ABSTRACT
Esophageal cancer is the sixth most common cause of cancer-related mortality worldwide. The standard treatment for unresectable esophageal cancer is systemic chemotherapy. However, the survival benefit is limited, with a median overall survival of less than 10 months. The advent of immune checkpoint inhibitors (ICIs), including programmed cell death-1 antibodies, has revolutionized the treatment paradigm for esophageal cancer. Since demonstrating promising efficacy with manageable safety in several clinical trials, ICIs has finally reached the point where they can be used in various tumor stages in the clinical setting. ICIs are most promising treatments that can be expected to improve the prognosis in patients with esophageal cancer now and in the future. This review outlines the mechanisms, results of clinical trials, and prospects for future studies of ICIs in esophageal cancer. It also discusses clinical questions and challenges in the therapeutic development of ICIs.
Introduction
Esophageal cancer is the sixth most common cause of cancer-related mortality, with 544,000 deaths from esophageal cancer reported in 2020 worldwide.Citation1 The two most common histological subtypes of esophageal cancer are squamous cell carcinoma (SCC), which accounts for approximately 90% of cases, and adenocarcinoma (AC).Citation2 The highest prevalence of esophageal SCC is in East Asia and East Africa, while AC is more common in North America and Western Europe.Citation1,Citation3,Citation4 Patients with esophageal cancer usually have locally advanced or metastatic disease by the time they are aware of any symptoms.Citation5 Although multidisciplinary treatments, including surgical resection, chemotherapy, radiotherapy (RT), and chemoradiotherapy (CRT), have been developed for advanced esophageal cancer, the prognosis remains poor, with 5-year relative survival rates of 26% in patients with locally advanced disease and 5% in those with distant metastasis.Citation6 The standard treatment for unresectable esophageal cancer is systemic chemotherapy, based on fluoropyrimidine, platinum, taxane, and irinotecan, and additional RT for local disease. However, the survival benefit has been limited, with median overall survival (OS) of less than 10 months.Citation7,Citation8 Therefore, innovative treatments are needed to improve the prognosis. Molecular targeted agents such as anti-endothelial growth factor receptor antibodies have not improved survival, despite showing efficacy in patients with head and neck SCC.Citation8–11 However, immune checkpoint inhibitors (ICIs) have revolutionized the treatment paradigm for esophageal cancer. This review provides an overview of the development and efficacy of ICIs in patients with esophageal cancer.
Immune checkpoints and their blockade
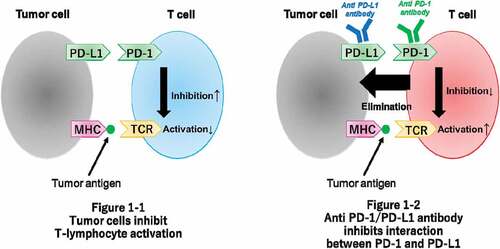
A schematic diagram of immune checkpoints and their blockade is provided in . Immune checkpoints, such as programmed cell death-1 (PD-1), which is an inhibitory receptor, are expressed on the surface of activated lymphocytes, and its ligands, programmed death ligand-1 (PD-L1) and programmed death ligand-2 (PD-L2), are expressed on the surface of tumor cells. These immune checkpoints maintain the balance of T-cell activation, immune tolerance, and immune-mediated tissue damage.Citation12–14 Overexpression of PD-L1 is associated with tumor progression because cancer cells exploit the PD-1/PD-L1 and PD-1/PD-L2 pathways to create an immunosuppressive environment.Citation15,Citation16 Furthermore, cancer cell-mediated upregulation of anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) on the surface of T-cells increases recruitment of immunosuppressive T-cells and serves as a co-inhibitory pathway to evade the host immune response.
Based on these mechanisms, immune checkpoint blockade has been developed and has emerged as a promising anti-cancer treatment. Immune checkpoint inhibitors (ICIs), such as anti-PD-1 and anti-PD-L1 antibodies, activate the immune response induced by anti-tumor T-cells in response to inhibition of the PD-1/PD-L1 and PD-1/PD-L2 pathways. The anti-CTLA-4 antibody is another ICI that is involved in peripheral tolerance and prevention of autoimmunity and reactivates the immune response induced by T-cells.Citation17
Expression of PD-L1, a therapeutic target, has been evaluated for its correlation with the efficacy of ICI therapy and as a potential biomarker in several clinical trials. The tumor proportion score (TPS) and combined positive score (CPS) have been used in most of the clinical trials. TPS is defined as the number of PD-L1-positive tumor cells divided by the total number of tumor cells. The CPS is defined as the number of PD-L1-positive tumor cells, macrophages, and lymphocytes divided by the total number of tumor cells. Recent clinical trials have also used the tumor area positivity (TAP) score, which is defined as the total percentage of the tumor area covered by tumor cells with any membrane staining above background and tumor-associated immune cells with any staining above background.
Clinical trials with ICI monotherapy in the late-line setting
First, ICIs revolutionized the treatment of melanoma, and their efficacy has been demonstrated in other cancers, including those involving the gastrointestinal system.Citation15–20 It has been reported that the prognosis of patients with esophageal cancer and high PD-L1 expression is poor, and PD-L1 expression of ≥1% has been detected in approximately 50% of patients with advanced SCC.Citation21 Therefore, esophageal SCC is a potential therapeutic target for ICIs and might be expected to respond well to immune agents.Citation22
The favorable efficacy and safety of ICIs was first demonstrated as salvage monotherapy in patients with advanced esophageal cancer in single-arm, Phase II clinical trials. After these promising results, head-to-head Phase III clinical trials of each ICI in comparison with standard chemotherapy were conducted in the second-line setting. An overview of the results of these trials is provided in .
Table 1. Results of clinical trials in which an ICI was used as monotherapy in a third-line or later setting.
Table 2. Results of clinical trials that compared ICI monotherapy with chemotherapy in a second-line setting.
Pembrolizumab
Pembrolizumab is a recombinant humanized immunoglobulin monoclonal anti-PD-1 antibody. The KEYNOTE-180 trial was a global, single-arm, Phase II study that evaluated the efficacy and safety of pembrolizumab monotherapy in patients with SCC or AC who had previously received more than two lines of treatment. Clinical correlation between the efficacy and PD-L1 expression were also assessed using CPS. This trial included 121 patients (63 [52%] with SCC and 58 [48%] who were PD-L1-positive [defined as a CPS ≥10]). The primary endpoint was the overall response rate (ORR), which was 9.9% (95% confidence interval [CI] 5.2–16.7) in all patients, 14.3% (95% CI 6–25) in patients who were PD-L1-positive, 14.3% (95% CI 6.7–25.4) in patients with SCC, and 5.2% (95% CI 1.1–14.4) in those with AC. Median OS was 5.8 months (95% CI 4.5–7.2) in all patients, 6.3 months (95% CI 4.4–9.3) in those who were PD-L1-positive, 6.8 months (95% CI 5.4–8.9) in patients with SCC, and 3.9 months (95% CI 3.2–6.3) in patients with AC. Median progression-free survival (PFS) was 2.0 months (95% CI 1.9–2.1) in all patients, 2.0 months (95% CI 1.9–2.2) in those with PD-L1-positive disease, 2.1 months (95% CI 2.0–2.4) in those with SCC, and 1.9 months (95% CI 1.8–2.0) in those with AC.Citation23 These findings indicated that pembrolizumab was more effective in SCC than in AC and that the CPS might have the potential to be a biomarker of the efficacy of this agent.
The KEYNOTE-181 trial was a global, open-label, randomized Phase III study that compared the efficacy and safety of pembrolizumab monotherapy with that of standard chemotherapy (paclitaxel, docetaxel, or irinotecan) as second-line treatment in patients with advanced SCC or AC. The primary endpoint was OS in patients with PD-L1-positive disease (CPS ≥ 10), those with SCC, and in all patients. The study included 628 patients (401 [64%] with SCC and 222 [35%] with PD-L1-positive disease). Median OS was longer with pembrolizumab than with chemotherapy in patients with PD-L1-positive disease (9.3 months vs. 6.7 months; hazard ratio [HR] 0.69, 95% CI 0.52–0.93; p = .0074) but not in patients with SCC (8.2 months vs. 7.1 months; HR 0.78, 95% CI 0.63–0.96; p = .0095) or in all patients (7.1 months vs. 7.1 months; HR 0.89, 95% CI 0.75–1.05; p = .0560). Furthermore, there was no significant benefit in terms of OS in patients with PD-L1-positive disease after appropriate adjustment in the final analysis. However, non-prespecified subgroup analysis showed a trend of better median OS on pembrolizumab than on chemotherapy in patients with PD-L1-positive SCC (10.3 months vs. 6.7 months; HR 0.64, 95% CI 0.46–0.90). However, no benefit was noted in terms of median OS in patients with PD-L1-positive AC (6.3 months vs. 6.9 months; HR 0.93, 95% CI 0.52–1.65).Citation18 Based on these results, the US Food and Drug Administration (FDA) approved pembrolizumab as second-line therapy for advanced esophageal SCC in patients with a CPS ≥10 in 2019.
Nivolumab
Nivolumab is a humanized immunoglobulin monoclonal anti-PD-1 antibody. The ATTRACTION-1 trial was a single-arm, Phase II study that evaluated the efficacy and safety of nivolumab monotherapy in 64 Japanese patients with SCC who had previously received fluoropyrimidine, platinum, and taxane therapy. PD-L1 expression was assessed using TPS. The primary endpoint was ORR, which was 17% (95% CI 10–28). Median OS was 10.8 months (95% CI 7.4–13.3) and median PFS was 1.5 months (95% CI 1.4–2.8).Citation24 These findings suggested that nivolumab is an effective late-line treatment in patients with SCC. A subgroup analysis in this study indicated that cutoff TPS values of 1% and 5% and tumor-infiltrating lymphocytes may be promising biomarkers.Citation25
The ATTRACTION-3 trial was a global, open-label, randomized Phase III study in which nivolumab monotherapy was compared with standard chemotherapy (paclitaxel or docetaxel) in patients with advanced SCC who had previously received fluoropyrimidine-based and platinum-based chemotherapy. The primary endpoint was OS in all patients. A subgroup analysis assessed the association between OS and PD-L1 expression using TPS. This study included 419 patients, 203 (48%) of whom had TPS ≥1. Median OS was significantly longer with nivolumab than with standard chemotherapy (10.9 months vs. 8.4 months; HR 0.77, 95% CI 0.62–0.96; p = .019). This median OS benefit was observed irrespective of TPS (10.9 months [95% CI 8.4–13.9] when TPS was <1% and 10.9 months [95% CI 8.0–14.2] when TPS was ≥1%).Citation21 Based on these findings, the FDA approved nivolumab in 2020 for use in patients with esophageal SCC who have previously received fluoropyrimidine-based and platinum-based chemotherapy irrespective of PD-L1 expression status.
Tislelizumab
Tislelizumab is a humanized immunoglobulin monoclonal anti-PD-1 antibody that was developed in China. In early-phase clinical trials, tislelizumab demonstrated promising antitumor activity when administered as monotherapy or in combination with chemotherapy in patients with solid tumors, including esophageal cancer, and a safety profile similar to that of other anti-PD-1 antibodies, such as pembrolizumab and nivolumab. PD-L1 expression was assessed using the TAP score.
The RATIONALE-302 trial was a global, open-label, randomized Phase III study that compared tislelizumab monotherapy versus standard chemotherapy (paclitaxel, docetaxel or irinotecan) in patients with advanced SCC in the second-line setting. The primary endpoint was OS in all patients and a key secondary endpoint was OS in patients with PD-L1 positivity (TAP score ≥10%). The study included 512 patients (157 [30%] with a TAP score ≥10%). Median OS was significantly longer with tislelizumab than with chemotherapy in all patients (8.6 months vs. 6.3 months; HR 0.70; 95% CI 0.57–0.85; p = .001) and in patients with TAP score ≥10% (10.3 months vs. 6.8 months; HR 0.54, 95% CI 0.36–0.79; p = .0006). However, there was no significant difference in median PFS (1.6 months vs. 2.1 months; HR 0.83, 95% CI 0.67–1.01). The ORR also favored tislelizumab over chemotherapy in all patients (20.3% vs. 9.8%) and in patients with TAP score ≥10% (28.1% vs. 11.8%).Citation26
Safety of ICI monotherapy
In view of the mechanism of action of ICIs, there are concerns about potential immune-related adverse events (irAEs). These irAEs can involve multiple body organs, and the most common irAEs with ICIs have been identified to be endocrine (hypothyroidism and hyperthyroidism), gastrointestinal (diarrhea and colitis), pulmonary (pneumonitis), dermatological (rash and pruritus), and hepatic (elevated liver enzymes).Citation27,Citation28 The majority of these irAEs are self-limiting or resolve with immunosuppressive therapy, such as corticosteroids. Persistent irAEs that do not resolve with corticosteroids require treatment with a tumor necrosis factor-α receptor antagonist such as infliximab or mycophenolate, which is an inhibitor of purine synthesis in T-cells and B-cells.Citation29,Citation30 Only a small minority of irAEs do not respond to these immune modulators. Therefore, fatal irAEs are exceedingly rare for the anti-PD-1 antibody, with an incidence of less than 0.5% in a meta-analysis of studies of ICI monotherapy in several types of cancer, and most often secondary to pneumonitis.Citation27,Citation28
There have been no new safety signals with ICIs in esophageal cancer versus other types of cancer. irAEs had an incidence of approximately 20% in the trials with pembrolizumab, the most common being hypothyroidism and pneumonitis. In the studies of nivolumab (in which there was no clear classification of irAEs or common AEs), the incidence of serious AEs of any grade was 15%–16% and that of grade ≥3 AEs was 10%–17%. One of the most common serious treatment-related AEs was interstitial lung disease, which had an incidence of 2%–5%. In the KEYNOTE-181 and ATTRACTION-3 trials, which compared the efficacy and safety of ICI monotherapy with that of chemotherapy, the incidence of treatment discontinuation as a result of AEs and that of treatment-related deaths were similar between ICI and chemotherapy. Furthermore, the incidence of any treatment-related AEs and that of grade ≥3 AEs were lower with an ICI than on chemotherapy. ICI monotherapy appears to be better tolerated than chemotherapy with fewer adverse events. However, severe irAEs may occur in a small percentage of patients and should be kept in mind.
Clinical trials with ICI plus chemotherapy in the first-line setting
After the effectiveness of ICIs was confirmed in the late-line setting, their efficacy as first-line agents were investigated. Since the first report on the efficacy of an ICI plus chemotherapy as first-line treatment for non-small cell lung cancer (NSCLC) in 2018,Citation31 this combination therapy has been assessed in several types of cancer. Cytotoxic chemotherapy may enhance the immune response achieved by an ICI via potential immunogenic effects, including destruction of tumor cells and increased potential for antigen cross-presentation by dendritic cells,Citation32 inhibition of myeloid-derived suppressor cells,Citation33 an increased ratio of cytotoxic lymphocytes to regulatory T-cells,Citation34 and blocking of the STAT6 pathway to enhance the activity of dendritic cells.Citation35 In view of these mechanisms, an ICI combined with standard chemotherapy was expected to improve the prognosis in patients with esophageal cancer. In the next section, we outline the results of the clinical investigations of ICI plus chemotherapy in the front-line setting and summarize them in .
Table 3. Results of clinical trials than compared an ICI plus chemotherapy with chemotherapy alone in a first-line setting.
Pembrolizumab plus chemotherapy
KEYNOTE-590 was a global, double-blind, randomized, placebo-controlled Phase III study that compared the efficacy and safety of pembrolizumab plus chemotherapy (fluorouracil and cisplatin) versus chemotherapy alone as first-line treatment in patients with unresectable SCC or AC. The primary endpoints were OS and PFS in all patients, in those with SCC who were PD-L1-positive (CPS ≥10), in those with SCC regardless of CPS status, and in those who were PD-L1 positive irrespective of histology. The study included 749 patients (286 [38%] with SCC and PD-L1 positivity, 548 [73%] with SCC, and 383 [51%] with PD-L1-positivity). Median OS was significantly longer in patients with SCC and PD-L1-positivity who received pembrolizumab plus chemotherapy than in those who received chemotherapy alone (13.9 months vs. 8.8 months, HR 0.57, 95% CI 0.43–0.75; p < .0001), those with SCC (12.6 months vs. 9.8 months, HR 0.72, 95% CI 0.6–0.88; p = .0006), those with PD-L1-positivity (13.5 months vs. 9.4 months, HR 0.62, 95% CI 0.49–0.78; p < .0001), and in all patients (12.4 months vs. 9.8 months, HR 0.73, 95% CI 0.62–0.86; p < .0001). Median PFS was also longer with pembrolizumab plus chemotherapy than with chemotherapy alone in patients with SCC (6.3 months vs. 5.8 months, HR 0.65, 95% CI 0.54–0.78; p < .0001), in those with PD-L1-positivity (7.5 months vs. 5.5 months, HR 0.51, 95% CI 0.41–0.65; p < .0001), and in all patients (6.3 months vs. 5.8 months, HR 0.65, 95% CI 0.55–0.76; p < .0001). The ORR was also improved in all patients (45% vs. 29.3%; p < .0001). Furthermore, OS (HR 0.86, 95% CI 0.68–1.10) and PFS (HR 0.80, 95% CI 0.64–1.01) tended to be better even in patients who were PD-L1 negative (CPS <10).Citation36 Therefore, the FDA approved pembrolizumab plus fluoropyrimidine and platinum-based chemotherapy as first-line treatment for patients with advanced or metastatic esophageal cancer irrespective of histology and PD-L1 expression in March 2021.
Nivolumab plus chemotherapy and nivolumab plus ipilimumab
The CheckMate-648 trial was a global, open-label, randomized Phase III study that investigated the efficacy and safety of not only nivolumab plus chemotherapy (fluorouracil and cisplatin) but also nivolumab plus ipilimumab (anti-CTLA-4 antibody) versus chemotherapy alone. Most of the study population had SCC. The coprimary endpoints were PFS and OS in patients who were PD-L1-positive (TPS ≥ 1) and in all patients. This study included 970 patients (951 [98%] with SCC and 473 [49%] who were PD-L1 positive). Median OS was significantly longer in patients who received nivolumab plus chemotherapy than in those who received chemotherapy alone, both in patients with PD-L1 positivity (15.4 months vs. 9.1 months, HR 0.54, 99.5% CI 0.37–0.80; p < .001) and in all patients (13.2 months vs. 10.7 months, HR 0.74, 99.1% CI 0.58–0.96; p = .002). However, median PFS was significantly longer with nivolumab plus chemotherapy than with chemotherapy alone only in patients who were PD-L1 positive (6.9 months vs. 4.4 months, HR 0.65, 98.5% CI 0.46–0.92; p = .002). The ORR was higher with nivolumab plus chemotherapy than with chemotherapy alone both in patients who were PD-L1-positive (53% vs. 20%) and in all patients (47% vs. 27%).
When nivolumab plus ipilimumab was compared with chemotherapy alone, median OS was significantly longer both in patients who were PD-L1 positive (13.7 months vs. 9.1 months, HR 0.64, 98.6% CI 0.46–0.90; p = .001) and in all patients (12.7 months vs. 10.7 months, HR 0.78, 98.2% CI 0.62–0.98; p = .01). However, median PFS was not longer with nivolumab plus ipilimumab than with chemotherapy alone, even in patients who were PD-L1 positive (4.0 months vs. 4.4 months, HR 1.02, 98.5% CI 0.73–1.43; p = .90); the ORR was also higher in these patients (35% vs. 20%), but was similar in all patients (28% vs. 27%). In fact, no OS or PFS benefit was observed with a nivolumab-containing regimen in comparison with chemotherapy alone in patients with TPS < 1.Citation37 Based on these results, the FDA approved nivolumab plus chemotherapy and nivolumab plus ipilimumab as first-line treatments for advanced or metastatic esophageal SCC irrespective of PD-L1 expression in April 2021.
Tislelizumab plus chemotherapy
The efficacy and safety of tislelizumab plus chemotherapy was compared with that of chemotherapy alone in RATIONALE-306, which was a global, double-blind, randomized, placebo-controlled Phase III study in patients with SCC. Standard chemotherapy was fluoropyrimidine plus platinum or paclitaxel plus platinum, which is a widely used first-line treatment in China. The primary endpoint was OS in all patients. This study included 649 patients (236 [36%] with PD-L1 positivity [TAP score ≥10%]). Median OS was significantly longer with tislelizumab plus chemotherapy than with chemotherapy alone in all patients (17.3 months vs. 10.6 months, HR 0.66, 95% CI 0.54–0.80; p < .0001). A median OS benefit was also observed both in patients with TAP score ≥10% (16.8 months vs. 10.0 months, HR 0.61, 95% CI 0.44–0.85; p = .0017) and in those with a TAP score <10% (16.7 months vs. 10.4 months, HR 0.72, 95% CI 0.55–0.94). Median PFS was also significantly longer with tislelizumab plus chemotherapy than with chemotherapy alone in all patients (7.3 months vs. 5.6 months, HR 0.62, 95% CI 0.52–0.75; p < .0001). The ORR was also significantly higher with tislelizumab plus chemotherapy than with chemotherapy alone in all patients (63.5% vs. 42.4%; p < .0001).Citation38
Other anti-PD-1 antibodies plus chemotherapy
The efficacy and safety of various other humanized immunoglobulin monoclonal anti-PD-1 antibodies used in combination with chemotherapy have been compared with those of chemotherapy alone in the first-line setting in three randomized Phase III studies, namely, ESCORT-1st (camrelizumab),Citation39 ORIENT-15 (sintilimab),Citation40 and JUPITER-06 (toripalimab).Citation41 These clinical trials were conducted only in China (although ORIENT-15 was a global trial, only 3% of the study population was from outside of China), targeted only patients with SCC, and standard chemotherapy consisted of paclitaxel plus platinum (only 6%–7% of patients received fluoropyrimidine plus platinum in ORIENT-15). In these trials, the primary endpoints of OS and PFS were met in all patients irrespective of PD-L1 status. Median OS was significantly longer with ICI plus chemotherapy than with chemotherapy alone in ESCORT-1st (15.3 months vs. 12.0 months, HR 0.70, 95% CI 0.56–0.88; p = .001), ORIENT-15 (16.7 months vs. 12.5 months, HR 0.63, 95% CI 0.51–0.78; p < .001), and JUPITER-06 (17 months vs. 11 months, HR 0.58, 95% CI 0.43–0.78; p = .0004). The HR for PFS in these studies ranged from 0.56 to 0.58 and the ORR with ICI plus chemotherapy from 66% to 72.1%.
Safety of ICI plus chemotherapy and nivolumab plus ipilimumab
The incidence of grade ≥3 treatment-related AEs and serious AEs tended to be slightly higher with ICI plus chemotherapy than with chemotherapy alone (2%–11% higher and 7%–10% higher, respectively, in the above-mentioned trials). Addition of an ICI was associated with a 4%–15% increase in discontinuation of treatment because of AEs but not with an increase in treatment-related mortality (1%–2% with ICI plus chemotherapy and with chemotherapy alone). Grade ≥3 irAEs occurred at a rate of 7%–10% with ICI plus chemotherapy. The most commonly reported irAEs were hypothyroidism and skin symptoms, such as rash and pruritus. Most irAEs were grade 1–2 and manageable. Furthermore, it seems that chemotherapy does not increase the likelihood of irAEs because their incidence in KEYNOTE-180 (≥third-line), KEYNOTE-181 (second-line), and KEYNOTE-590 (first-line with chemotherapy) was 20.7%, 23.2%, and 26%, respectively. However, a higher incidence of irAEs, in particular endocrine and skin AEs, was observed with nivolumab plus ipilimumab than with nivolumab plus chemotherapy in CheckMate-648. The median time to onset of most irAEs tended to be shorter with nivolumab plus ipilimumab than with nivolumab plus chemotherapy (endocrine, 13.0 weeks vs. 8.2 weeks; gastrointestinal, 5.1 weeks vs. 9.1 weeks; hepatic, 7.9 weeks vs. 5.0 weeks; pulmonary, 31.2 weeks vs. 11.9 weeks; renal, 10.1 weeks vs. 7.1 weeks; and skin, 5.9 weeks vs. 3.9 weeks).Citation42
There was a higher incidence of toxicity when an ICI was combined with anti-cancer therapy, including chemotherapy, immunotherapy, targeted therapy, and RT, than when an ICI was used alone.Citation43 Furthermore, the incidence of AEs was significantly higher on anti-PD-1 or anti-PD-L1 plus anti-CTLA-4 combinations than on anti-PD-1 or anti-PD-L1 monotherapy in several meta-analyses.Citation44–48 Close monitoring and early recognition of pertinent symptoms and signs are required to ensure appropriate management when these agents are used in combination therapy.
Future prospects for ICI therapy
Given the consistent observation of a survival benefit, high ORR, and manageable safety with ICI plus chemotherapy in the clinical trials described above, these combinations have become established as new standard first-line treatments for unresectable esophageal cancer. Even with the use of ICIs, median OS only reaches at least 1 year. These results cannot be considered satisfactory, and development of novel combination treatments that include an ICI are underway to improve the prognosis.
ICI plus tyrosine kinase inhibitor therapy
Combination of an ICI and a tyrosine kinase inhibitor (TKI) has been tested in various types of cancer. JapicCTI -195063 is an ongoing Phase Ib study of pembrolizumab plus futibatinib (TAS-120) in patients with advanced or metastatic esophageal cancer or NSCLC and overexpression of fibroblast growth factor receptor (FGFR)1–4. Futibatinib is a highly selective irreversible FGFR1–4 inhibitor and was approved by the FDA in March 2022 for unresectable interhepatic cholangiocarcinoma harboring FGFR2 gene rearrangements, including gene fusions. Inhibition of FGFR may sensitize tumors to ICI by direct action on cancer cells or by altering the tumor microenvironment.Citation49
A Phase II study (NCT04704154) of nivolumab plus regorafenib in patients with recurrent or metastatic solid tumors, including esophageal cancer, is also under way. Regorafenib is a potent inhibitor of angiogenic and oncogenic kinase and blocks the activity of vascular endothelial growth factor receptors 1–3, FGFR, platelet-derived growth factor receptor, and the oncogenes KIT, RET, RAF, and BRAF. This TKI reduces expression of tumor-associated macrophages, which may contribute to resistance to ICIs.Citation50
The Phase III LEAP-014 trial (NCT04949256) is presently evaluating the efficacy and safety of a new investigational treatment consisting of pembrolizumab plus fluoropyrimidine and platinum in addition to lenvatinib, which is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1–3, FGFR1–4, platelet-derived growth factor receptor, and the oncogenes RET and KIT. This combination is based on the findings in an in vivo model that lenvatinib substantially decreased expression of tumor-associated macrophages and enhanced activation of the interferon signaling pathway, resulting in increased anti-tumor activity via PD-1 inhibition.Citation51
ICI plus radiotherapy
RT plays an important role in the treatment of esophageal cancer regardless of resectability. Combination of RT/CRT with an ICI has been under development because radiation enhances of the antigen-specific immune response, including death of inflammatory tumor cells, activation of dendritic cells, cross-presentation of antigens, and activation and proliferation of cytotoxic T-cells.Citation52
Since the PACIFIC trial demonstrated significantly longer OS with durvalumab (anti-PD-L1 antibody) following definitive CRT in patients with unresectable stage III NSCLC,Citation53 a combination of definitive CRT with ICI for unresectable locally advanced esophageal cancer has been developed. The Phase II and III KEYNOTE-975 (NCT04210115),Citation54 NOBEL (UMIN000035889), and KUNLUN (NCT04550260) trials are presently assessing the efficacy and safety of definitive CRT plus pembrolizumab, nivolumab, and durvalumab, respectively. Furthermore, the Phase II CRUCIAL trial (NCT03437200) is currently recruiting and will evaluate the efficacy and safety of FOLFOX-based definitive CRT plus nivolumab followed by sequential nivolumab monotherapy and CRT plus nivolumab and ipilimumab followed by sequential nivolumab and ipilimumab.
ICI therapy in the perioperative setting
While preoperative chemotherapy with cisplatin plus 5-fluorouracil (CF) followed by surgery has been established as standard therapy for resectable locally advanced esophageal cancer in Japan,Citation55 neoadjuvant CRT has also established one of the standard treatments from the results of CROSS trial (carboplatin plus paclitaxel with RT) and CALGB 9781 trial (CF with RT) in Western countries.Citation56,Citation57 The addition of ICI to these chemotherapy or CRT in the neoadjuvant setting for esophageal SCC has also been under development.
The Phase II NIC-ESCC2019 trial, which evaluated the safety and efficacy of camrelizumab plus chemotherapy (cisplatin plus nab-paclitaxel) as neoadjuvant therapy, showed pathologic complete response (pCR) rate of 31.4% and manageable safety.Citation58 Phase II Keystone-001 trial (NCT0438917) and Phase III Keystone-002 trial (NCT04807673) are currently ongoing in China which evaluate the efficacy and safety of pembrolizumab plus chemotherapy (cisplatin plus paclitaxel) over CRT in neoadjuvant setting.Citation59,Citation60 The Phase I PALACE-1 trial, which evaluated the safety of pembrolizumab in combination with neoadjuvant CRT (carboplatin plus paclitaxel with RT), showed high pCR rate of 55.6% and manageable safety,Citation61 and subsequent Phase II PALACE-2 trial (NCT04435197) is underway.Citation62 However, the pCR rate of same regimen was poor at 23.1% in the Phase II study (NCT02844075) which conducted in Korea.Citation63 In Japan, CF plus docetaxel (DCF) became established as a novel standard neoadjuvant treatment in 2022 based on the results of a three-arm Phase III NExT (JCOG 1109) trial which evaluated whether DCF or CF plus RT conferred a survival benefit over CF in the neoadjuvant setting.Citation64 The FRONTiER trial (JCOG1804E) is underway to evaluate the efficacy and safety of addition of nivolumab to these preoperative therapies,Citation65 and it has been reported that the pCR rate of 33.3% in combination of DCF with nivolumab in Phase I part.Citation66 In preoperative setting, the efficacy of additional ICI to chemotherapy or CRT remains controversial because of lack of consistency and difference of standard chemotherapy in each trial, and further investigations are needed.
In postoperative adjuvant setting, the Phase III CheckMate-577 trial, which targeted on both SCC and AC, demonstrated significant improvement of median disease-free survival with adjuvant nivolumab over placebo in patients without a pCR to neoadjuvant CRT followed by curative surgery (22.4 months vs. 11.0 months, HR 0.69, 95% CI 0.56–0.86; p = .0003).Citation67 Adjuvant nivolumab was approved by the FDA for resected esophageal cancer in May 2021.
Challenges in the development of ICI therapy
There are several challenges to overcome with the ongoing development of ICI therapy. First, it remains uncertain that CPS and TPS are useful as biomarkers. The CPS is likely to be useful for identifying the population in which pembrolizumab might demonstrate clinical efficacy based on the results of KEYNOTE-180 and KEYNOTE-181. In fact, there was a trend of better survival in patients with a CPS ≥10 than in those with a CPS <10 in KEYNOTE-590. On the other hand, in SCC only, it has been shown that TPS is more valuable predict factor of ICI benefit than CPS from the meta-analysis of the results in Phase III trials which described above.Citation68 Although CPS/TPS should be used as one of the few predictors in clinical practice, it is not perfect enough to discourage the use of ICI because of low CPS/TPS. In addition, tumor mutation burden (TMB) and microsatellite instability (MSI) are also known to be biomarkers of the response to ICIs. Higher expression levels of TMB and MSI induce expression of immunogenic neoantigen, which promotes a response to ICIs. Pembrolizumab has already been approved for clinical use in patients with TMB-high cancer and those with MSI-high cancer based on the results of KEYNOTE-016 Citation69 and KEYNOTE-158.Citation70,Citation71 These biomarkers should be considered separately because MSI-high status is reportedly independent of the CPS.Citation72 It remains necessary to search for new biomarkers and combinations of biomarkers.
Second, the effectiveness of ICI therapy in patients with esophageal AC remains controversial because of the limited numbers of patients with this disease in the clinical trials reported to date. The proportion of patients with AC was 36% in KEYNOTE-181, 27% in KEYNOTE-590, and only 2% in CheckMate-648. There was no improvement in OS with pembrolizumab monotherapy in comparison with chemotherapy in patients with AC irrespective of the CPS in KEYNOTE-181. However, OS was improved when pembrolizumab was added to chemotherapy in patients with AC regardless of the CPS in KEYNOTE-590. However, the median OS in patients who received chemotherapy alone was 2 months shorter in those with CPS <10 than in those with CPS ≥10 (10.7 months vs. 8.4 months) but was comparable with that in those who received pembrolizumab plus chemotherapy regardless of the CPS (12.1 months vs 12.7 months). This difference in survival according to the CPS score in the chemotherapy group is difficult to explain. Moreover, any survival benefit conferred by addition of an ICI was consistently attenuated in patients with advanced gastric cancer (usually AC) and low PD-L1 expression (CPS <5 or <10) in CheckMate-649 and KEYNOTE-062.Citation73–75 More robust evidence is required in these populations.
Third, passage obstruction and inadequate oral intake as a result of the primary tumor irrespective of metastasis are common problems that interfere with treatment of patients in clinical practice. These patients are suitable for treatment with RT and/or CRT. Since the ORR has been improved by ICI plus chemotherapy, it is difficult to determine whether the best treatment option is RT or an ICI in addition to chemotherapy for these patients because it remains unclear if the response rates in the clinical trials are directly applicable to the primary tumor given that it is a non-targeted lesion and difficult to measure. Furthermore, these patients are usually not candidates for clinical trials. The CPS/TPS might be a useful predictor of efficacy when choosing between RT and an ICI. Further clinical evidence of benefit from combining RT and/or CRT with ICI therapy is needed.
Conclusion
Based on the results of recent clinical trials, ICIs have finally reached the point where they can be used clinically in patients with various stages of esophageal cancer. However, there are still clinical questions and challenges that need to be resolved during the ongoing clinical development of the ICIs. ICIs are undoubtedly promising drugs that can be expected to improve the prognosis in patients with esophageal cancer in the future.
Disclosure statement
YN has nothing to declare. SY has received honoraria from ONO Pharmaceuticals and Bristol-Myers Squibb. KK has received research funds from ONO, BMS, MSD, Beigeen, Shionogi, and Oncolys Biopharma as well as honoraria from ONO, Eli Lilly, and Taiho.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers In 185 countries. CA Cancer J Clin. 2021;71(3):1–12. doi:10.3322/caac.21660.
- Thrift AP. The epidemic of esophageal carcinoma: where are we now? Cancer Epidemiol. 2016;41:88–95. doi:10.1016/j.canep.2016.01.013.
- Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Network. 2019;17(7):855–83. doi:10.6004/jnccn.2019.0033.
- Shah MA. Update on metastatic gastric and esophageal cancer. J Clin Oncol. 2015;33(16):1760–69. doi:10.1200/JCO.2014.60.1799.
- Ingelfinger JR, Rustgi AK, El-Serag HB, Ingelfinger JR. Esophageal carcinoma. N Engl J Med. 2014;371(26):2499–509. doi:10.1056/NEJMra1314530.
- American Cancer Society. Esophagus cancer, early detection, diagnosis, and staging; [ accessed 2022 Jun 30]. https://www.cancer.org/cancer/esophagus-cancer/detection-diagnosis-staging/survival-rates.html.
- Hiramoto S, Kato K, Shoji H, Okita N, Takashima A, Honma Y, Iwasa S, Hamaguchi T, Yamada Y, Shimada Y, et al. A retrospective analysis of 5-fluorouracil plus cisplatin as first-line chemotherapy in the recent treatment strategy for patients with metastatic or recurrent esophageal squamous cell carcinoma. Int J Clin Oncol. 2018;23(3):466–72. doi:10.1007/s10147-018-1239-x.
- Moehler M, Maderer A, Thuss -, Brenner B, Meiler J, Ettrich TJ, Hofheinz R-D, Al-Batran SE, Vogel A, Mueller L, et al. Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER). Ann Oncol. 2020;31(2):228–35. doi:10.1016/j.annonc.2019.10.018.
- Crosby T, Hurt CN, Falk S, Gollins S, Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI, et al. Chemotherapy with or without cetuximab in patients with oesophageal cancer (SCOPE1): a multicenter, phase 2/3 randomised trial. Lancet Oncol. 2013;14(7):627–37. doi:10.1016/S1470-2045(13)70136-0.
- Suntharalingam M, Winter K, Ilson D, Dicker AP, Kachnic L, Konski A, Chakravarthy AB, Anker CJ, Thakrar H, Horiba N, et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: the NRG oncology RTOG 0436 Phase 3 randomized clinical trial. JAMA Oncol. 2017;3(11):1520–28. doi:10.1001/jamaoncol.2017.1598.
- Lorenzen S, Schuster T, Porschen R, Al-Batran S-E, Hofheinz R, Thuss-Patience P, Moehler M, Grabowski P, Arnold D, Greten T, et al. Cetuximab plus cisplatin–5-fluorouracil versus cisplatin–5-fluorouracil alone in first-line metastatic squamous cell carcinoma of the esophagus: a randomized phase II study of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2009;20(10):1667–73. doi:10.1093/annonc/mdp069.
- Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligand in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–45. doi:10.1038/ni1443.
- Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–24. doi:10.1093/intimm/dxm057.
- Nurieva RI, Liu X, Dong C. Yin-Yang of costimulation: crucial controls of immune tolerance and function. Immunol Rev. 2009;229(1):88–100. doi:10.1111/j.1600-065X.2009.00769.x.
- Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. 2018;36(28):2836–44. doi:10.1200/JCO.2017.76.6212.
- Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153–67. doi:10.1038/nri.2017.108.
- Brahmer JR, Drake CG, Woller I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, et al. Phase I study of single-agent anti–programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi:10.1200/JCO.2009.26.7609.
- Raufi AG, Klempner SJ. Immunotherapy for advanced gastric and esophageal cancer: preclinical rationale and ongoing clinical investigations. J Gastrointest Oncol. 2015;6(5):561–69. doi:10.3978/j.issn.2078-6891.2015.037.
- Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu C-H, Doi T, Moriwaki T, Kim S-B, Lee S-H, et al. randomized phase III KEYNOTE-181 Study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol. 2020;38(35):4138–48. doi:10.1200/JCO.20.01888.
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–55. doi:10.1126/science.aar4060.
- Kato K, Cho BC, Takahashi M, Okada M, Lin C-Y, Chin K, Kadowaki S, Ahn M-J, Hamamoto Y, Doki Y, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomized, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–17. doi:10.1016/S1470-2045(19)30626-6.
- Chen L, Sun J, Wu H, Zhou S-M, Tan Y, Tan M, Shan B-E, Lu B-F, Zhang X-G. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60(7):1047–55. doi:10.1007/s00262-011-1017-3.
- Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim S-B, Tajika M, Kim HT, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of esophagus. The phase 2 KEYNOTE-180 study. JAMA Oncol. 2019;5(4):546–50. doi:10.1001/jamaoncol.2018.5441.
- Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, Hironaka S, Hara H, Satoh T, Iwasa S, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, Phase 2 trial. Lancet Oncol. 2017;18(5):631–39. doi:10.1016/S1470-2045(17)30181-X.
- Kato K, Doki Y, Ura T, Hamamoto Y, Kojima T, Tsushima T, Hironaka S, Hara H, Kudo T, Iwasa S, et al. Long-term efficacy and predictive correlates of response to nivolumab in Japanese patients with esophageal cancer. Cancer Sci. 2020;111(5):1676–84. doi:10.1111/cas.14380.
- Shen L, Kato K, Kim SB, Ajani JA, Zhao K, He Z, Yu X, Shu Y, Luo Q, Wang J, et al. Tislelizumab versus chemotherapy as second-line treatment for advanced or metastatic esophageal squamous cell carcinoma (RATIONALE-302): a randomized phase III study. J Clin Oncol. 2022;40(26):3065–76. JCO2101926. doi:10.1200/JCO.21.01926.
- Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune check point inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–85. doi:10.1093/annonc/mdx286.
- Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, Shen C, Duma N, Vera Aguilera J, Chintakuntlawar A, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008–19. doi:10.1001/jamaoncol.2019.0393.
- Gangadher TC, Vonderheide RH. Mitigating the toxic effects of anti-cancer immunotherapy. Nat Rev Clin Oncol. 2014;11(2):91–99. doi:10.1038/nrclinonc.2013.245.
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–68. doi:10.1200/JCO.2017.77.6385.
- Gaudhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab Plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92. doi:10.1056/NEJMoa1801005.
- Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. doi:10.1038/cdd.2013.67.
- Wang Z, Till B, . Chemotherapeutic agent-mediated elimination of myeloid-derived suppressor cells. Oncoimmunology. 2017;6:e1331807. doi:10.1080/2162402X.2017.1331807.
- Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, Farsaci B, Donahue R, Gulley JL, Schlom J, et al. Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology. 2013;2(10):e27025. doi:10.4161/onci.27025.
- Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJH, Nierkens S, Schreibelt G, de Boer A, Van Herpen CML, Kaanders JH, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest. 2011;121(8):3100–08. doi:10.1172/JCI43656.
- Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges J-P, Li Z, Kim S-B, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–71. doi:10.1016/S0140-6736(21)01234-4.
- Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu C-H, Adenis A, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–62. doi:10.1056/NEJMoa2111380.
- Yoon HH, Kato K, Raymond E, Hubner R, Shu Y, Pan Y, Jiang Y, Zhang J, Park S, Kojima T, et al. RATIONALE-306: randomized, global, placebo-controlled, double-blind Phase 3 study of tislelizumab plus chemotherapy versus chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma. Esmo Wgi. 2022;33:S375. Abstract LBA-1. doi:10.1016/j.annonc.2022.04.439.
- Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, Zhang Y, Zhao K, Chen Z, Gao S, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma. The ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–25. doi:10.1001/jama.2021.12836.
- Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang L, Wang B, Shun G, Ji Y, Cao G, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. doi:10.1136/bmj-2021-068714.
- Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): a multi-center phase 3 trial. Cancer Cell. 2022;40(3):277–88.e3. doi:10.1016/j.ccell.2022.02.007.
- Chau I, Ajani JA, Doki Y, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu C, Adenis A, et al. Nivolumab plus chemotherapy or ipilimumab vs chemotherapy as first-line treatment for advanced esophageal squamous cell carcinoma: expanded efficacy and safety analyses from CheckMate 648. Esmo Wgi. 2022;33:S379–80. Abstract O-3. doi:10.1016/j.annonc.2022.04.444.
- Zhou X, Yao Z, Bai H, Duan J, Wang Z, Wang X, Zhang X, Xu J, Fei K, Zhang Z, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitor-based combination therapies in clinical trials: a systematic review and meta-analysis. Lancet Oncol. 2021;22(9):1265–74. doi:10.1016/S1470-2045(21)00333-8.
- Yang Y, Gin G, Pang Y, Huang Y, Wang W, Zhang H, Tuo G, Wu P, Wang Z, Zhu Z. Comparative efficacy and safety of nivolumab and nivolumab plus ipilimumab in advanced cancer: a systematic review and meta-analysis. Front Pharmacol. 2020;11:40. doi:10.3389/fphar.2020.00040.
- Da L, Teng Y, Wang N, Zaguirre K, Liu Y, Qi Y, Song F. Organ-specific immune-related adverse events associated with immune checkpoint inhibitor monotherapy versus combination therapy in cancer: a meta-analysis of randomized controlled trials. Front Pharmacol. 2020;10:1671. doi:10.3389/fphar.2019.01671.
- Park R, Lopes L, Cristancho CR, Riano IM, Saeed A. Treatment -related adverse events of combination immune checkpoint inhibitors: systematic review and meta-analysis. Front Oncol. 2020;10:258. doi:10.3389/fonc.2020.00258.
- Almutairi AR, McBride A, Slack M, Erstad BL, Abraham I. Potential immune-related adverse events associated with monotherapy and combination therapy of ipilimumab, nivolumab, and pembrolizumab for advanced melanoma: a systematic review and meta-analysis. Front Oncol. 2020;10:91. doi:10.3389/fonc.2020.00091.
- Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, Li X, Zhu H, Zhong X, Pan J, et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): a systematic review and meta-analysis. BMC Cancer. 2019;19(1):559. doi:10.1186/s12885-019-5785-z.
- Koshkin VS, Sonpavda GP, Hwang C, Mellado B, Tomlinson G, Shimura M, Chisamore MJ, Gil M, Loriot Y. Futibatinib plus pembrolizumab in patients (pts) with advanced or metastatic urothelial carcinoma (mUC): preliminary safety results from a phase 2 study. ASCO Genitourinary Cancer Symposium. 2022;40(6_suppl):501. Abstract #501. doi:10.1200/JCO.2022.40.6_suppl.501.
- Doleschel D, Hoff S, Koletnik S, Rix A, Zopf D, Kiessling F, Lederle W. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Explained Clin Cancer Res. 2021;40(1):288. doi:10.1186/s13046-021-02043-0.
- Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, Ito J, Tachino S, Hori Y, Matsuki M, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14(2):e0212513. doi:10.1371/journal.pone.0212513.
- Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498–509. doi:10.1016/S1470-2045(15)00007-8.
- Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2343–50. doi:10.1056/NEJMoa1809697.
- Shah MA, Bennouna J, Doi T, Shen L, Kato K, Adenis A, Mamon HJ, Moehler M, Fu X, Cho BC, et al. KEYNOTE-975 study design: a Phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with esophageal carcinoma. Future Oncol. 2021;17(10):1143–53. doi:10.2217/fon-2020-0969.
- Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68–74. doi:10.1245/s10434-011-2049-9.
- Eyck BM, van Lanschot JJB, Hulshof MCCM, van der Wilk BJ, Shapiro J, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, et al. Ten-Year outcome of neoadjuvant chemoradiotherapy plus surgery for esophageal cancer: the Randomized controlled CROSS trial. J Clin Oncol. 2021;39(18):1995–2004. doi:10.1200/JCO.20.03614.
- Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D, Mayer R. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–92. doi:10.1200/JCO.2007.12.9593.
- Liu J, Li J, Lin W, Shao D, Depypere L, Zhang Z, Li Z, Cui F, Du Z, Zeng Y, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): amulticenter, phase 2 study. Int J Cancer. 2022;151(1):128–37. doi:10.1002/ijc.33976.
- Shang X, Zhao G, Liang F, Zhang C, Zhang W, Liu L, Li R, Duan X, Ma Z, Yue J, et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage III) esophageal squamous cell carcinoma: a study protocol for a prospective, single-arm, single center, open-label, phase-II trial (Keystone-001). Ann Transl Med. 2022;10:229.
- Shang X, Zhang W, Zhao G, Liang F, Zhang C, Yue J, Duan X, Ma Z, Chen C, Pang Q, et al. Pembrolizumab combined with neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy followed by surgery for locally advanced oesophageal squamous cell carcinoma: protocol for a multicentre, prospective, randomized-controlled, Phase III clinical study (Keystone-002). Front Oncol. 2022;12:831345.
- Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, Wu Y, Feng X, Qi W, Chen K, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (PALACE-1). Eur J Cancer. 2021;144:232–41. doi:10.1016/j.ejca.2020.11.039.
- Zheng Y, Li C, Yu B, Zhao S, Li J, Chen X, Li H. Preoperative pembrolizumab combined with chemoradiotherapy for esophageal squamous cell carcinoma: trial design. JTCVS Open. 2021;9:293–99. doi:10.1016/j.xjon.2021.11.003.
- Lee S, Ahn BC, Park SY, Kim DJ, Lee CG, Cho J, Kim JH, Kim HR, Kim Y-H, Park SR, et al. A phase II trial of preoperative chemoradiotherapy and pembrolizumab for locally advanced esophageal squamous cell carcinoma (ESCC). 2019. Ann Oncol. 2019;30(v754):v754. doi:10.1093/annonc/mdz266.018.
- Kato K, Ito Y, Daiko H, Ozawa S, Ogata T, Hara H, Kojima T, Abe T, Bamba T, Watanabe M, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. 2022. ASCO Gastrointestinal Cancer Symposium.40(4_suppl): 238. Abstract #238. 10.1200/JCO.2022.40.4_suppl.238
- Yamamoto S, Kato K, Daiko H, Kojima T, Hara H, Abe T, Tsubosa Y, Nagashima K, Aoki K, Mizoguchi Y, et al. Feasibility study of nivolumab as neo adjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol. 2020;16(19):1351–57. doi:10.2217/fon-2020-0189.
- Matsuda S, Yamamot S, Kato K, Daiko H, Kojima T, Hara H, Abe T, Tsubosa Y, Kawakubo H, Nagashima K, et al. FRONTiER: a feasibility trial of nivolumab with neoadjuvant CF or DCF, FLOT therapy for locally advanced esophageal carcinoma (Jcog1804e)—short-term results for cohorts C and D. ASCO Gastrointestinal Cancer Symposium. 40(4_suppl): 286. Abstract #286. 10.1200/JCO.2022.40.4_suppl.286
- Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lièvre A, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. 2021;384(13):1191–203. doi:10.1056/NEJMoa2032125.
- Yoon HH, Jin Z, Kour O, Kankeu Fonkoua LA, Shitara K, Gibson MK, Prokop LJ, Moehler M, Kang Y-K, Shi Q, et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer. Systematic review and meta-analysis of 17 Phase 3 randomized clinical trials. JAMA Oncol. 2022;8(10):1456. doi:10.1001/jamaoncol.2022.3707.
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. doi:10.1056/NEJMoa1500596.
- Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–65. doi:10.1016/S1470-2045(20)30445-9.
- Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord J-P, Geva R, Gottfried M, Penel N, Hansen AR, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi:10.1200/JCO.19.02105.
- Shitara K, Ajani JA, Moehler M, Garrido M, Gallardo C, Shen L, Yamaguchi K, Wyrwicz L, Skoczylas T, Bragagnoli AC, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942–48. doi:10.1038/s41586-022-04508-4.
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomized, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi:10.1016/S0140-6736(21)00797-2.
- Shitara K, Cutsem EV, Bang YJ, Fuchs C, Wyrwicz L, Lee K-W, Kudaba I, Garrido M, Chung HC, Lee J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer. The KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6(10):1571–80. doi:10.1001/jamaoncol.2020.3370.
- Zhao JJ, Yap DWT, Chan YH, Tan BKJ, Teo CB, Syn NL, Smyth EC, Soon YY, Sundar R. Low programmed death-ligand 1–expressing Subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J Clin Oncol. 2022;40(4):392–402. doi:10.1200/JCO.21.01862.