ABSTRACT
The COVID-19 pandemic has severely affected adolescents. Safe and effective vaccines are pivotal tools in controlling this pandemic. We reviewed the safety profile of the BNT162b2 COVID-19 vaccine in adolescents using mostly real-world data to assist decision-making. We used random-effects model meta-analysis to derive pooled rates of single or grouped adverse events following immunization (AEFI) after each primary and booster dose, as well as after combining all doses. Reporting on over one million participants with safety data were included. The most-reported local and systemic AEFIs were pain/swelling/erythema/redness and fatigue/headache/myalgia, respectively. AESIs were rarely reported but were more frequent after the second dose than they were after the first and the booster doses. Health impact was less common among adolescents after receiving BNT162b2 vaccine. Rare life-threatening AEFIs were reported across all doses in real-world studies. Our findings highlight the significance of enhancing national and regional vaccination programs to ensure public confidence.
Introduction
A safe and efficacious vaccine is pivotal for mitigating the socioeconomic and health impact of the coronavirus disease 2019 (COVID-19) pandemic among adolescents.Citation1 There is overwhelming evidence demonstrating the impact of COVID-19 on adolescents’ daily lives. Indirectly, these impacts include constraints for remote learning, low levels of physical activity, high exposure to indoor air pollution, mental health issues, social life disruption (such as no contact with extended family members), premature pregnancy, and malnutrition in countries where children rely on meals from school feeding programmes.Citation2 Directly, these impacts include spreading SARS-CoV-2 infection in the community, symptomatic COVID-19,Citation3 hospitalizations,Citation4 multisystem inflammatory syndrome in children (MISC-C),Citation5 long COVID,Citation6 and death.Citation7
BNT162b2,Citation8 a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine that encodes the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) full-length spike protein, is developed by BioNTech and Pfizer. It was one of the first vaccines to receive emergency approval from various regulatory agencies around the world, including the World Health Organization (WHO), for use in adolescents. In clinical trials with teenagers, the BNT162b2 vaccination revealed a very high level of safety and efficacy.Citation9 For instance, although adolescents who received immunizations reported greater local and systemic adverse effects than those who received a placebo, these were mild to moderate in severity and resolved within one to two days. Pain at the injection site and headache were the most often reported local and systemic adverse effects, respectively. In general, systemic effects were reported more frequently following the second vaccine dose compared to the first. There was no difference in reactogenicity between participants who were SARS-CoV-2–positive at baseline and those who were SARS-CoV-2–negative at baseline, and even after six months of follow-up, no major side effects, such as myocarditis, were reported.Citation10 Causality assessment is a WHO-proposed algorithm for determining a causal association between vaccinations and adverse events following immunization (AEFIs), particularly for serious AEFIs.Citation11,Citation12 However, this has rarely been applied in many COVID-19 vaccine post-marketing safety studies,Citation13 instead depending on passive data reporting systems, which are recognized to have a significant risk of reporting bias.
Global,Citation14,Citation15 regional,Citation16 and national studies,Citation17,Citation18 have demonstrated a high level of vaccine hesitancy among the general population,Citation19–21 with safety concerns being one of the most significant predictors of vaccine hesitancy. Vaccine hesitancy, the willingness to delay or to refuse a vaccine despite its accessibility, has been classified by the WHO among the top ten threats to global health.Citation22 The unprecedented endeavor to produce a safe vaccination for COVID-19 in such a short period of time may consequently be hampering vaccine uptake due to vaccine hesitancy.Citation23 Based on prior studies of vaccine safety surveillance,Citation24,Citation25 and studies of COVID-19,Citation26 safety monitoring remains critical to building and sustaining public confidence, trust and engagement.Citation27,Citation28 These outcomes will safeguard the global effort and mechanism for vaccine equity by ensuring the success of a mass immunization program, particularly in an era where social media has magnified misinformation.Citation29 However, synthesized post-marketing evidence regarding the safety of the BNT162b2 vaccination in adolescents, providing pooled effects across large samples, is needed to facilitate informed decision making at both the individual and population level. To this end, we conducted a comprehensive systemic review that included clinical trial and real-world data to describe the safety profile of BNT162b2 in adolescents after mass vaccination to protect against COVID-19 infection.
Materials and methods
Criteria for considering studies for this review
Types of studies
We included randomized and non-randomized interventional studies (i.e., post-authorization surveillance data), regardless of the method or unit of allocation, as well as observational studies (i.e., prospective or retrospective cohort studies), case-control and cross-sectional studies. Follow-up of participants in the included studies could be of any duration. We excluded reviews, case series and case reports, as well as studies in non-human subjects.
Types of participants
We included adolescent participants (aged 12 to 17 years) from the general population, i.e., predominantly healthy individuals. We included studies recruiting a subset of eligible participants (i.e., children and adolescents) if results were reported separately for the eligible subset of participants. If this was not feasible, we included such studies if 90% or more of the sample were children in the age range 12–17 years. We excluded studies where participants were children under the age of 12 years or adults (aged ≥18 years). In addition, we excluded studies that focused on specific populations (e.g., people with type 1 diabetes, hematologic or solid organ transplants) and those focusing on specific outcomes (e.g., myocarditis or Bell’s palsy only).
Types of interventions
We included studies investigating any injectable BNT162b2 vaccine intended for the prevention, or mitigation, of symptoms of SARS-CoV-2-infection. Studies investigating one or more primary doses (typically dose 1 or 2) as well as boosters of these vaccines were all eligible for inclusion. Studies were included if interventions were comparatively analyzed with either a placebo vaccine or no receipt of a SARS-CoV-2 vaccine. We excluded studies reporting on other COVID-19 vaccines only (e.g., mRNA1273 or CoronaVac).
Types of outcomes
Our outcomes of interest were broadly categorized as those related to vaccine safety profile (see Supplementary Table S4 for definition). Overall, outcomes related to safety were assessed at any time point and included:
any adverse events following immunization (AEFIs),
any adverse events (AEs); serious, non-serious and leading to discontinuation,
any adverse events of special interest (AESIs),
local injection site reactions,
local reactions (e.g., erythema, pain or swelling),
systemic reactions (e.g. myalgia, rash, abdominal pain, itching, arthralgia, muscle pain, chills, fatigue, fever, headache, nausea, vomiting or diarrhea),
any related events,
any life-threatening related events,
need for medical attention due to adverse reaction (e.g. antipyretic or analgesic treatment, hospitalization, inability to perform daily activities or attend school/work, telehealth consults, attendance to clinic or emergency room or taking medication for side effects),
serious adverse events (SAEs) (e.g. chest pain, tachycardia, dyspnea, myocarditis, myopericarditis or severe allergic reactions),
non-serious AEs,
AEs leading to discontinuation from the study,
AEFI by system organ class (SOC) (e.g. infections and infestations; general disorders and administration site conditions; nervous system disorders; sleeping disturbances; musculoskeletal and connective tissue disorders; respiratory, thoracic and mediastinal disorders; cardiac disorders; arrhythmias; injury, poisoning and procedural complications; vascular disorders; hemorrhagic diseases; thromboembolism; reproductive system and breast disorders; hepato-renal syndrome; gastrointestinal disorders or psychiatric disorders),
unsolicited or unbalanced AEFIs (e.g. autoimmune diseases, Guillain-Barré syndrome; acute aseptic arthritis or Bell’s palsy),
hypersensitivity,
lymphadenopathy, and
death
Then, we classified all adverse events as local and systemic AEFI, serious AEFI, AESI, health impact, and unsolicited AEFI by SOC following any dose of BNT162b2 in adolescents. We excluded studies focusing solely on vaccine efficacy or effectiveness that lacked a clear report of the safety profile of the vaccine, or whose safety profile did not correspond to the targeted age group.
Search methods for the identification of studies
We used a comprehensive search strategy designed to identify the maximum number of eligible studies regardless of language or publication status, within a restrictive timeframe.
Electronic sources
We identified evidence through a systematic search of MEDLINE (PubMed), Embase (Ovid), Web of Science and Cochrane library (CENTRAL). The search was initially designed for MEDLINE (PubMed), but it has since been adapted for use with other sources. The MEDLINE (PubMed) search approach is detailed in Box 1 of the supplemental materials. The search was undertaken initially on 15 February 2022 and subsequently on the 15th of each month until 30 May 2022. To maximize sensitivity, the techniques did not differentiate between “safety” and “efficacy or effectiveness.”
Searching other resources
We checked the references of included studies, the WHO database, and the Centers for Disease Control and Prevention (CDC) website as well as the Advisory Committee on Immunization Practices (ACIP) meetings to identify any references to additional studies that could be potentially eligible.
Data collection and analysis
Selection of studies
All search records were imported into Covidence software (Veritas Health Innovation, Melbourne, Australia, available at www.covidence.org.) for de-duplication and screening. Two review authors (PK and LB) independently screened titles and abstracts of these to identify potentially eligible full-text studies. Any discrepancies between reviewers were resolved through discussion, with adjudication by a third reviewer if necessary. Full-text reports were retrieved for each record that was considered potentially eligible. Two review authors (PK and LB) independently screened these full texts to identify studies for inclusion in the review, with any discrepancies again resolved through consensus discussion with support from a third reviewer, if needed. The reasons for excluding full-text reports were documented, with duplicate records also excluded. Where multiple publications reported on the same study, we collated these to ensure that the study, rather than individual reports, was the unit of analysis. In the case where a full report of a study was not available (e.g., conference proceedings) and there was insufficient information to decide regarding eligibility, the study was categorized as ‘awaiting classification’ until the next search update. Given the potential impact of safety profile on vaccine confidence, trust, and vaccine uptake, we did not include preprint papers unless these had been peer-reviewed at the time of the last search. The flow of studies was recorded and summarized in a PRISMA flow diagram.
Data extraction and management
Study characteristics and outcome data for vaccine safety were extracted by a single review author (LB, AB or MK). A second reviewer (PK) checked data extraction to ensure consistency. Extractions were done using a pre-piloted extraction form set up in Covidence, which included the following fields:
identification of the study: country in which the study was conducted and time period over which the study was conducted,
methods: aim of the study and study design, participants-mean age and age group of participants included in the study, total number of participants included, number of participants per group (if reported),
interventions: follow-up period after vaccine administration (first and second dose), primary doses or booster administered, and
safety outcomes: type of AEFI and number of events in each group and sub-age-group as well.
At any point of time, discrepancies were resolved through consensus or adjudication by a senior review author (PK). Characteristics of included studies were summarized in a table, using descriptive information extracted from studies.
Assessment of risk of bias in included studies
Two reviewers (LB and MK) assessed the risk of bias (RoB) of included studies using the Cochrane RoB tool for randomized evidence,Citation30 and the ROBINS-I tool for non-randomized studies.Citation31 One senior reviewer (AB) conducted all RoB assessments as the independent duplicate reviewer. Standard domains for each tool were used, and an overall risk of bias was determined using a worst-domain scenario approach. As a result, we judged the overall risk of bias for each study as ‘low risk,’ ‘unclear risk’ or ‘high risk’ based on the worst-risk individual domain and reported the main reason in the summary table for RoB. Any discrepancies in individual domain judgments as well as overall judgments were resolved through consensus or adjudication by a third review author (PK).
Measures of treatment effect
For the vaccine safety outcomes, dichotomous data on events were extracted using the number of events in each group as the numerator and the number of participants randomized, or exposed to, each group as the denominator.
Data synthesis
We conducted meta-analyses to summarize reported data across studies using the meta, metafor (metaprop function and RevMan5 layout) packages in R (version 3.6.3, R foundation for Statistical Computing, Vienna, Austria). For the safety profile, the primary outcomes were the proportion of any reported AEFI after receiving the BNT162b2 COVID-19 vaccine. We examined rates of local and systemic AEFIs, serious AEFI, AESI and unsolicited AEFI by SOC as well as any related health impact. We performed meta-analyses of proportions to estimate the pooled rate of safety outcomes. To generate pooled proportions, a random-effects model meta-analysis using the inverse variance approach was conducted, with the pooled proportions calculated using the arcsine transformation,Citation32 and the Clopper-Pearson methodCitation33 to calculate 95% confidence intervals (95% CIs). Heterogeneity was evaluated by the χ2 test on Cochran’s Q statistic,Citation34 which was quantified by I2. The I Citation2 statistic estimates the percentage of total variation across studies due to true between-study differences rather than chance. We explored the reasons for variations across studies and examined whether the proportion of AEFI varied by dose. This was done by assessing AEFI following the primary doses (first or second) and booster dose separately, before comparing all doses together (i.e., first and second primary dose plus booster). We also explored whether some variation was due to sample size of included studies by excluding or including studies with sample size below 100 for the age group of interest. We assessed the presence of publication bias using the Egger test of funnel plot asymmetry,Citation35 with p < .10 indicating significant publication bias. If event-related information was not clearly reported, findings were summarized narratively.
Results
Characteristics of included studies
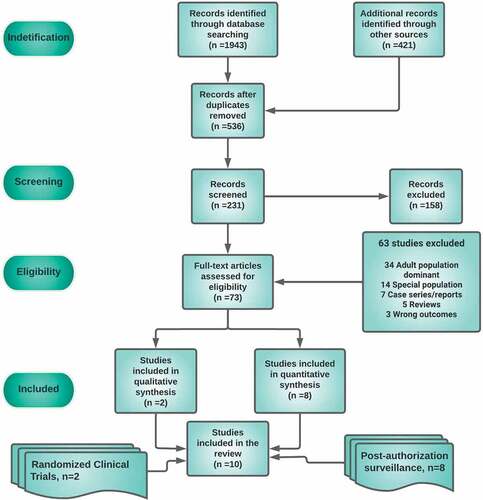
We identified 1,943 records from the searches. After removing duplicates, we screened 536 records and found 73 potentially eligible studies whose full-text articles were assessed for eligibility; 10 of these were included in this systematic review (). The safety data collection period of included studies had a maximal range of six months, with most studies reporting data from day zero (the date of injection) to seven days. Two studies reported data going from day zero to 2 months, and one study from day zero to six months. The main characteristics of included studies, both clinical trials and post-authorization studies, are reported in Supplementary Table S5.
Quality methods of included studies
Risk of bias by study design
Observational studies
The risk of bias in five included observational studies reporting on safety was conducted using ROBINS-I (Supplementary Table S1).Citation36–40 Three of these studies had a high overall risk of bias: two of these studies did not report adjusting their analyses for potential confounding (Alamer 2021; Hause 2021), with Alamer additionally having a high risk of selection and ascertainment bias. The study by Kitagawa 2022 had a high overall risk of bias due to methodological limitations related to selection and ascertainment biases; all three studies also had a number of domains at unclear risk of bias. The two remaining studies had an unclear overall risk of bias: both due to uncertainty around the potential for bias relating to attrition, ascertainment, and generalizability; with Hause 2022 additionally having an unclear judgment for selective reporting and Klein 2021 for potentially underpowered analyses of rare events.
Cohort studies
Three included studies with a cohort design reported on safety and were also assessed using ROBINS-I (Supplementary Table S2).Citation41–43 Two of these studies had a high overall risk of bias, with Chan 2022 judged to be at high risk for selection and ascertainment bias and June Choe 2021 judged to be at high risk for confounding as well as selection and attrition biases. Both studies were also judged to be at unclear risk of selective outcome reporting and limited generalizability. Furthermore, it was unclear to what extent Chan 2022 was biased with regards to the incorrect intervention classification. The third study had unclear overall risk of bias (Lai 2022) as residual confounding, misclassification bias and selective outcome reporting could not be ruled out, and generalizability was limited.
Randomized trials
The two included randomized trials (RCTs) reporting on safety were assessed using Cochrane’s RoB tool (Supplementary Table S3);Citation9,Citation10 both were judged as having unclear overall RoB. This was due to both studies not explicitly stating how randomization sequences were protected; Thomas 2021 was additionally judged to be at unclear risk of attrition bias.
Adverse events following immunization (AEFI) in included studies
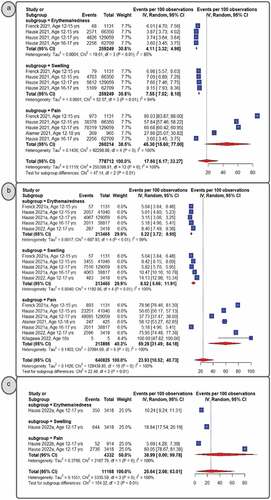
Local AEFI
displays the pooled rate of local AEFIs following primary dose 1 (panel A), primary dose 2 (panel B), and the booster dose (panel C). Pain (at the injection site) was the most often reported AEFI per dosage, followed by swelling and erythema (or redness). Overall, they were reported most frequently after the booster dose (25.64%, 95% CI: 2.08–63.01) followed by the second dose (23.93%, 95% CI: 10.52–40.73) and the first dose (17.6%, 95% CI: 6.17–33.27). This was consistent when considering any reported local reaction (Supplementary Figure S1). Similar trends were found for erythema and edema, with children reporting a progressive, but low, pooled incidence from the initial dose to the booster dose. The pooled proportion for pain increased with the primary second dose (59.28%, 95% CI: 31.49–84.18) when compared to the booster dose (38.99%, 95% CI: 0.00–99.78) and the primary first dose (45.30%, 95% CI: 15.60–77.00), for which children reported less pain when receiving BNT162b2 vaccine. Pain (at the injection site) remained the most frequently reported local AEFI (46.89%, 95% CI: 28.5–65.68), even when combining AEFIs for all three doses. This was followed by swelling (8.94%, 95% CI: 6.70–11.46) and erythema (or redness) (5.22%, 95% CI: 3.92–6.08). (Supplementary Figures S2-5, respectively).
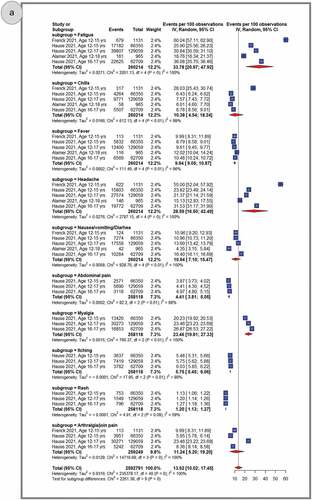
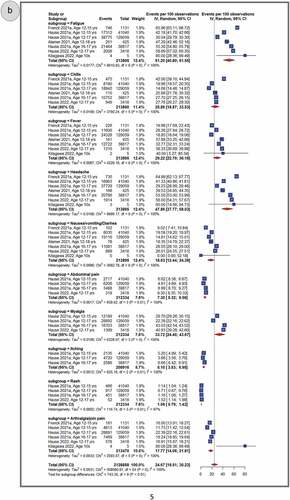
Systemic AEFI
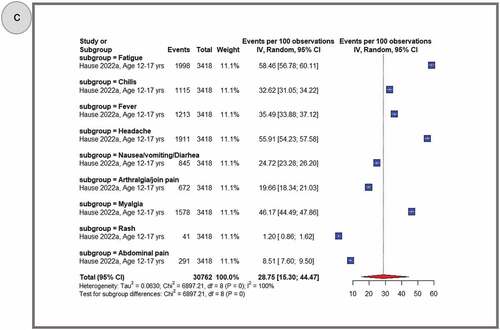
shows pooled proportions of the top 10 reported systemic AEFIs after primary dose 1 (panel A), primary dose 2 (panel B) and the booster dose (panel C). In general, adolescents experienced more systemic AEFIs after the booster dose (28.75%, 95% CI: 15.30–44.47) than after the primary second (24.67%, 95% CI: 19.51–30.23) and primary first (13.52%, 95% CI: 10.02–17.45) doses. The three most common systemic AEFI across all the three doses were fatigue (primary dose 1: 33.78%, 95% CI: 20.97–47.92; primary dose 2: 51.20%, 95% CI: 40.80–61.55; booster dose: 58.46%, 95% CI: 56.78–60.11), headache (primary dose 1: 28.59%, 95% CI: 16.50–42.49; primary dose 2: 47.86%, 95% CI: 37.77–58.03; booster dose: 55.91%, 95% CI: 54.23–57.58), and myalgia (primary dose 1: 23.46%, 95% CI: 19.81–5.05; primary dose 2: 33.72%, 95% CI: 24.45–43.67). After pooling AEFIs for all doses, the most frequently reported adverse reactions were fatigue (43.76%, 95% CI: 34.77–52.96); headache (40.01%, 95% CI: 30.65–49.75); myalgia (27.34%, 95% CI: 21.20–33.96); fever (20.71%, 95% CI: 14.38–27.86); chills (19.25%, 95% CI:12.85–26.59); nausea, vomiting or diarrhea (15.80%, 95% CI: 11.78–20.28); arthralgia or join pain (14.87%, 95% CI:11.7–18.99); abdominal pain (5.5%, 95% CI, 4.16–7.01); itching (5.42%, 95% CI, 4.60–6.30); and rash (1.09%, 95% CI, 0.93–1.27) (Supplementary Figures S6-16). A similar trend was observed during sensitivity analyses that excluded studies with sample sizes less than 100 and excluded data from clinical trials. (Supplementary Figures S56–61).
Serious adverse events
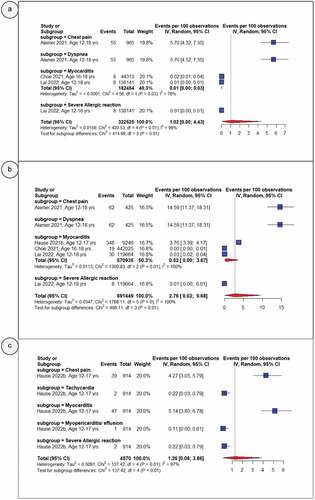
shows pooled proportions of the serious AEs or AESIs after primary dose 1 (panel A), primary dose 2 (panel B) and the booster dose (panel C). While serious AEs or AESIs were infrequently reported among vaccinated adolescents, these AEFIs were more common after the primary second dose (2.76%, 95% CI: 0.03–9.68) than they were after the primary first (1.02%, 95% CI: 0.00–4.43) and booster (1.26%, 95% CI: 0.08–3.86) doses. Considering a pooled proportion across all doses, any serious AEs or AESIs remained less commonly reported among vaccinated adolescents (1.72%, 95% CI: 0.24–4.50) (Supplementary Figures S23). Similarly, the most frequently reported serious AEs or AESIs across all doses were dyspnea (9.62%, 95% CI: 2.78–19.96), chest pain (7.59%, 95% CI: 2.84–14.37), myocarditis (5.95%, 95% CI: 0.00–25.47), tachycardia (0.22%, 95% CI: 0.03–0.79), myopericarditis (1.09%, 95% CI: 0.03–6.08), and severe allergic reactions (0.64%, 95% CI: 0.37–0.99) (Supplementary Figures S17-22).
Health impact
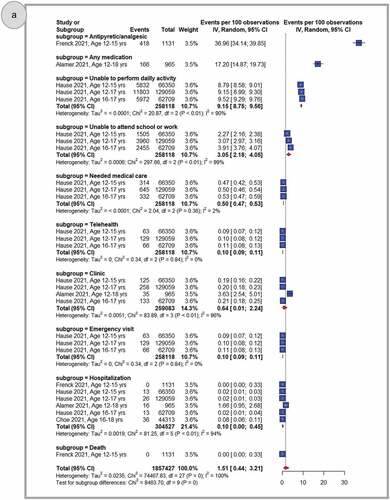
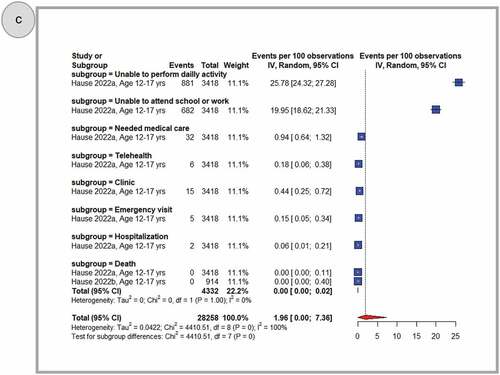
shows pooled proportions of any related health impact (need for medication, impact on activities, medical visit, hospitalization, and death) after primary dose 1 (panel A), primary dose 2 (panel B) and the booster dose (panel C). In general, adolescents who received the BNT162b2 COVID-19 vaccine reported less negative health effects. Once reported, these were more common following the primary second dose (2.69%, 95% CI: 0.91–5.37) when compared to the primary first dose (1.51%, 95% CI: 0.44–3.21), or a booster dose (1.96%, 95% CI: 0.00–7.36). After considering all doses together, the use of any medication for side effects was widespread (31.40%, 95% CI: 6.92–63.64) and antipyretic use was frequently reported (43.93%, 95% CI: 30.52–57.81). Inability to perform daily activities (17.36%, 95% CI: 11.83–23.69), or to attend school or work (6.96%, 95% CI: 3.75–11.08), was reported less frequently when data from all three doses were combined. Instances of need for medical care (5.77%, 95% CI: 4.85–7.64), telehealth consultation (0.13%, 95% CI: 0.10–0.17), clinic visits (7.76%, 95% CI: 0.89–21.34) or emergency visits (1.38%, 95% CI: 1.05–1.75), hospitalization (1.65%, 95% CI: 0.01–6.00), and death (1.13%, 95% CI: 0.00–5.17) were also very rarely observed (Supplementary Figures S23-33)
AESI and unsolicited AEFI by SOC
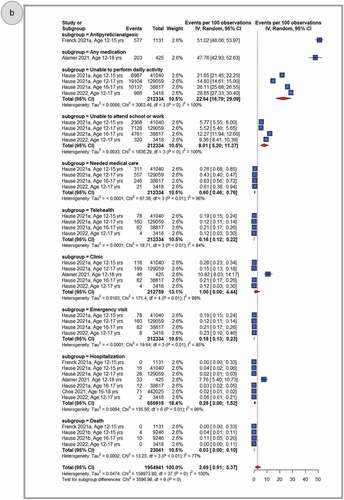
depicts the pooled proportion of observed AESIs and unsolicited AEFIs by SOC following primary dose 1 (panel A), primary dose 2 (panel B) and the booster dose (panel C) of BNT162b2 in adolescents. Overall, reported AESIs and unsolicited AEFIs by SOC were rarely reported among adolescents who received BNT162b2 vaccine. Observations varied from zero percent, after primary doses 1 and 2, to 0.52% (95% CI, 0.07–1.41) after a booster dose. Cardiovascular diseases, nervous system disorders or sleep disturbances, respiratory, thoracic, and mediastinal disorders, musculoskeletal disorders, and infections and infestations were the most frequently reported AEFIs by SOC, albeit at a very low rate (1% for pooled rate across three doses). Across primary doses 1 and 2, the pooled rate of any AESI was 0.05% (95% CI: 0.02–0.09). AESIs such as Guillain-Barré syndrome (0.01 per 100,000 observations, 95% CI: 0.00–1.32) and Bell’s palsy (1.76 per 100,000 observations, 95% CI: 0.14–5.21) remained less common even after pooling data from the three doses (Supplementary Figures S34-52)
Safety outcomes specific to clinical trials
Discontinuation (0.18%, 95% CI: 0.02–0.64), any life-threatening event (0.09, 95% CI: 0.00–0.049), and any related event (2.92%, 95% CI: 2.02–4.07) (Supplementary Figures S53–55) were less common among vaccinated adolescents when compared with placebo group. Decreased appetite, lethargy, asthenia, malaise, nocturnal sweats, and hyperhidrosis were among the new adverse events due to BNT162b2 that had not been detected in prior investigations.Citation10
Discussion
In these meta-analyses, primarily including real-world studies from geographically and socioeconomically diverse populations, the safety profile of the BNT162b2 vaccination in adolescents following primary and booster doses is described in detail for the first time, to our knowledge. Overall, we found that AEFIs were reported more frequently after the booster dose than after the second and initial doses. Solicited local and systemic AEFIs were very common at all doses; however, when graded, they were predominantly categorized as mild to moderate. Serious AEFIs, AESIs, and unsolicited AEFIs by SOC were also recorded at a lower rate compared to AEFIs, when comparing these across all doses. While many adolescents reported the need to take medication to alleviate AEFIs, and the use of antipyretics were mentioned by more than half of them, only a small percentage of them were hospitalized or had their daily lives affected. Death was recorded far less frequently following vaccination.
We found that the majority of local and systemic AEFIs reported in post-authorization studies were also reported during clinical trials, and that they were detected much less frequently during mass vaccination than in the clinical trial setting. This may be due to the regulated environment that enables highly controlled surveillance during RCTs as well as the level of underreporting during passive surveillance.Citation44–46 Furthermore, we also noted that local and systemic AEFIs were more likely to be reported at a high rate compared to the adult population.Citation10 Young people being more likely to exhibit reactogenicity when compared to adults is a well-known phenomenon in vaccinology and should not be used as evidence against the vaccine’s safety profile. A recent systematic review comparing the rates of solicited AEs in the active and placebo groups of COVID-19 vaccines similarly found that AEs were more prevalent in the younger population. In addition, authors concluded that their findings are in accordance with the expectancy theory of nocebo effects, and that the AEs associated with COVID-19 vaccines may be related to the nocebo effect.Citation47 Consequently, increased awareness of the nocebo effect in vaccination studies might contextualize the safety findings in the presence of expected negative effects, thereby increasing COVID-19 vaccine uptake and infection protection.Citation47
In contrast, serious AEFIs were recorded at a much higher rate after mass vaccination compared to the initial clinical trial (during the blinded phase),Citation9 and even after six months of surveillance following the RCT cohort (open-label phase).Citation10 There are numerous possible explanations for these disparities. First, the incidence of myocarditis is so low that it is less likely to be observed in a selected, relatively small group. Second, myocarditis is over-reported during passive surveillance since anyone can record a side effect regardless of their background. Third, once recorded at the hospital, myocarditis is reported as-is, without any clinical confirmation (and most of time without biopsy). Fourth, the rate of myocarditis may be exclusively associated with COVID-19 infection in some cases, as confirmation of COVID-19 prior to vaccination is rarely reported in passive surveillance data. Myocarditis and related symptoms should be analyzed extensively once reported, and efforts should be made to confirm cases (myocarditis) using the WHO causality assessment to further enhance risk-benefit approach, particularly in this age group. For instance, between 9 December 2021 and 20 February 2022, 64 preliminary reports of myocarditis were received via the Vaccine Adverse Event Reporting System (VAERS); 47 of these were deemed serious; 32 (68.1%) of these reports were validated by provider interview or medical record review as meeting the Centers for Disease Control and Prevention (CDC) working criteria of myocarditis.Citation38 All 32 cases included adolescent males, and of the 27 (84.4%) hospitalized patients, as of 20 February 2022, all had been discharged, 18 had recovered, and nine were recovering.
Similar approaches to confirm cases should be taken for any AEFIs with health impacts. During the clinical trial, for instance, withdrawal owing to adverse events was rarely reported, and neither life-threatening adverse events nor hospitalizations were observed. Despite their rarity, we found that a few adolescents had been hospitalized. It remains unclear, however, if hospitalizations were due to the vaccine, as was reported by some. After evaluating the V-safe data,Citation3,Citation38 2 adolescents (roughly 0.9%) were reported as having received medical treatment during the week following booster vaccination; the majority (n = 15; 0.4%) received care through clinic appointments. One (0.03%) teenager was hospitalized for treatment of a new-onset migraine during the week after a booster dose; it was not possible to ascertain if hospitalization was a result of vaccination. Similarly, few adolescents had their everyday activities or schooling affected after immunization, though this was likely under-reported in earlier studies. During the first phases of vaccination (clinical trials reporting on first and second primary doses), remote education may have concealed most effects related to life disruptions, such as inability to attend school or work.
In many earlier investigations, only short-term surveillance data were available, and various unsolicited AEFIs were not reported. In addition, other AESIs, such as Bell’s palsy, Guillain-Barré syndrome and anaphylaxis were recorded across the different BNT62b2 vaccine doses, but most of them were not reported in the clinical trial. While their pooled rate remained extremely low across all doses, their numerical values merit national and international monitoring. With regards to other COVID-19 vaccines, a case series and nested case-control study conducted in the general population in Hong Kong reported an increase of 4.8 cases per 100,000 vaccinated with CoronaVac against 2.0 cases per 100,000 vaccinated with BNT162b2, indicating an overall increased risk of Bell’s palsy after vaccination with CoronaVac.Citation48 While anaphylaxis after BNT162b2 may be more prevalent in certain populations, such as people of Japanese ethnicity,Citation49 a recent meta-analysis has showed that the risk of anaphylactic reaction, anaphylactoid reaction, anaphylactic shock, and anaphylactoid shock is not exclusive to mRNA COVID-19 vaccinations;Citation50 thus, immunization sites must be prepared to provide aid in all circumstances. Moreover, in a study of individuals with a history of allergy, the incidence and severity of AEFIs following BNT162b2 immunization were higher, but anaphylaxis or death were not observed. These findings suggest that patients with a history of allergic reactions can tolerate the BNT162b2 mRNA COVID-19 vaccination.Citation51 Additionally, as stated in a similar study, allergic individuals can be immunized using an algorithm that can be implemented in a variety of medical institutions and includes a referral center, a risk assessment questionnaire, and a setting for immunization of severely allergic patients under medical supervision.Citation52 Very little research has focused on unsolicited AEFIs by SOC. Our pooled rates favored a very infrequent incidence across the three BNT162b2 doses, with most cases involving cardiovascular issues and infections or infestations. As with other serious AEFIs, there was no obvious distinction between cardiac-related vaccination events and infections caused by COVID-19 itself throughout the epidemic. For illustration, according to data from 40 health care institutions participating in a major research network in the United States, the risk for cardiac complications was considerably greater after COVID-19 than after mRNA COVID-19 immunization; this was the case for males and females of all ages.Citation53
Vaccination programs should prioritize AEFI reporting systems and support staff in doing in-depth analysis. Our study highlights two significant issues. Firstly, investigations are mostly done through passive surveillance, with active surveillance studies far fewer. However, passive surveillance systems are affected by underreporting or lack of information and clinical data.Citation44,Citation45 Based on the preceding findings, hospital-based surveillance should complement public health surveillance. Following the detection of an increase in aseptic meningitis associated with the Urabe mumps vaccine strain at several Canadian children’s hospitals, the need for a hospital-based active surveillance system to reliably detect serious AEFIs was previously recognized (Canadian Immunization Monitoring Program Active; IMPACT).Citation54 This signal, which was not detected by the passive system, led to the substitution of the Urabe vaccination with a safer alternative. A similar network was established in Australia through the Pediatric Active Enhanced Disease Surveillance (PAEDS). Their active surveillance has improved data precision, enhanced regular surveillance, bridged research gaps, and enabled scaled public health response supports. In both IMPACT and PAEDS, trained surveillance nurses at each hospital are supervised by volunteer pediatric doctors who serve as site investigators. Secondly, we found a geographical disparity in the reporting of AEFI, with most data coming from high-income regions. Decolonizing global vaccination surveillance is an important milestone for the epidemiology of AEFIs. Several African nations, for instance, are vaccinating adolescents, but we were unable to obtain published safety data from this region. Hospital-based surveillance systems in middle- and low-income countries, such as the Influenza Monitoring of Vaccine Effectiveness Network (I-MOVE), Global Rotavirus and Invasive Bacterial Vaccine Preventable Diseases Surveillance Networks (IB-VPD), and AEFI surveillance network in the Americas, might also be leveraged. Rather than starting a de novo system, the combination of these can form surveillance initiatives comparable to IMPACT or PAEDS. Such an initiative may be supplemented globally by the WHO global safety database (VigiBase).Citation54,Citation55
Even though our study has the unique advantage of combining millions of real-world observations with those from clinical trials (during the blind and open-label period), investigating effects across all three vaccine doses, including populations from several WHO regions, and assessing effects over a relatively long time compared to other studies in the literature, certain limitations should be mentioned. These include the consideration of passive data reporting systems, such as the VAERS system, which allows health professionals and laypeople to report safety data. Importantly, relying on such data can enhance under-reporting bias, as individuals experiencing symptoms are more likely to report than those who did not, making it difficult for studies to have a clear denominator. As a result, we attempted to focus on the most frequently reported AEFI, according to the clinical trial, and ensured that sensitivity analyses were performed both with and without clinical trial data. These analyses showed no significant difference in the pooled proportion. Similarly, studies exhibited a high degree of heterogeneity attributable mostly to differences in the data collection methods. To mitigate such a threat, we have considered assessing the risk of bias by design to understand the overall quality of the included studies and have conducted sensitivity analyses excluding very small studies (including fewer than 100 participants). These analyses showed that small studies tended to inflate the pooled estimate. In addition, we excluded studies reporting solely on immunocompromised individuals, individual cases, those that did not include age range data, and those whose authors did not respond to requests for sharing individual data. While some studies did indeed incorporate data on comorbidities, others did not. This could bias the true estimate in either direction, depending on ‘where’ comorbidities are – for example, if many more persons with comorbidities were present in the placebo group, then the effect would possibly be overestimated; conversely, if many more persons with comorbidities were present in the vaccine group, the effect would possibly be biased toward the null. Future research should focus on answering questions regarding the safety of specific populations. In terms of specific outcomes, we excluded studies focusing primarily on specific AESIs, such as myocarditis or Bell’s palsy, from our meta-analyses due to the lack of reported denominators. Future research should examine resolving this constraint to enable a more precise risk-benefit analysis, so vaccine uptake may be improved. Finally, it should be noted that all included studies were either at high or unclear overall risk of bias, indicating methodological limitations of the evidence base. Non-randomized studies were frequently at high or unclear risk of confounding as they did not adjust for potential confounders or did so inadequately. Selection bias as well as bias related to ascertainment of outcomes were also often identified as domains of high risk; this was mainly a function of how these studies were designed. Attrition bias was difficult to determine in observational studies, also due to the way in which studies were conducted and the passive nature of data collection. This is accepted as a feature of this study design, and it is recommended that future studies be planned as cohort studies where follow-up is more feasible. Generalizability was also an area of concern in most observational and cohort studies; this is mitigated by the pooling of studies conducted in diverse settings and populations. While the extraordinary and often emergency-response circumstances under which many of these studies were conducted are acknowledged, future non-randomized studies would be strengthened by measuring and properly adjusting for confounding variables; having clearly defined sampling frames; and specifying outcome definitions, preferably adjudicated by qualified medical personnel against validated criteria. The two included randomized studies were both at unclear risk of bias due to uncertainties relating to allocation concealment, with one trial additionally at unclear risk of attrition bias as the participant flow in the age group eligible for this review was not clearly reported. It is recommended that future randomized studies explicitly report measures taken to protect the randomization sequence through concealment of allocation, as well as the flow of participants (with accompanying numbers and reasons for attrition), in the overall sample as well as all subgroups of interest, throughout the trial.
Conclusion
Our findings corroborate the safety profile of the Pfizer-BioNTech vaccine for COVID-19 in adolescents following primary and booster doses obtained from previous clinical trials. Local and systemic AEFIs are common, although these are most often mild to moderate in severity. Severe AEFIs, AESIs, and AEFIs by SOC can occur after any dose, but most often occur after a booster dose; however, these are extremely uncommon across studies. Life-threatening AEFIs are extremely rare, as are hospitalizations and deaths. Our results, primarily based on real-world research, provide evidence for healthcare decision-making around COVID-19 vaccination in adolescents and highlight a need for continuous worldwide surveillance through registries. This need is especially acute in low- and middle-income countries (LMICs) where such surveillance information is scant and COVID-19-related child deaths have occurred at a comparatively high rate when viewed against other WHO regions.
Data sharing
All data generated or analyzed during this study are included in this published article and the appendix.
Author contributions
PK, CSW and GG conceived the study. PK and TM did the literature search. PK and LB did the study selection. AB, LB, MK, and PK extracted the relevant information. PK accessed and verified the data. PK and JT synthesized the data. PK wrote the first draft of the paper. CSW and GG supervised the overall work. All authors critically revised successive drafts of the paper. All authors had full access to all the data in the study, read, and approved the final manuscript, and had final responsibility for the decision to submit for publication
Supplemental Material
Download PDF (4.3 MB)Acknowledgments
We acknowledge the South African Medical Research Council for supporting the work of some of the authors and for providing funding for open access publication of this study. AB is partly supported by the Research, Evidence and Development Initiative (READ-It). READ-It (project number 300342-104) is funded by UK aid from the UK government; however, the views expressed do not necessarily reflect the UK government’s official policies
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2144039.
Additional information
Funding
References
- Considering the impact of COVID-19 on children [Internet]. [accessed 2022 Jun 19]. https://www.who.int/europe/activities/considering-the-impact-of-covid-19-on-children
- COVID-19 and children [Internet]. UNICEF DATA. [accessed 2022 Jun 19]. https://data.unicef.org/covid-19-and-children/
- Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Antonelli M, Murray B, Kläser K, Kerfoot E, Chen L, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5(10):1–17. doi:10.1016/S2352-4642(21)00198-X.
- Cloete J, Kruger A, Masha M, du Plessis NM, Mawela D, Tshukudu M, Manyane T, Komane L, Venter M, Jassat W, et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Health. 2022;6(5):294–302. doi:10.1016/S2352-4642(22)00027-X.
- Sacco K, Castagnoli R, Vakkilainen S, Liu C, Delmonte OM, Oguz C, Kaplan IM, Alehashemi S, Burbelo PD, Bhuyan F, et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat Med. 2022;28(5):1050–62. doi:10.1038/s41591-022-01724-3.
- Stephenson T, Pereira SMP, Shafran R, de Stavola BL, Rojas N, McOwat K, Simmons R, Zavala M, O’Mahoney L, Chalder T, et al. Physical and mental health 3 months after SARS-CoV-2 infection (long COVID) among adolescents in England (CLoCk): a national matched cohort study. Lancet Child Adolesc Health. 2022;6(4):230–39. doi:10.1016/S2352-4642(22)00022-0.
- Nachega JB, Sam-Agudu NA, Machekano RN, Rabie H, van der Zalm MM, Redfern A, Dramowski A, O’Connell N, Pipo MT, Tshilanda MB, et al. Assessment of clinical outcomes among children and adolescents hospitalized with COVID-19 in 6 sub-saharan African countries. JAMA Pediatr. 2022;176(3):e216436. doi:10.1001/jamapediatrics.2021.6436.
- Vogel AB, Kanevsky I, Che Y, Swanson KA, Muik A, Vormehr M, Kranz LM, Walzer KC, Hein S, Güler A, et al. Bnt162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283–89. doi:10.1038/s41586-021-03275-y.
- Frenck RWJ, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, Perez JL, Walter EB, Senders S, Bailey R, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021;385(3):239–50. doi:10.1056/NEJMoa2107456.
- Thomas SJ, Moreira EDJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–73. doi:10.1056/NEJMoa2110345.
- Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification , 2nd ed., 2019 update [Internet]. [accessed 2022 Sep 28]. https://www.who.int/publications-detail-redirect/9789241516990
- SYSTEMATIC USE OF CAUSALITY ASSESSMENT IN AEFI SURVEILLANCE: a 2013-2016 pilot study in puglia [Internet]. [accessed 2022 Sep 28]. http://www.embj.org/embj/causality-assessment-aefi-surveillance/
- Lee CW, Sa S, Hong M, Kim J, Shim SR, Han HW. Adverse events and safety profile of the COVID-19 vaccines in adolescents: safety monitoring for adverse events using real-world data. Vaccines. 2022;10(5):744. doi:10.3390/vaccines10050744.
- Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, Kimball S, El-Mohandes A. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27(2):225–28. doi:10.1038/s41591-020-1124-9.
- Solís Arce JS, Warren SS, Meriggi NF, Scacco A, McMurry N, Voors M, Syunyaev G, Malik AA, Aboutajdine S, Adeojo O, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. 2021;27(8):1385–94. doi:10.1038/s41591-021-01454-y.
- Harapan H, Anwar S, Yufika A, Sharun K, Gachabayov M, Fahriani M, Husnah M, Raad R, Abdalla RY, Adam RY, et al. Vaccine hesitancy among communities in ten countries in Asia, Africa, and South America during the COVID-19 pandemic. Pathog Glob Health. 2022;116(4):236–43. doi:10.1080/20477724.2021.2011580.
- Wiysonge CS, Alobwede SM, de Marie C, Katoto P, Kidzeru EB, Lumngwena EN, Cooper S, Goliath R, Jackson A, Shey MS. Cross-cultural adaptation and validation of the 5C scale to identify factors associated with COVID-19 and influenza vaccine hesitancy among healthcare workers in cape town, South Africa – a protocol. Expert Rev Vaccines. 2022;11:1–11. doi:10.12688/f1000research.123332.1.
- Dinga JN, Sinda LK, Titanji VPK. Assessment of vaccine hesitancy to a COVID-19 vaccine in Cameroonian adults and its global implication. Vaccines (Basel). 2021;9(2):175. doi:10.3390/vaccines9020175.
- Temsah M-H, Alhuzaimi AN, Aljamaan F, Bahkali F, Al-Eyadhy A, Alrabiaah A, Alhaboob A, Bashiri FA, Alshaer A, Temsah O, et al. Parental attitudes and hesitancy about COVID-19 vs. routine childhood vaccinations: a national survey. Front Public Health. 2021;9:752323. doi:10.3389/fpubh.2021.752323.
- Goldman RD, Krupik D, Ali S, Mater A, Hall JE, Bone JN, Thompson GC, Yen K, Griffiths MA, Klein A, et al. Caregiver willingness to vaccinate their children against COVID-19 after adult vaccine approval. Int J Environ Res Public Health. 2021;18(19):10224. doi:10.3390/ijerph181910224.
- Chen F, He Y, Shi Y. Parents’ and guardians’ willingness to vaccinate their children against COVID-19: a systematic review and meta-analysis. Vaccines (Basel). 2022;10(2):179. doi:10.3390/vaccines10020179.
- Ten health issues WHO will tackle this year [Internet]. [accessed 2021 Sep 27]. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
- Katoto PDMC, Parker S, Coulson N, Pillay N, Cooper S, Jaca A, Mavundza E, Houston G, Groenewald C, Essack Z, et al. Predictors of COVID-19 vaccine hesitancy in South African local communities: the VaxScenes study. Vaccines. 2022;10(3):353. doi:10.3390/vaccines10030353.
- Karafillakis E, Simas C, Jarrett C, Verger P, Peretti-Watel P, Dib F, De Angelis S, Takacs J, Ali KA, Pastore Celentano L, et al. HPV vaccination in a context of public mistrust and uncertainty: a systematic literature review of determinants of HPV vaccine hesitancy in Europe. Hum Vaccin Immunother. 2019;15(7–8):1615–27. doi:10.1080/21645515.2018.1564436.
- Wiyeh AB, Cooper S, Nnaji CA, Wiysonge CS. Vaccine hesitancy ‘outbreaks’: using epidemiological modeling of the spread of ideas to understand the effects of vaccine related events on vaccine hesitancy. Expert Rev Vaccines. 2018;17(12):1063–70. doi:10.1080/14760584.2018.1549994.
- Wagner AL, Huang Z, Ren J, Laffoon M, Ji M, Pinckney LC, Sun X, Prosser LA, Boulton ML, Zikmund-Fisher BJ. Vaccine hesitancy and concerns about vaccine safety and effectiveness in Shanghai, China. Am J Prev Med. 2021;60(1):S77–86. doi:10.1016/j.amepre.2020.09.003.
- Hou Z, Tong Y, Du F, Lu L, Zhao S, Yu K, Piatek SJ, Larson HJ, Lin L. Assessing COVID-19 vaccine hesitancy, confidence, and public engagement: a global social listening study. J Med Internet Res. 2021;23(6):e27632. doi:10.2196/27632.
- Tram KH, Saeed S, Bradley C, Fox B, Eshun-Wilson I, Mody A, Geng ED. Dissent, and distrust: understanding distinct drivers of coronavirus disease 2019 vaccine hesitancy in the United States. Clin Infect Dis [Internet]; 2021 Sep 28;74(8):1429–41. doi:10.1093/cid/ciab633.
- Wiyeh AB, Cooper S, Jaca A, Mavundza E, Ndwandwe D, Wiysonge CS. Social media and HPV vaccination: unsolicited public comments on a facebook post by the western cape department of health provide insights into determinants of vaccine hesitancy in South Africa. Vaccine. 2019;37(43):6317–23. doi:10.1016/j.vaccine.2019.09.019.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343(oct18 2):d5928. doi:10.1136/bmj.d5928.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919.
- Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods. 2019;10(3):476–83. doi:10.1002/jrsm.1348.
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857–72. doi:10.1002/(SICI)1097-0258(19980430)17:8<857:AID-SIM777>3.0.CO;2-E.
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi:10.1002/sim.1186.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi:10.1136/bmj.315.7109.629.
- Alamer E, Alhazmi A, Qasir NA, Alamer R, Areeshi H, Gohal G, Qadri M, Hashem AM, Algaissi A. Side effects of COVID-19 Pfizer-BioNtech mRNA vaccine in children aged 12–18 years in Saudi Arabia. Vaccines (Basel). 2021;9(11):1297. doi:10.3390/vaccines9111297.
- Hause AM. COVID-19 vaccine safety in children aged 5–11 years — United States , November 3–December 19 2021. MMWR Morb Mortal Wkly Rep [Internet]. 2021 [accessed 2022 Feb 7]; 70. https://www.cdc.gov/mmwr/volumes/70/wr/mm705152a1.htm
- Hause AM. Safety monitoring of COVID-19 vaccine booster doses among persons aged 12–17 Years — United States, December 9, 2021–February 20, 2022. MMWR Morb Mortal Wkly Rep [Internet]. 2022 [accessed 2022 Mar 10]; 71. http://www.cdc.gov/mmwr/volumes/71/wr/mm7109e2.htm
- Kitagawa H, Kaiki Y, Sugiyama A, Nagashima S, Kurisu A, Nomura T, Omori K, Akita T, Shigemoto N, Tanaka J, et al. Adverse reactions to the BNT162b2 and mRNA-1273 mRNA COVID-19 vaccines in Japan. J Infect Chemother. 2022;28(4):576–81. doi:10.1016/j.jiac.2021.12.034.
- Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, Donahue JG, Kharbanda EO, Naleway A, Nelson JC, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390–99. doi:10.1001/jama.2021.15072.
- Chan EWW, Leung MTY, Lau LKW, Leung J, Lum D, Wong R-M, Li X, Chui CSL, Wan EYF, Wong CKH, et al. Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNtech) messenger RNA COVID-19 vaccine: a prospective cohort study. Int J Infect Dis. 2022;116:47–50. doi:10.1016/j.ijid.2021.12.354.
- June Choe Y, Yi S, Hwang I, Kim J, Park Y-J, Cho E, Jo M, Lee H, Hwa Choi E. Safety and effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents. Vaccine. 2022;40(5):691–94. doi:10.1016/j.vaccine.2021.12.044.
- Lai FTT, Chua GT, Chan EWW, Huang L, Kwan MYW, Ma T, Qin X, Chui CSL, Li X, Wan EYF, et al. Adverse events of special interest following the use of BNT162b2 in adolescents: a population-based retrospective cohort study. Emerg Microbes Infect. 2022;11(1):885–93. doi:10.1080/22221751.2022.2050952.
- Tafuri S, Fortunato F, Gallone MS, Stefanizzi P, Calabrese G, Boccalini S, Martinelli D, Prato R. Systematic causality assessment of adverse events following HPV vaccines: analysis of current data from Apulia region (Italy). Vaccine. 2018;36(8):1072–77. doi:10.1016/j.vaccine.2018.01.018.
- Varallo FR, de Oliveira Paim Guimarães S, Abjaude SAR, de Carvalho Mastroianni P. Causes for the underreporting of adverse drug events by health professionals: a systematic review. Rev Esc Enferm USP. 2014;48(4):739–47. doi:10.1590/S0080-623420140000400023.
- VAERS. Guide to Interpreting VAERS Data [Internet]. [accessed 2022 Sep 28]. https://vaers.hhs.gov/data/dataguide.html
- Amanzio M, Mitsikostas DD, Giovannelli F, Bartoli M, Cipriani GE, Brown WA. Adverse events of active and placebo groups in SARS-CoV-2 vaccine randomized trials: a systematic review. Lancet Reg Health Eur. 2022;12:100253. doi:10.1016/j.lanepe.2021.100253.
- Wan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, Gao L, Yu Q, Lam ICH, Chun RKC, et al. Bell’s palsy following vaccination with mRNA (Bnt162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22(1):64–72. doi:10.1016/S1473-3099(21)00451-5.
- Hashimoto T, Ozaki A, Bhandari D, Sawano T, Sah R, Tanimoto T. High anaphylaxis rates following vaccination with the Pfizer BNT162b2 mRNA vaccine against COVID-19 in Japanese healthcare workers: a secondary analysis of initial post-approval safety data. J Travel Med. 2021;28(7):taab090. doi:10.1093/jtm/taab090.
- Sobczak M, Pawliczak R. The risk of anaphylaxis behind authorized COVID-19 vaccines: a meta-analysis. Clinical and Molecular Allergy. 2022;20(1):1. doi:10.1186/s12948-022-00167-y.
- Inoue S, Igarashi A, Morikane K, Hachiya O, Watanabe M, Kakehata S, Sato S, Ueno Y. Adverse reactions to BNT162b2 mRNA COVID-19 vaccine in medical staff with a history of allergy. Respir Investig. 2022;60(2):248–55. doi:10.1016/j.resinv.2021.11.007.
- Shavit R, Maoz-Segal R, Iancovici-Kidon M, Offengenden I, Haj Yahia S, Machnes Maayan D, Lifshitz-Tunitsky Y, Niznik S, Frizinsky S, Deutch M, et al. Prevalence of allergic reactions after Pfizer-BioNtech COVID-19 vaccination among adults with high allergy risk. JAMA Netw Open. 2021;4(8):e2122255. doi:10.1001/jamanetworkopen.2021.22255.
- Block JP. Cardiac complications after SARS-CoV-2 Infection and mRNA COVID-19 vaccination — PCORnet, United States, January 2021–January 2022. MMWR Morb Mortal Wkly Rep [Internet]. 2022 [accessed 2022 Jun 20] 71. http://www.cdc.gov/mmwr/volumes/71/wr/mm7114e1.htm
- Top KA, Macartney K, Bettinger JA, Tan B, Blyth CC, Marshall HS, Vaudry W, Halperin SA, McIntyre P. Active surveillance of acute paediatric hospitalisations demonstrates the impact of vaccination programmes and informs vaccine policy in Canada and Australia. Euro Surveill. 2020;25(25):1900562. doi:10.2807/1560-7917.ES.2020.25.25.1900562.
- Centre UM. About VigiBase [Internet]. [accessed 2022 Jun 20]. https://who-umc.org/vigibase/