 25 patients (31.6%) was unknown. Focal seizures (67 patients, 84.8%), generalized seizures (2 patients, 2.5%), and unknown seizure types (10 patients, 12.7%) were detected among the admitted PWE.
Out of 79 patients, 39 patients (49.4%) were in monotherapy: 11 patients (13.9%) were treated with sodium valproate, 10 patients (12.7%) with oxcarbazepine, 7 patients (8.8%) with lamotrigine, 5 patients (6.3%) with levetiracetam, and 4 patients (5.1%) with carbamazepine. Twenty patients (25.3%) took a combination of ≥ 2 ASMs. The average number of ASMs being used was 1.0 (2.0). Twenty-two patients (27.8%) did not take ASMs before vaccination. Among them, 13 patients did not take ASMs due to a rare seizure frequency of less than one time per year, in which 11 patients had freedom from seizures for more than 2 years; 6 patients treated themselves by Chinese traditional medicine, and 3 patients only took antipsychotic drugs because of the significant symptoms of mental disturbance and infrequent seizures (approximately 2–3 times per year) ().
Table 1. Demographic and clinical characteristics of the patients with epilepsy.
The seizure frequency was less than once per year in 16 patients (20.3%), more than once per year in 21 patients (26.9%), more than once every 3 months in 9 patients (11.4%), more than once per month in 23 patients (29.1%), and more than once per week in 10 patients (12.7%). The baseline monthly seizure frequency before vaccination was 0.5 (1.0) ().
Changes in seizures after COVID-19 vaccination in PWE
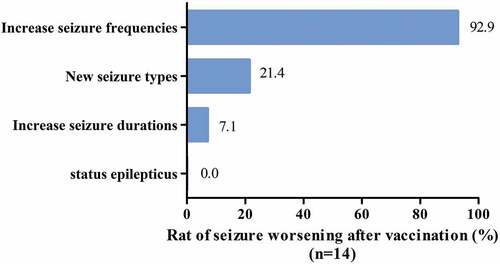
After COVID-19 vaccination, no changes in seizures were observed in 65 patients (82.3%), and 14 patients (17.7%) experienced worsening seizures. Among the patients with worsening seizures, 13 (13/14, 92.9%) had increased seizure frequencies, and three (21.4%) experienced changes in seizure types compared to the prevaccine period. Among them, one patient had focal seizure progression to bilateral tonic-clonic seizures; one patient experienced perceptual disturbance with automatism, which manifested as episodic stupor and groping; and one patient experienced autonomic seizures, which manifested as episodes of palpitation with dizziness; all of these observations corresponded to first-time manifestations. Further examinations showed that no new structural changes were found in brain magnetic resonance imaging (MRI) reexamination, the blood tests were normal, and the epileptic discharges on electroencephalogram (EEG) were more active than before vaccination. One patient experienced worsening seizures with longer seizure duration than before vaccination, and no patients experienced status epilepticus after vaccination ().
Figure 1. The patterns and their occurrence rate of seizures worsening after vaccination.
In addition, the side effects of vaccination in our cohort were all mild and fleeting. Sixteen patients (20.3%) experienced endurable pain at the injection site and muscular pain after vaccination, 3 patients (3.8%) exhibited transient dizziness and fatigue, and one patient (1.3%) experienced a single vomiting episode after the second dose. None of the patients experienced fever after vaccination. Other local and systemic side effects were not found.
Comparison of clinical characteristics among patients with worsening seizures after COVID-19 vaccination
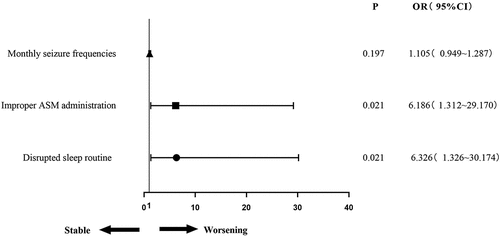
Compared with patients with stable seizures after vaccination, patients with worsening seizures after vaccination had higher baseline monthly seizure frequencies before vaccination (P = .012) (). No differences were observed between the two groups concerning sex, age, disease duration, history of FS, etiology, seizure type, the number of ASMs taken, or seizure frequency patterns.
Table 2. Univariate comparison between the changes of seizures after COVID-19 vaccination.
Seizure predisposing factors, including not receiving treatment with ASMs or improper ASM administration within 1 month before and after vaccination (including missed medication, self-reduction, or withdrawal), as well as disrupted sleep routine, fatigue, excessive use of electronic products, and the consumption of alcohol or beverages containing caffeine and other excitatory substances after vaccination, were reported in this cohort. None of the 14 patients with seizure worsening reported fever or infection after vaccination. Univariate analysis suggested that improper ASM administration (P = .003) and a disrupted sleep routine (P = .016) were associated with worsening seizures after vaccination ().
Table 3. Univariate comparison between the changes of seizures after COVID-19 vaccination.
All the comparisons are summarized in and .
Factors correlated with seizure exacerbation after vaccination
The clinical characteristics with significant differences in the univariate analysis (P < .05) were included in a multivariate logistic regression analysis. The results suggested that improper ASM administration [odds ratio (OR) 6.186, confidence interval (CI) 1.312–29.170, P = .021] and disrupted sleep routine (OR 6.326, CI 1.326–30.174, P = .021) were associated with seizure worsening ().
Discussion
The fear of worsening epileptic seizures after COVID-19 vaccination was the most important reason for vaccination refusal by most PWE.Citation9,Citation10 This study is one of the few clinical observational studies focusing on seizure changes in PWE after COVID-19 vaccination and their influencing factors, especially seizure exacerbation after COVID-19 vaccination. This is also the only observational study of the Chinese population. In this study, only 17.7% of PWE experienced worsening seizures after COVID-19 vaccination, suggesting that patients with poor seizure control before vaccination (high seizure frequency) may experience seizure worsening after vaccination, while improper ASM administration and a disrupted sleep routine were associated with seizure exacerbation after vaccination.
Correlations between the main epilepsy-related clinical characteristics of the PWE in this study and seizure exacerbation after COVID-19 vaccination were not found, including the epilepsy duration, history of FS, seizure type, and treatment regimens before vaccination. This finding is consistent with a clinical observational cohort study in Italy conducted in May 2022.23 Similarly, we also observed that patients with poor seizure control were more likely to have worsening seizures, although multivariate analysis did not show a relatively strong correlation between them because of the small study sample. In PWE with high seizure frequencies, the seizure threshold is speculated to be low, and the inflammatory reaction may play an important role in this condition. Based on the pathophysiological processes of inflammatory diseases of the central nervous system (CNS) (such as autoimmune encephalitis and multiple sclerosis; the disease course from the acute phase to the recovery phase is usually 3 months), and concerning the possibility of the occurrence of CNS immune-inflammatory adverse reactions induced by vaccines, we designated 90 days as the minimum observation period after COVID-19 vaccination. Three patients experienced changes in seizure types in the present study. Further examination, including brain MRI, EEG and blood test, could not identify the cause, especially the cause related to immune-inflammation.
Although the COVID-19 vaccine has been administered on a large scale worldwide, the observation time is still short, clinical studies on COVID-19 vaccination in PWE are scarce, and basic research is lacking. Studies on other types of vaccines in PWE, such as the clinical study of Arnheim-Dahlström et al. in 2012 on the risk of seizures after influenza vaccination, have shown that the risk of seizures in PWE did not increase after vaccination.Citation27 In 2002, Barlow WE et al. studied associations between diphtheria-pertussis-tetanus (DPT) and measles-mumps-rubella (MMR) vaccines and epileptic seizures and showed that the DPT and MMR vaccine did not increase the risk of epilepsy.Citation28 On the other hand, due to many reports of neurological complications after COVID-19 infection, the adverse reactions of the nervous system after COVID-19 vaccination have also received increasing attention. The results of a case‒control study on neurological complications following COVID-19 vaccination and COVID-19 infection in the United Kingdom showed that the risk of neurological adverse reactions after COVID-19 vaccination was far lower than that after COVID-19 infection.Citation29 In October 2021, an observational study in Singapore targeting neurological diseases after receiving the COVID-19 mRNA vaccine showed no evidence of a causal association between the vaccine and any neurological diseases (including epilepsy).Citation30 The results of this study also validated these results from another perspective. We believe that the COVID-19 vaccine should not be refused because of concerns about the risk of adverse neurological reactions.
In this study, most patients with aggravated seizures reported clear predisposing factors, and some patients had two to three predisposing factors at the same time. The regression analyses showed that improper ASM administration before and after vaccination and a disrupted sleep routine were significantly correlated with seizure worsening. Studies have shown that low drug adherence can significantly increase the mortality rate and the incidence of other diseases in PWE.Citation31,Citation32 Related studies have also confirmed that a lack of sleep is an independent triggering factor for epilepsy.Citation33,Citation34 A survey from Italy showed that changes in sleep habits were one of the main factors contributing to epileptic seizure worsening during the COVID-19 pandemic.Citation35 Similarly, a survey in the United States showed that the main reasons for the increase in seizures reported during the COVID-19 pandemic were attributed to sleep disturbances and reduced exercise.Citation36 An expert consensus by the Chinese Association Against Epilepsy on COVID-19 vaccination in PWE recommends that PWE should monitor their symptoms before and after vaccination and take ASMs regularly before and after vaccination to avoid seizure triggers such as sleep deprivation, alcohol consumption, and dramatic mood swings.Citation37
Conclusion
The present study demonstrated that COVID-19 vaccines have a good safety and tolerability profile in PWE, and only a small percentage of patients had transient seizure worsening, but no evidence indicated that vaccination per se had a correlation with aggravating epilepsy. Uncontrolled epilepsy, improper ASM administration and unhealthy living habits are likely to be important factor of seizure recurrence after COVID-19 vaccination. However, our data support COVID-19 vaccination for a wider population of PWE.
Because of the limitations of monocentric, small sample and retrospective studies, further multicenter studies with large sample sizes, prospective designs, and longer follow-up times are needed to verify this finding.
Supplemental Material
Download PDF (274.8 KB)Acknowledgements
The authors wish to thank Professor Jian Qin, School of Public Health, Guangxi Medical University for Clinical data analysis support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2141519.
Additional information
Funding
References
- World Health Organization. WHO coronavirus (COVID-19) dashboard with vaccination data. https://covid19.who.int.2022-06-08.
- Jalkanen P, Kolehmainen P, Häkkinen HK, Huttunen M, Tähtinen PA, Lundberg R, Maljanen S, Reinholm A, Tauriainen S, Pakkanen SH, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12(1):1. doi:10.1038/s41467-021-24285-4.
- Wang X, Zhao X, Song J, Wu J, Zhu Y, Li M, Cui Y, Chen Y, Yang L, Liu J, et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerging Microbes Infect. 2022;11(1):477–7. doi:10.1080/22221751.2022.2030200.
- Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20(1):1–15. doi:10.1186/s12916-022-02397-y.
- Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ (Clin Res Ed). 2022;376:e069761. doi:10.1136/bmj-2021-069761.
- Cabezudo-García P, Ciano-Petersen NL, Mena-Vázquez N, Pons-Pons G, Castro-Sánchez MV, Serrano-Castro PJ. Incidence and case fatality rate of COVID-19 in patients with active epilepsy. Neurology. 2020;95(10):e1417–25. doi:10.1212/WNL.0000000000010033.
- Horváth RA, Sütő Z, Cséke B, Schranz D, Darnai G, Kovács N, Janszky I, Janszky J. Epilepsy is overrepresented among young people who died from COVID-19: analysis of nationwide mortality data in Hungary. Seizure. 2022;94:136–41. doi:10.1016/j.seizure.2021;11.013.
- Siahaan YM, Ketaren RJ, Hartoyo V, Hariyanto TI. Epilepsy and the risk of severe coronavirus disease 2019 outcomes: a systematic review, meta-analysis, and meta-regression. Epilepsy Behav. 2021;125:108437. doi:10.1016/j.yebeh.2021.108437.
- Qiao S, Zhang R-R, Yang T-T, Wang Z-H, Fang X-Q, Fang C-Y, Geng J-H, Zhang D-M, Qu L-X, Cao L-L, et al. Attitudes to being vaccinated against COVID-19: a survey of people with epilepsy in China. Front Neurol. 2021;12:743110. doi:10.3389/fneur.2021.743110.
- Li N, Chu C, Lin W. A survey of hesitancy and response to the COVID-19 vaccine among patients with epilepsy in northeast China. Front Neurol. 2021;12:778618. doi:10.3389/fneur.2021.778618.
- Puteikis K, Mameniškienė R. Factors associated with COVID-19 vaccine hesitancy among people with epilepsy in Lithuania. Int J Environ Res Public Health. 2021;18(8):4374. doi:10.3390/ijerph18084374.
- Lin K, Huang H, Fang S, Zheng G, Fu K, Liu N, Du H. Should patients with epilepsy be vaccinated against coronavirus disease 2019? A systematic review and meta-analysis. Epilepsy Behav. 2022;134:108822. doi:10.1016/j.yebeh.2022.108822.
- von Wrede R, Pukropski J, Moskau-Hartmann S, Surges R, Baumgartner T. COVID-19 vaccination in patients with epilepsy: first experiences in a German tertiary epilepsy center. Epilepsy Behav. 2021;122:108160. doi:10.1016/j.yebeh.2021.108160.
- Massoud F, Ahmad SF, Hassan AM, Alexander KJ, Al–hashel J, Arabi M. Safety and tolerability of the novel 2019 coronavirus disease (COVID-19) vaccines among people with epilepsy (PWE): a cross-sectional study[j]. Seizure. 2021;92:2–9. doi:10.1016/j.seizure.2021.08.001.
- Özdemir HN, Dere B, Gökçay F, Gökçay A. Are COVID-19 vaccines safe for people with epilepsy? A cross-sectional study. Neurol Sci. 2022;43(6):3489–96. doi:10.1007/s10072-022-05956-6.
- Lu L, Zhang Q, Xiao J, Zhang Y, Peng W, Han X, Chen S, Yang D, Sander JW, Zhou D, et al. COVID-19 vaccine take-up rate and safety in adults with epilepsy: data from a multicenter study in China. Epilepsia. 2022;63(1):244–51. doi:10.1111/epi.17138.
- International League against epilepsy. COVID-19 vaccines and people with epilepsy; ( Updated: 2022 Feb 21). https://www.Ilae.org/patient-care/covid-19-and-epilepsy/covid-19-vaccines-and-people-with-epilepsy.
- Department of Health & Social Care of UK. Independent report. Joint committee on vaccination and immunization: advice on priority groups for COVID-19 vaccination. 2020 Dec 30. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020.2022-06-26.
- de Picker LJ, Dias MC, Benros ME, Vai B, Branchi I, Benedetti F, Borsini A, Leza JC, Kärkkäinen H, Männikkö M, et al. Severe mental illness and European COVID-19 vaccination strategies. Lancet Psychiatry. 2021;8(5):356–59. doi:10.1016/s2215-0366(21)00046-8.
- National Health Commission of the People’s Republic of ChinaTechnical guidelines for SARS-CoV-2 vaccination in China (1st edition). Int J Epidemiol Infect Dis. 2021;48(2):91–92. doi:10.3760/cma.j.cn331340-20210329-00061.(Chinese).
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J, Forsgren L, French JA, Glynn M, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. doi:10.1111/epi.12550.
- Guerrini R, Dravet C, Genton P, Belmonte A, Kaminska A, Dulac O. Lamotrigine and seizure aggravation in severe myoclonic epilepsy. Epilepsia. 1998;39(5):508–12. doi:10.1111/j.1528-1157.1998.tb01413.x.
- Romozzi M, Rollo E, Quintieri P, Dono F, Evangelista G, Consoli S, Veleno L, Anzellotti F, Calvello C, Costa C, et al. Impact of COVID-19 vaccine on epilepsy in adult subjects: an Italian multicentric experience. Neurol Sci. 2022;43(8):4627–34. doi:10.1007/s10072-022-06100-0.
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005-2009. Epilepsia. 2010;51(4):676–85. doi:10.1111/j.1528-1167.2010.02522.x.
- Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez E, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):522–30. doi:10.1111/epi.13670.
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51:1069–77. doi:10.1111/j.1528-1167.2009.02397.x.
- Arnheim-Dahlström L, Hällgren J, Weibull CE, Sparén P. Risk of presentation to hospital with epileptic seizures after vaccination with monovalent AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine (Pandemrix): self controlled case series study. BMJ (Clin Res Ed). 2012;345:e7594. doi:10.1136/bmj.e7594.
- Barlow WE, Davis RL, Glasser JW, Rhodes PH, Thompson RS, Mullooly JP, Black SB, Shinefield HR, Ward JI, Marcy SM, et al. The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med. 2001;345(9):656–61. doi:10.1056/NEJMoa003077.
- Patone M, Handunnetthi L, Saatci D, Pan J, Katikireddi SV, Razvi S, Hunt D, Mei XW, Dixon S, Zaccardi F, et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;27(12):2144–53. doi:10.1038/s41591-021-01556-7.
- Koh JS, Hoe RHM, Yong MH, Chiew HJ, Goh Y, Yong KP, Tu TM, Chan DWS, Tan BYQ, Yeo LLL, et al. Hospital-based observational study of neurological disorders in patients recently vaccinated with COVID-19 mRNA vaccines. J Neurol Sci. 2021;430:120030. doi:10.1016/j.jns.2021.120030.
- Malek N, Heath CA, Greene J. A review of medication adherence in people with epilepsy. Acta Neurol Scand. 2017;135(5):507–15. doi:10.1111/ane.12703.
- Faught E, Duh MS, Weiner JR, Guérin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM Study. Neurology. 2008;71(20):1572–78. doi:10.1212/01.wnl.0000319693.10338.b9.
- Samsonsen C, Sand T, Bråthen G, Helde G, Brodtkorb E. The impact of sleep loss on the facilitation of seizures: a prospective case-crossover study. Epilepsy Res. 2016;127:260–66. doi:10.1016/j.eplepsyres.2016.09.014.
- Ferlisi M, Shorvon S. Seizure precipitants (triggering factors) in patients with epilepsy. Epilepsy Behav. 2014;33:101–05. doi:10.1016/j.yebeh.2014.02.019.
- Assenza G, Lanzone J, Brigo F, Coppola A, Di Gennaro G, Di Lazzaro V, Ricci L, Romigi A, Tombini M, Mecarelli O. Epilepsy care in the time of COVID-19 pandemic in Italy: risk factors for seizure worsening. Front Neurol. 2020;11:737. doi:10.3389/fneur.2020.00737.
- Casassa C, Moss R, Goldenholz DM. Epilepsy during the COVID-19 pandemic lockdown: a US population survey. Epileptic Disord. 2021;23(2):257–67. doi:10.1684/epd.2021.1259.
- China association against epilepsy community management committeePatients with epilepsy and COVID-19 vaccines: advice from Chinese experts. J Epilepsy. 2021;7(7):323–26. doi:10.1111/ane.13417.(Chinese).