ABSTRACT
Emergency vaccination (EV) is used as effective postexposure prophylaxis (PEP) to control varicella outbreaks within 3–5 days. However, the advantages of a second dose of varicella vaccine (VarV) in students who had received one dose before an outbreak and the potential benefits of EV at more than 5 days after exposure have not been fully evaluated. This study evaluated the vaccine effectiveness (VE) of EV in preventing disease development during a varicella outbreak in Shanghai, China, in 2020. Questionnaires were used to obtain student demographic information, clinical manifestations, varicella history, vaccination status, and willingness to receive EV. The VE of EV was calculated as [1-relative risk (RR)] ×100%. Among the 1455 students included in this study, 31 cases were identified, resulting in an overall attack rate of 2.13%. There were 6 cases in unvaccinated students and 25 cases in one-dose-vaccinated students. A total of 788 students received one EV dose. The attack rates were 6.38% (6/94), 4.26% (19/446), 2.82% (2/71), and 0.56% (4/717) among unvaccinated students, students who received 1 dose of VarV, and students who received EV with the 1st and 2nd dose of VarV, respectively. Compared to that in unvaccinated students, the VE of EV with the 2nd dose of VarV was 88% (95% CI 49% to 97%). EV should be performed as soon as possible after exposure. Nevertheless, vaccination is still recommended at more than 5 days post exposure to control varicella outbreaks.
Introduction
Varicella is a highly contagious acute respiratory disease caused by primary varicella zoster virus (VZV) infection. It is characterized by systemic papules and pruritic varicella rashes, which are common in children and can lead to serious complications, such as pneumonia, encephalitis and even death.Citation1 According to conservative estimates in developed countries, varicella causes 4.2 million severe complications, leading to hospitalization and approximately 4200 deaths.Citation2 Students must be removed from the school setting immediately after the identification of a varicella rash and remain quarantined until their rash has crusted over; after exposure, those without evidence of varicella immunity need to be monitored, which contributes to high burdens on families, schools, and the government.Citation3–5
With the widespread use of varicella vaccines (VarVs) worldwide, the number of varicella cases has been substantially reduced.Citation2 In China, the single-dose VarV has been licensed for use in children aged 1–12 years since 1998, although it is not included in the National Immunization Program.Citation6 A total of 73.6% of children born between 2008 and 2012 in six provinces and 78% of students aged 3–17 years in Shanghai have received one dose of VarV.Citation7,Citation8 Although the coverage of a single dose of VarV has reached more than 70%, varicella outbreaks are still reported in some cities, even those with high economic productivity, in China.Citation9,Citation10
Emergency vaccination (EV) is used as effective postexposure prophylaxis (PEP) to control varicella outbreaks.Citation11 The US Advisory Committee on Immunization Practices states that EV, if provided within 3–5 days post exposure, is highly effective in preventing moderate or severe varicella disease and recommends a 2nd dose of VarV in individuals who previously received one dose of VarV as PEP for outbreak control. In China, this strategy was implemented in Beijing in 2006, in Guangzhou in 2012, and in Shanghai in 2013.Citation11–13 However, the advantage of 2nd dose of VarV in students who received one dose before the outbreak and the potential benefits of EV at more than 5 days after exposure have not been fully evaluated.
In this study, we assessed the effectiveness of a 2nd dose of VarV as an EV strategy in students who had received one dose of VarV before the outbreak and the effectiveness of 1 dose of VarV as an EV strategy in unvaccinated students. The results of this study provide empirical evidence to inform prevention and control measures for varicella outbreaks and support the implementation of routine two-dose vaccination in provinces of China where this strategy has not been adopted.
Materials and methods
Varicella surveillance
Mandatory reporting of varicella in Shanghai began in 2006 after the varicella vaccine was more widely used in children. Local health providers and physicians are required to report varicella cases directly to the Shanghai Municipal Center for Disease Control and Prevention (CDC) within 24 hours via an internet-based surveillance system. Kindergartens, and schools obligatorily report varicella outbreaks to the public health agency. Meanwhile, the district CDC actively monitors varicella outbreaks in institutions via the internet reporting system. In response to varicella outbreaks, public health staff conducts epidemiological investigations and implements control measures.
Outbreak setting
The varicella outbreak took place in a senior high school (called “School A”) located in Pudong New District of Shanghai from November to December 2020. There were a total of 1474 students in grades 1–3 in School A. There were four five-story school buildings located in this school, and each floor had 5 classrooms.
Outbreak control measures
All gathering activities were stopped, and morning examinations in all classes were carried out during the outbreak. The affected classes were transferred to an isolated area, and disinfection was performed by special personnel. All patients were quarantined and treated in a centralized manner. EV was recommended for all possibly exposed students and staff. Domestic varicella vaccines were used for EV, produced by the Shanghai Institute of Biological Products, Shanghai. The domestic vaccine (Shanghai) introduced the Oka strain VZV and contains similar concentrations of Oka strain VZV with >2000 plaque-forming units/dose (0.5 ml).
Study definitions
A case of varicella was defined as an acute maculopapular vesicular rash without another apparent cause in a student in school A from November 2 to 14 December 2020. Varicella and its complications were diagnosed by experienced doctors in the local hospital. Breakthrough varicella was defined as a varicella-like rash that developed >42 days after VarV. In this study, students were divided into four groups based on varicella vaccination status as follows: (1) unvaccinated; (2) 1 dose of VarV, before the outbreak and refusal of EV; (3) EV with the 1st dose of VarV in students with no VarV immunization history before the outbreak; and (4) EV with the 2nd dose of VarV in students who received one dose before the outbreak. After EV, it takes at least 4 days to produce sufficient antibodies against varicella-zoster virus infection.Citation8,Citation14 Therefore, the students receiving EV with the first dose of VarV and receiving EV with the second dose of VarV within 4 days after varicella exposure were classified into the unvaccinated and 1-dose VarV groups, respectively, during the outbreak.
Data collection
This retrospective study was conducted to assess the effectiveness of varicella vaccination. Investigators were trained to use a structured questionnaire to collect epidemiological information about the school during the outbreak through face-to-face interviews with school staff. The questionnaire, which included demographic information, clinical manifestations, vaccination status, varicella history, duration of isolation and willingness to receive EV, was completed by an investigator via a telephone interview with the student’s parents. Rash severity was categorized as mild (<50 lesions), moderate (50–499 lesions), or severe (≥500 lesions).
The data collection process was a part of the routine varicella surveillance program and exempt from ethical approval after review by the Ethics Committee of the Shanghai Municipal CDC.
Data analysis
Data were entered into Microsoft Excel 2010. Statistical analyses were performed using SPSS 18.0. Varicella severity was compared between the unvaccinated, one-dose VarV, 1st EV dose and 2nd EV dose using Pearson’s Chi-square test, Fisher’s exact test, or the Wilcoxon rank-sum test, as appropriate. A p value of <.05 was considered statistically significant.
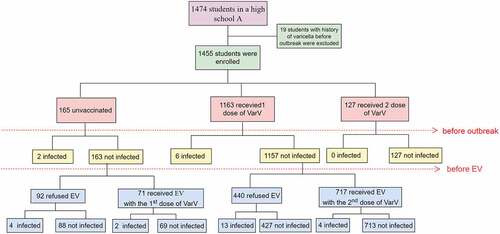
In this study, 1455 students were enrolled, and 19 students with a past medical history of varicella before the outbreak were excluded. An epidemiological curve was used to describe the distribution of varicella cases during the outbreak. The vaccine effectiveness (VE) of EV was calculated as [1-relative risk (RR)] ×100%, where RR is the incidence density calculated in this outbreak investigation. The at-risk period for each student began on 2 November 2020 (date of infection of the first patient) and ended on 14 December 2020 (21 days after the date of rash onset for the last infected student, as 21 days is the maximum incubation period for varicella). In addition, the additional reduction in varicella cases associated with the 2nd emergency vaccine dose relative to the 1 dose VarV was assessed. A 95% confidence interval (CI) of VE excluding 0 was considered statistically significant.
Result
Study population
Among the 1474 students in school A, 19 students with a history of varicella before the outbreak were excluded from the analysis (). No staff members or faculty members developed varicella during the outbreak; therefore, all analyses were restricted to students. The mean age of the 1455 students was 16.97 ± 0.91 years (range 15–19 years), and 49% were male. Among the 1455 students with no varicella history before the outbreak, 165 (11.34%) were unvaccinated, 1163 (79.93%) had received 1 dose of the vaccine, and 127 (8.73%) had received 2 doses of the vaccine, resulting in schoolwide vaccination coverage with ≥1 dose before the outbreak of 88.66%. Among the 1 dose recipients (n = 1163), the mean time from vaccination to the outbreak was 14 ± 2.54 years (range 1–17 years).
Outbreak
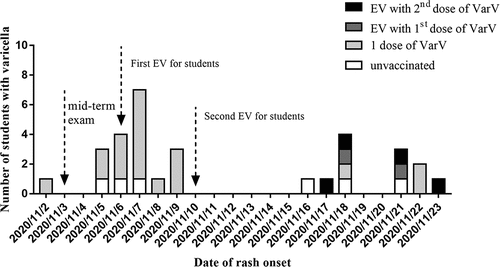
The outbreak lasted approximately 1.5 months, from November 2 through 14 December 2020. Among the 1455 students included in this study, a total of 31 cases were identified, for an overall attack rate of 2.13%. There were 6 cases in unvaccinated students and 25 cases in one-dose-vaccinated students (). The mean age of the patients was 17.89 ± 0.44 years, and 16 of them (51.61%) were male. The index case was an 18-year-old boy from class 7 in grade 3 who had previously received 1 dose of VarV. He developed a fever of 38.6°C on November 2 and a mild rash on November 5, approximately 14 years after varicella vaccination on 21 August 2006. The number of cases peaked in this outbreak at approximately one week after the index case ().
EV campaign
Before EV, two unvaccinated students and six 1-dose VarV recipients were diagnosed with varicella; 127 students had received 2 doses of VarV. Among the 1320 students who were eligible for EV with one dose of VarV, 788 received one dose of VarV (). The rate of EV among students who had received 1 dose of VarV before the outbreak was 61.97% (717/1157), which was higher than 43.56% (71/163) among unvaccinated students (p < .05). After EV, the attack rates were 6.38% (6/94), 4.26% (19/446), 2.82% (2/71), and 0.56% (4/717) among unvaccinated students, students who received 1 dose of VarV, students who received EV with the 1st dose of VarV and students who received EV with the 2nd dose of VarV, respectively.
Case characteristics
All cases were mild. None of the 1st-dose EV or 2nd-dose EV students had fever. In contrast, fever was reported in 33.33% (2/6) of unvaccinated students and 31.58% (6/19) of 1-dose VarV students (). There was no difference among the four groups of students in those who were absent (p = .26). Cases and noncases differed in terms of age (p < .05) and grade (p < .05) (). Most cases occurred among students in grade 3 (29, 93.55%), and the average age was 17.89 years (). Cases and noncases did not differ in terms of sex (p = .769) or region (p = .733) (). In the one-dose VarV recipients, the time since vaccination was associated with breakthrough varicella in this outbreak (p < .05) (). Among 31 cases, 23 were breakthrough varicella cases and had received one-dose VarV. The time from vaccination to outbreak longer, the number of breakthrough varicella cases higher. Among them, the time of 10 cases from vaccination were between 11 years to 15 years and the time of 13 cases from vaccination were more than 15 years.
Table 1. Disease severity of varicella according to vaccination status.
Table 2. Comparison of infected and noninfected students during a varicella outbreak in Shanghai, 2020.
EV effectiveness
Among the 31 varicella cases, 8 (25.81%) were infected before the EV campaign, and 23 (74.19%) were infected after the campaign (). After EV, the incidence rates were 0.89 cases/1000 person-days, 0.60 cases/1000 person-days, 0.57 cases/1000 person-days and 0.11 cases/1000 person-days among unvaccinated students, students who received 1 dose of VarV, students who received EV with the 1st dose of VarV and students who received EV with the 2nd dose of VarV, respectively (). Compared to that in unvaccinated students, the effectiveness of 1 dose of VarV was 33% (95% CI −108 to 78), of EV with the 1st dose of VarV was 36% (95% CI −254 to 88) and of EV with the 2nd dose of VarV was 88% (95% CI 49 to 97) (). The incremental VE rates (EV with the 2nd dose vs. 1 dose of VarV) were 81% (95% CI 42 to 94) and (EV with the 2nd dose vs. EV with the 1st dose) 80% (95% CI −8 to 96).
Table 3. Effectiveness of varicella vaccination during the outbreak in School A.
Discussion
In this study, compared to that in unvaccinated students, the VE of EV with the 2nd dose of VarV in students who had received one dose of VarV more than 5 years previously was 88%, which was higher than that in students who had received 1 dose of VarV (33%) and received EV with the 1st dose of VarV (36%). This result may be explained by the fact that the administration of a 2nd dose of VarV can more effectively induce protective antibody titers than the administration of one dose of VarV (99.6% vs. 85.7%).Citation15 As geometric mean antibody concentrations increase approximately 10-fold following administration of the second dose of VarV in children, boosting may help students who fail to respond to the priming immunization to mount a protective immune response.Citation16 In Germany, a second dose of MMRV within the second year of life (66% second-dose coverage) increased vaccine effectiveness from 62% to 94% in outbreak situations.Citation17 A long-term follow-up of clinical trials in which 2 doses were given in a short interval (3 months between doses) showed that 2 doses provide more protection than a single dose and is associated with higher anti-body titers (and presumably better protection from varicella).Citation15 The results of this study add to the limited data regarding the potential benefit of the administration of a second dose of VarV after exposure in persons who have previously received one dose of VarV. Additional data are needed to better understand the benefit of the administration of a second dose of VarV to one-dose recipients after exposure, as varicella outbreaks in the school setting have become an increasing challenge, even with high one-dose VarV coverage.
VarVs are not free in all provinces of China. Children under 12 years of age living in Shanghai were recommended to receive one dose of varicella vaccine at the age of 12 months before 2017. Previous studies have shown that vaccine coverage >85% can effectively prevent outbreaks of infectious diseases.Citation18 However, some studies reported that protection was incomplete even when varicella vaccine coverage reached 88.3–100%.Citation6,Citation19,Citation20 In this study, we found that despite a varicella vaccine coverage rate of 88.66%, a varicella outbreak still occurred in this school, probably due to the low effectiveness of the vaccine. In this outbreak, breakthrough cases accounted for 61.29% (19/31), and time since vaccination before the outbreak was associated with the onset of varicella. One explanation might be that immunity wanes over time; the initial response to varicella vaccination is attenuated, and the effectiveness of a one-dose vaccine is reduced over time. This finding suggested that single varicella vaccination did not reduce the risk of varicella infection among high-risk children and was consistent with the results of several previous studies, including those demonstrating that breakthrough cases were common during varicella outbreaks.Citation21,Citation22 Despite the high VE of the single-dose varicella vaccine, this vaccine does not provide sufficient herd immunity to inhibit local VZV transmission and prevent outbreaks completely.
In 2006, the Advisory Committee on Immunization Practices (ACIP) of the US revised the varicella vaccine guidelines and recommended that children receive the first dose of VarV between 12 and 15 months of age and the second dose between 4 and 6 years of age.Citation18 Since the implementation of the universal two-dose program in the US, reductions in both the varicella disease burden and the number of outbreaks had been observed.Citation22,Citation23 Two-dose varicella vaccination schedules have also been implemented in national immunization plans in several countries, including Cyprus, Germany, Greece and Luxembourg. Studies from other countries all suggested that two-dose varicella vaccination was very effective in preventing varicella.Citation24,Citation25 To assess the safety of two-dose varicella vaccination, the ACIP analyzed reports of adverse events (AEs) in children who received a second dose of VarV between 2006 and 2014. The safety data from the ACIP on two-dose varicella vaccination are reassuring; reported AEs after the second dose of VarV were mild, self-limiting, and similar in frequency to AEs reported after the first dose of the vaccination, with no new or unexpected safety concerns.Citation26 Studies conducted in Argentina and India indicated that both one-dose and two-dose varicella vaccines were safe.Citation27,Citation28 The abovementioned studies clearly showed that two doses of VarV are helpful in reducing the number of cases and the severity of disease. In our study, there were no infection cases among students who had received two doses of VarV before the outbreak. A second dose of vaccine may be important not only to prevent breakthrough varicella and continuous transmission of the virus, but also to potentially lower the subsequent risk of developing herpes zoster by decreasing the number of latent infections with wild-type VZV.Citation24 Therefore, we advise evaluating the VE of two-dose varicella vaccination and revising the immunization schedule in China. The implementation of a two-dose VarV strategy is expected to reduce the number of varicella outbreaks and protect children’s physical health, as its safety has been stated clearly.
Fortunately, a voluntary two-dose VarV schedule has been recommended in Shanghai, China, since November 2017 and was included in the immunization program of Shanghai in August 2018. The schedule includes a first dose at 12 months followed by a second dose at 4 years. Several studies showed that conducting PEP campaigns during school outbreaks reduced the attack rate of varicella in school age children, and the VE of VarV as PEP during school outbreaks was significant (47.0–85.3%).Citation5,Citation8,Citation11,Citation12 In addition, the incidence of varicella decreased by 30.2% after the implementation of VarV as PEP in Guangzhou, China, in 2012Citation12. Therefore, the administration of VarV as PEP is an appropriate varicella outbreak intervention in countries where a two-dose VarV schedule has not yet been adopted.
This study is subject to some limitations. The case definition criterion was based on clinical diagnosis rather than laboratory confirmation. Additionally, the exposures examined in our study mainly focused on school contacts, and some transmission events may have occurred outside the school (for instance, during after-school activities).
In conclusion, administering a 2nd dose of VarV to students who have previously received one dose of VarV and the 1st dose of VarV to students who have no VarV immunization history are appropriate interventions for outbreak control in countries where routine two-dose VarV immunization had not been implemented. We recommend that PEP control measures should be conducted in schools experiencing varicella outbreaks to contain transmission. In the future, a cost effectiveness analysis of EVs will be conducted to provide a basis for measure optimization. Moreover, the implementation of a two-dose VarV strategy is expected to reduce the number of varicella outbreaks and protect children’s physical health. Therefore, two-dose varicella vaccination should be included in the national childhood immunization program.
Abbreviations
| EV | = | Emergency Vaccination |
| PEP | = | Postexposure Prophylaxis |
| VarV | = | Varicella Vaccine |
| VE | = | Vaccine Effectiveness |
| CI | = | Confidence Interval |
| RR | = | Relative Risk |
Author contributions
The study protocol was designed by LMZ, FY and XCY. Data collection was done by WQZ and FY. Data analysis was completed by LMZ, DPF and XST. LMZ and XCY prepared the manuscript which was reviewed by all authors.
Acknowledgments
We highly appreciate the help of the school nurses and the clinical practitioners in this outbreak investigation and the participation of the parents and students.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gershon AA. Is chickenpox so bad, what do we know about immunity to varicella zoster virus, and what does it tell us about the future? J Infect. 2017;74:S27–6. doi:10.1016/S0163-4453(17)30188-3.
- Varicella and herpes zoster vaccines: WHO position paper, June 2014–recommendations. Vaccine. 2016; 34: 198–99. doi:10.1016/j.vaccine.2014.07.068.
- Meszner Z, Molnar Z, Rampakakis E, Yang HK, Kuter BJ, Wolfson LJ. Economic burden of varicella in children 1-12 years of age in Hungary, 2011-2015. BMC Infect Dis. 2017;17:495. doi:10.1186/s12879-017-2575-6.
- Leung J, Rue A, Lopez A, Ortega-Sanchez IR, Harpaz R, Guris D, Seward J. Varicella outbreak reporting, response, management, and national surveillance. J Infect Dis. 2008;197(2):S108–13. doi:10.1086/522138.
- Wu Q, Liu J, Wang Y, Zhou Q, Wang X, Xuan Z, Zhang L, Gao Y, Chen B, Hu Y. Effectiveness of second-dose varicella vaccination as post-exposure prophylaxis: a prospective cohort study. Clin Microbiol Infect. 2019;25:872–77. doi:10.1016/j.cmi.2018.11.013.
- Wang Z, Yang H, Li K, Zhang A, Feng Z, Seward JF, Bialek SR, Wang C. Single-dose varicella vaccine effectiveness in school settings in China. Vaccine. 2013;31:3834–38. doi:10.1016/j.vaccine.2013.06.075.
- Yue C, Li Y, Wang Y, Liu Y, Cao L, Zhu X, Martin K, Wang H, An Z. The varicella vaccination pattern among children under 5 years old in selected areas in China. Oncotarget. 2017;8:45612–18. doi:10.18632/oncotarget.17317.
- Wu QS, Liu JY, Wang X, Chen YF, Zhou Q, Wu AQ, Wang L. Effectiveness of varicella vaccine as post-exposure prophylaxis during a varicella outbreak in Shanghai, China. Int J Infect Dis. 2018;66:51–55. doi:10.1016/j.ijid.2017.10.016.
- Suo L, Lu L, Wang Q, Yang F, Wang X, Pang X, Marin M, Wang C. Varicella outbreak in a highly-vaccinated school population in Beijing, China during the voluntary two-dose era. Vaccine. 2017;35:4368–73. doi:10.1016/j.vaccine.2017.06.065.
- Zhu YF, Li YF, Du Y, Zeng M. Epidemiological characteristics of breakthrough varicella infection during varicella outbreaks in Shanghai, 2008-2014. Epidemiol Infect. 2017;145:2129–36. doi:10.1017/S0950268817000772.
- Cao Z, Chen D, Yang Y, Zhang D. Effectiveness of post-exposure prophylaxis during varicella outbreaks among primary and middle school students in Shanghai: an analysis of three-year surveillance data. Vaccine. 2018;36:5754–59. doi:10.1016/j.vaccine.2018.08.004.
- Li T. Varicella emergency vaccination seemed instrumental in declining chickenpox incident in Guangzhou, Southern China. Rev Inst Med Trop São Paulo. 2013;55:55. doi:10.1590/S0036-46652013000300016.
- Ma R, Sun MP, Sun M, Hou WJ, Jiang GY, Peng XH, Wu J. Effectiveness on post-exposure vaccination of varicella and its influencing factors in elementary schools in Beijing. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2009;30:559–63.
- Nguyen MD, Perella D, Watson B, Marin M, Renwick M, Spain CV. Incremental effectiveness of second dose varicella vaccination for outbreak control at an elementary school in Philadelphia, Pennsylvania, 2006. Pediatr Infect Dis J. 2010;29:685–89. doi:10.1097/INF.0b013e3181d9f657.
- Kuter B, Matthews H, Shinefield H, Black S, Dennehy P, Watson B, Reisinger K, KIM LL, Lupinacci L, Hartzel J, et al. Ten Year follow-up of healthy children who received one or two injections of varicella vaccine. 2004;23:132–37. doi:10.1097/01.inf.0000109287.97518.67
- Bonanni P, Gershon A, Gershon M, Kulcsar A, Papaevangelou V, Rentier B, Sadzot-Delvaux C, Usonis V, Vesikari T, Weil-Olivier C, et al. Primary versus secondary failure after varicella vaccination: implications for interval between 2 doses. Pediatr Infect Dis J. 2013;32:e305–13. doi:10.1097/INF.0b013e31828b7def.
- Spackova M, Wiese-Posselt M, Dehnert M, Matysiak-Klose D, Heininger U, Siedler A. Comparative varicella vaccine effectiveness during outbreaks in day-care centres. Vaccine. 2010;28:686–91. doi:10.1016/j.vaccine.2009.10.086.
- Zhong JM, Zhang M, Huang ZY, Qiu GP, Rao F, Lu ZH, Chen T, Zhang Q-L. A persistent outbreak of varicella in a primary school in Dongguan City, Guangdong Province, China. J Int Med Res. 2020;48:300060519887847. doi:10.1177/0300060519887847.
- Lu L, Suo L, Li J, Zhai L, Zheng Q, Pang X, Bialek SR, Wang C. A varicella outbreak in a school with high one-dose vaccination coverage, Beijing, China. Vaccine. 2012;30:5094–98.
- Lopez AS, Guris D, Zimmerman L, Gladden L, Moore T, Haselow DT, Loparev VN, Schmid DS, Jumaan AO, Snow SL. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics. 2006;117:e1070–7. doi:10.1542/peds.2005-2085.
- Seward JF, Marin M, Vazquez M. Varicella vaccine effectiveness in the US vaccination program: a review. J Infect Dis. 2008;197(2):S82–9. doi:10.1086/522145.
- Marin M, Meissner HC, Seward JF. Varicella prevention in the United States: a review of successes and challenges. Pediatrics. 2008;122:e744–51. doi:10.1542/peds.2008-0567.
- Kattan JA, Sosa LE, Bohnwagner HD, Hadler JL. Impact of 2-dose vaccination on varicella epidemiology: connecticut–2005-2008. J Infect Dis. 2011;203:509–12. doi:10.1093/infdis/jiq081.
- Shapiro ED, Vazquez M, Esposito D, Holabird N, Steinberg SP, Dziura J, LaRussa PS, Gershon AA. Effectiveness of 2 doses of varicella vaccine in children. J Infect Dis. 2011;203:312–15. doi:10.1093/infdis/jiq052.
- Siedler A, Rieck T, Tolksdorf K. Strong additional effect of a second varicella vaccine dose in children in Germany, 2009-2014. J Pediatr. 2016;173:202–6 e2. doi:10.1016/j.jpeds.2016.02.040.
- Su JR, Leroy Z, Lewis PW, Haber P, Marin M, Leung J, Woo EJ, Shimabukuro TT. Safety of second-dose single-antigen varicella vaccine. Pediatrics. 2017:139. doi:10.1542/peds.2016-2536.
- Fridman D, Monti A, Bonnet MC, Armoni J, Stamboulian D. Safety of a second dose of varicella vaccine administered at 4 to 6 years of age in healthy children in Argentina. Hum Vaccin. 2011;7:1066–71. doi:10.4161/hv.7.10.17816.
- Mitra M, Chowdhury J, Basu S, Halder PP, Mukherjee M, Karadkhele A, Puppalwar G, Jain R. Evaluation of immunogenicity, safety and breakthrough following administration of live attenuated varicella vaccine in two doses three months apart regimen in Indian children. Ther Adv Vaccines Immunother. 2020;8:2515135520937216. doi:10.1177/2515135520937216.