ABSTRACT
Inflammatory and immunological skin diseases such as psoriasis, systemic sclerosis, dermatomyositis and atopic dermatitis, whose abnormal skin manifestations not only affected life quality but also caused social discrimination, have been wildly concerned. Complex variables such as hereditary predisposition, racial differences, age and gender can influence the prevalence and therapeutic options. The population of patients with unsatisfactory curative effects under current therapies is growing, it’s advisable to seek novel and advanced therapies that are less likely to cause systemic damage. Mesenchymal stem cells (MSCs) have been proven with therapeutic benefits in tissue regeneration, self-renewal and differentiation abilities when treating refractory skin disorders in preclinical and clinical studies. Here we highlighted the immune modulation and inflammation suppression of MSCs in skin diseases, summarized current studies, research progress and related clinical trials, hoping to strengthen the confidence of promising MSCs therapy in future clinical application.
Introduction
Skin is the largest organ of the body and the first physiological defense line against infection and maintains skin homeostasis and the skin consists of the epidermis, dermis and subcutaneous layer.Citation1 The epidermis is a layered flat epithelium with keratinocytes and tight junction, which form the first defense against outer invasions. Functional keratinocytes help to modulate the inflammatory and immune response through various methods like generating pro-inflammatory cytokines or promoting Th1 response when Toll-like receptors are activated.Citation2 Skin barrier dysfunction dissolves tight junctions of stratum granulosum that allow dendrites extension and antigens presentation.Citation3 The dermis is a dense connective tissue with many elastic and collagen fibers, blood vessels, and nerves, which allows immune cells migration. Substances secreted by sweat and sebaceous glands also help skin defense.Citation4
External stimuli like antigens or irritants and internal abnormalities like persistent skin inflammation increase the risk of skin barrier dysfunction. Skin diseases could be genetic and caused by other factors like environmental triggers, systemic diseases, and medications. The complex interplays make some skin diseases easy to relapse and difficult to cure. Psoriasis and atopic dermatitis (AD) are common chronic inflammatory skin diseases with substantial systemic burden and physical comorbidities. Due to their complex risk factors, multi-faceted evaluations before medical treatment are essential.Citation5,Citation6 Systemic sclerosis and dermatomyositis are autoimmune connective tissue diseases with abnormal skin manifestations. Because the autoantigens are not tissue-specific, multiple organs disorders are also involved.Citation7
A group of cells derived mainly from the third germ layer-mesoderm with the capability of differentiation and connective tissues buildingCitation8 was recommended the name of multipotent mesenchymal stromal cells by the International Society for Cellular Therapy (ISCT) in 2006.Citation9 Organs like bone marrow, umbilical cord, placental tissues, and adipose tissue are extensive sources of MSCs with different potentials of regulatory properties. Self-renewal, immunoregulation, anti-inflammatory, and low immunogenicity are the key properties of MSCs which make it possible in clinical management and avoiding allogeneic rejection.Citation10,Citation11 Autologous or allogeneic MSCs have different advantages in medical practice.Citation12 However, different MSC type has its pros and cons. For example, though bone marrow stem cells are easy to isolate, the yield of them are relatively low and the differentiation capacity is limited. Human umbilical cord stem cells are primitive and strong in proliferation and differentiation but they can only be harvested from the donor once due to its origin.Citation13 High yield and the capacity of maintain longer phenotype in culture make adipose-derived stem cells extensive for application, but the problem of limited differentiation capacity still exist.Citation14–16 So an ideal MSC type is also worth studying in specific clinical treatment.
MSCs could regulate primary and acquired immune responses by interacting with specific effector cells or secreting bioactive factors for their benefit. For example, studies have shown that contact between MSCs and serum could activate the complement system Citation17,Citation18 but the secretion of factor H which is a regulator of complement activation by MSCs themselves could rescue the effect.Citation19 The extra addition of factor H to MSCs could also prevent complement-mediated damage.Citation20 These indicate autologous MSCs application and local modulation of the complement system may be ideal therapies to guarantee cellular viability and function. MSCs could increase phagocyte activity of neutrophils,Citation21 regulate neutrophil phenotype,Citation22 and balance tissue homeostasis by reduction of neutrophil oxidative burst via intercellular adhesion molecule 1 mediated engulfment of neutrophils.Citation23 TLR3 is overexpressed in human-induced pluripotent stem cells (iPSC), which serves as a negative regulator of the NF-KB p65 signaling pathway.Citation24 MSCs could sense combinations of multi-inflammatory cytokines like TNF-α, IL-1β and, IFN -γ and respond by modulating cytokine secretion and activating distinct pathways.Citation25 MSCs could induce metabolic shifts in differentially polarized macrophage and further promote macrophage differentiation,Citation26 as well as enhance M2 macrophage polarization.Citation27
MSC therapy has arisen interest in skin diseases due to its potential in immune modulation and anti-inflammatory capacity, which help skin lesions healing and re-epithelization by secreting various growth factorsCitation28 and its safety and efficacy have been partially established.Citation29 Exosomes are secreted small bio-vesicles enriched with some cell-specific biological substances. MSC exosomes have shown the potential in diseases treatment with the properties of MSC.Citation30–32 For example, MSC exosomes could regulate mast cell activation under inflammatory conditionsCitation33 and suppress mast cell activation by PGE2 production and EP4 activation.Citation34 What’s more, during MSC culture, there are plenty of proteins and lipid mediators secreted in the MSC-conditioned medium and could be preserved without losing efficacy. Such cell-free products have been used for treating skin diseases.Citation35,Citation36
MSC therapy in psoriasis
Psoriasis is a common immune-mediated chronic inflammatory skin disease that causes erythematous, itchy scaly patches with a high incidence, a long course of the disease, and a tendency to relapse. The imbalance between the Th1/Th17 and chemokines and cytokines like IL-17, IL-23, TNFα, and IFN-γ promote psoriasis pathogenesis which could be briefly explained as dysfunction of keratinocytes, immune and inflammatory cells.Citation37 Some cases are almost incurable for life, which may lead to adult comorbidities like psoriatic arthritis and atherosclerotic cardiovascular disease.Citation38 Targeted phototherapy and prescribing biologics targeting key immune pathways in the psoriasis pathogenesis like infliximab (TNF-α),Citation39 ustekinumab (IL-12/23),Citation40 secukinumab (IL-17)Citation41 and guselkumab (IL-23),Citation42 significantly improve the life quality of patients with moderate to severe psoriasis.Citation43 However, not all patients respond to biological drugs and may experience adverse effects without any clinical improvement.Citation44
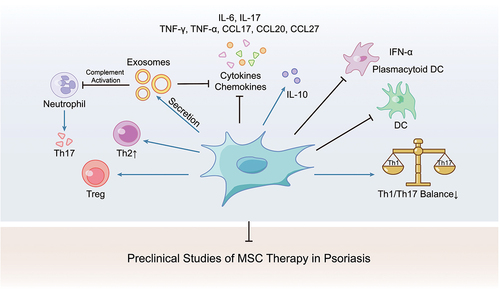
Preclinical studies about MSC infusion in psoriasis models were carried out for several years (). For example, subcutaneous application of extracellular superoxide dismutase transduced MSCs has been proven to prevent disease development in mice,Citation45 and MSC alone could inhibit proinflammatory cytokines or chemokines like IL6, IL7, TNF-α, CCL17, CCL20, and CCL27.Citation46 A recent study has proved that MSCs could reduce the production of type I interferon (IFN-I) by plasmacytoid dendritic cells (pDCs) and rebalance the Th1, Th2, and Th17 responses.Citation47 Topical application of MSC exosomes could also alleviate psoriasis-associated inflammation by inhibiting complement activation.Citation48
Figure 1. MSC therapy could rebalance the amount and function of immune cells and suppress inflammatory mediators in psoriasis model.
A male patient who had received various psoriasis treatments for 25 years without ideal efficacy showed significant improvement after intravenous transplantation (3.0 × 106 cells/mL) and local transplantation (1.0 × 106 cells/mL) of umbilical cord-derived MSCs transplantation.Citation49 And in the long follow-up of 4 to 5 years, a female patient showed no psoriatic relapse signs after three infusions and two more consolidating infusions of umbilical cord-derived MSCs (1.0 × 106/kg for each time). Unexpected psoriasis relief was observed in a male patient who had suffered psoriasis for 12 years during the treatment of newly diagnosed diffuse large B-cell lymphoma (stage IV) with standard lymphoma chemotherapies and autologous hematopoietic stem cell transplantations.Citation50
Several clinical trials also show confidence in the potential of MSC application in psoriasis treatment from a clinical perspective (). Both intravenous and subcutaneous injections were adopted as delivery routes. The dose of (0.5–3.0) *106 cells/kg was commonly used by intravenous injection, while the dose of (0.1–2.0) *108 cells was adopted in subcutaneous injection. At least 12 weeks of follow-up is necessary for efficacy assessment of intravenous injection and 4 weeks for subcutaneous injection. MSC exosome has been made as a kind of ointment for topical treatment and the follow-up of 20 days. PASI (Psoriasis Area and Severity Index) score is the most common criterion as a primary outcome in different trials.
Table 1. Clinical trials of MSC therapy in psoriasis.
MSC therapy in systemic sclerosis
Systemic sclerosis (SSc) is a rare multisystem disease that causes progressive fibrosis of the skin and visceral organs, characterized by localized or diffuse skin thickening and fibrosis, microvascular dysfunction, and immune system abnormally activation.Citation51 The chronic course of disease and complications of multiple organ dysfunction significantly reduce the life span and life quality of patients.Citation52 Briefly, the disease starts with the activation of autoimmune response, leading to damage of endothelial and subsequent production of autoantibody and cytokines. Macrophages, immune cells including T and B lymphocytes, and dendritic cells are the main functionally abnormal cells in skin lesions.Citation53,Citation54 Excessive fibrosis produced by myofibroblasts further aggravates organ and blood vessel damage. Based on the pathogenesis, therapies like immunosuppression, antifibrotic agents, and immunomodulators have been applied to relieve pain or improve organ functionCitation55 and the therapeutic effect of intravenous immunoglobulins has also been proved.Citation56 However, it’s still a challenge to comprehensive assessment for individual-based treatment precisely because of the multiple organ complications in SSc. A safer therapy without many side effects will bring hope to SSc patients.
Promotion of angiogenesis,Citation57 secretion of substances in vascular repairCitation58 and anti-fibrosis effectsCitation59–61 make stem cells great therapeutic potentials in SSc. The advantages of adipose stem cells (ADSCs) in low immunogenicity and easy acquirementCitation62 achieve its role in SSc treatment.Citation63,Citation64 In the bleomycin-induced fibrosis mice model, intravenous application of ADSCs prevents lung and skin fibrosis, and accelerates wound healing.Citation65 In clinical applications, autologous fat grafting could correct and improve facial beauty by recreating fullnessCitation66 and injection of autologous adipose-derived stromal vascular fraction in fingers significantly improves hand disability.Citation67 Though these findings were enlightening, the capacity of adipocytic progenitors of differentiation into myofibroblasts might aggravate fibrosisCitation68 and exposure to MSCs in SSc may increase their myofibroblast differentiating potential.Citation69
A female SSc patient who developed acute gangrene of the upper and lower limbs recovered revascularization after three infusions of autologous mesenchymal stem cells.Citation70 And study has found that allogeneic MSCs retain their therapeutic effects under the oxidative environment of SSc.Citation71
Most of the current clinical studies deliver autologous or allogeneic MSCs by intravenous infusion. And various MSC types from adult umbilical cord, Wharton’s jelly, adipose tissue and bone marrow are adopted. At least 12 weeks for skin score evaluation and adverse events monitoring is needed. The most reported common dose of MSCs was 1.0 × 106 cells ().
Table 2. Clinical trials of MSC therapy in systemic sclerosis.
MSC therapy in dermatomyositis
Dermatomyositis is a rare, multi-system, autoimmune, and inflammatory myopathy characterized by typical heliotrope rash, Gottron’s sign and papules, splinter hemorrhage of the nail fold, and symmetric, proximal muscle weakness.Citation72 Patients can present single or several cutaneous manifestations and there is no clinical parallelism between cutaneous and muscle manifestations. Perivascular infiltration of CD4+ lymphocytes can be found in skin biopsies.Citation73 Though there is no exact reason, potential pathogenic factors like MHC polymorphisms,Citation74 environmental factors, and abnormal activation of the immune system are all threatening for susceptible individuals. Myositis-specific antibodies like anti-Mi2, anti-MDA5, and anti-TIF1 are associated with the diagnosis and prognosis of DM.Citation75 Existent therapies are symptom management. For example, intravenous immunoglobulin, and corticosteroids alone or combined with immunosuppressive agents like azathioprine and methotrexate, or intravenous immunoglobulins are the main therapiesCitation76 to improve the condition. But clinical relapses and non-responders are still the headaches.
Early between May 2008 and July 2009, 10 patients with drug-resistant polymyositis or dermatomyositis received allogeneic mesenchymal stem cell transplantation with the dose of 1.0 × 106 cells/kg of body weight by intravenous infusion. Ideal clinical responses were demonstrated but whether a single MSC application could be the replacement was not sure because none of the patients stopped immunosuppressive therapy in the follow-up.Citation77 Recently, a long-time follow-up study of three patients with the diagnosis of refractory juvenile dermatomyositis showed complete remission after autologous hematopoietic stem cell transplantation.Citation78 A case reported a patient whose cutaneous symptoms and morphology of bone marrow aspirate were significantly improved after allogeneic hematopoietic stem cell transplantation.Citation79
There are currently two clinical trials based on human umbilical cord-derived MSC therapy for dermatomyositis by intravenous infusion (). The Dose Limiting Toxicity is the main primary outcome in the follow-up. But the ideal dosage and frequency need more clinical clues.
Table 3. Clinical trials of MSC therapy in dermatomyositis.
MSC therapy in atopic dermatitis
Atopic dermatitis (AD) is an intractable, chronic and relapsing skin disease with unpleasant pruritus. Approximately 20% of children and 10% of adults are affected by AD in high-income countries. Complex causes like epidermal barrier dysfunction and allergen exposure result in innate and adaptive immune disorders and abnormal inflammatory response.Citation80 Because AD is easy to relapse and difficult to cure, the major aims of disease management are symptom improvement and long-term control. Topical treatments are effective in mild atopic dermatitis patients while phototherapy, topical steroids and systemic immunosuppressant drugs are most commonly used in moderated to severe patients. Concerns exist like skin thickness decrease by overusing corticosteroid cream or ointment, side effects on the immune system by calcineurin inhibitors, and the increased risk of skin cancer by long-term light therapy. Biological agents like IL4 Rα antagonists Dupilumab,Citation81,Citation82 IL13 inhibitor tralokinumab,Citation83 JAK1 inhibitor upadacitinibCitation84 and abrocitinib,Citation85 JAK1 and JAK2 inhibitor baricitinib,Citation86 have shown good therapy potential in moderate-to-severe atopic dermatitis, but full course is still a heavy financial burden to patients and their families.
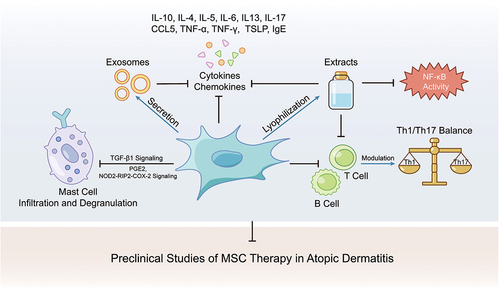
The therapeutic effects of MSC have been demonstrated by several studies (). For example, MSC infusion could suppress mast cell degranulation by increasing PGE2 and TGF-β1,Citation87 rebalance Th1, Th17, and Th2,Citation88 and decrease cytokines or chemokines like IL-1β, IL4, IL5, IL6, IL13, IL17, CCL5, TNF-α, IFN-γ, TSLP and IgE.Citation89–91 Even MSC-derived exosomes, conditioned medium and extracts were found to relieve atopic dermatitis.Citation36,Citation92,Citation93 Researchers also found either preconditioned with mast cell granules, poly I: C, or IFN-γ could improve MSC therapeutic effects.Citation94,Citation95 Both intravenous and subcutaneous MSC infusion were effective in mouse models.Citation8,Citation91,Citation94
Figure 2. The anti-inflammatory effect and immune modulation of MSC or its cell-free products in atopic dermatitis model.
In current clinical trials, injection of human umbilical cord-derived stem cells intravenously is the most common therapy with the dosage varies from 2.5 × 107 to 3.0 × 108 cells. 31 patients with moderate-to-severe AD have completed a clinical trial in which they received MSCs subcutaneously with a low dose of 2.5 × 107 cells and a high dose of 5.0 × 107 cells, respectively. The therapeutic effects of AD manifestation presented a dose-dependent manner.Citation96 Other 5 patients without ideal respondence to conventional treatments were administrated with 1.0 × 106 cells/kg MSCs intravenously and showed good clinical improvement in the follow-up.Citation97 Various studies with a larger number of patients, more reasonable grouping and MSC dose selection criteria are working together for clinical efficacy demonstration ().
Table 4. Clinical trials of MSC therapy in atopic dermatitis.
Discussion
Long-term and sustained treatments for chronic and refractory skin diseases can cause unwilling side effects and maybe a heavy economic stress. MSCs are promising in tissue repairing, immune and inflammatory modulation. Though there are effective biological agents available for skin diseases like psoriasis and atopic dermatitis, many MSC therapy related clinical trials are ongoing. with the expect for more effective and safe alternatives with persistent curative effects. For diseases which is not common but severe like systemic sclerosis and dermatomyositis and systemic diseases, MSC therapy will be benefit more from these properties.
Though the safety and efficacy of MSCs in clinical application have been confirmed by emerging studies, administration route, standard dose, injection speed and frequency still need more evidence in applying under certain circumstances. It’s also worth considering under individual-based treatment that multidisciplinary judgment is a must when in combination with other diseases. A deep understanding of profound mechanisms of MSC therapy may in turn help find novel therapeutic targets for a certain disease.
Authors’ contributions
CH collected references, summarized clinical trials and wrote the manuscript. SC drew the graphical summaries. HC reviewed and checked the article. The authors have approved the final manuscript.
Acknowledgments
This study was supported by grants from the Key science and technology R&D project of Zhejiang Province (2021C03077).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chambers ES, Vukmanovic-Stejic M. Skin barrier immunity and ageing. Immunology. 2020;160(2):116–14. doi:10.1111/imm.13152.
- Matejuk A. Skin immunity. Arch Immunol Ther Exp (Warsz). 2018;66(1):45–54. doi:10.1007/s00005-017-0477-3.
- Brandner JM, Zorn-Kruppa M, Yoshida T, Moll I, Beck LA, De Benedetto A. Epidermal tight junctions in health and disease. Tissue Barriers. 2015;3(1–2):e974451. doi:10.4161/21688370.2014.974451.
- Tan Y, Tey HL, Chong SZ, Ng LG. Skinny deeping: uncovering immune cell behavior and function through imaging techniques. Immunol Rev. 2022;306(1):271–92. doi:10.1111/imr.13049.
- Darlenski R, Kazandjieva J, Hristakieva E, Fluhr JW. Atopic dermatitis as a systemic disease. Clin Dermatol. 2014;32(3):409–13. doi:10.1016/j.clindermatol.2013.11.007.
- Sidbury R, Kodama S. Atopic dermatitis guidelines: diagnosis, systemic therapy, and adjunctive care. Clin Dermatol. 2018;36(5):648–52. doi:10.1016/j.clindermatol.2018.05.008.
- Traineau H, Aggarwal R, Monfort JB, Senet P, Oddis CV, Chizzolini C, Barbaud A, Francès C, Arnaud L, Chasset F. Treatment of calcinosis cutis in systemic sclerosis and dermatomyositis: a review of the literature. J Am Acad Dermatol. 2020;82(2):317–25. doi:10.1016/j.jaad.2019.07.006.
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–50. doi:10.1002/jor.1100090504.
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Dj P, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8(4):315–17. doi:10.1080/14653240600855905.
- Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond). 2005;2(1):8. doi:10.1186/1476-9255-2-8.
- Koppula PR, Chelluri LK, Polisetti N, Vemuganti GK. Histocompatibility testing of cultivated human bone marrow stromal cells - a promising step towards pre-clinical screening for allogeneic stem cell therapy. Cell Immunol. 2009;259(1):61–65. doi:10.1016/j.cellimm.2009.05.014.
- Li C, Zhao H, Cheng L, Wang B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021;11(1):187. doi:10.1186/s13578-021-00698-y.
- Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2(2):155–62. doi:10.1007/s12015-006-0022-y.
- Matsiko A, Levingstone TJ, O’Brien FJ. Advanced strategies for articular cartilage defect repair. Materials (Basel). 2013;6(2):637–68. doi:10.3390/ma6020637.
- Zhang J, Liu Y, Chen Y, Yuan L, Liu H, Wang J, Liu Q, Zhang Y. Adipose-derived stem cells: current applications and future directions in the regeneration of multiple tissues. Stem Cells Int. 2020;2020:8810813. doi:10.1155/2020/8810813.
- Musiał-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. 2019;28(7):801–12. doi:10.1177/0963689719837897.
- Li Y, Lin F. Mesenchymal stem cells are injured by complement after their contact with serum. Blood. 2012;120(17):3436–43. doi:10.1182/blood-2012-03-420612.
- Li Y, Fung J, Lin F. Local inhibition of complement improves mesenchymal stem cell viability and function after administration. Mol Ther. 2016;24(9):1665–74. doi:10.1038/mt.2016.142.
- Tu Z, Li Q, Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010;19(11):1803–09. doi:10.1089/scd.2009.0418.
- Li Y, Qiu W, Zhang L, Fung J, Lin F. Painting factor H onto mesenchymal stem cells protects the cells from complement- and neutrophil-mediated damage. Biomaterials. 2016;102:209–19. doi:10.1016/j.biomaterials.2016.05.055.
- Taghavi-Farahabadi M, Mahmoudi M, Hashemi SM, Rezaei N. Evaluation of the effects of mesenchymal stem cells on neutrophils isolated from severe congenital neutropenia patients. Int Immunopharmacol. 2020;83:106463. doi:10.1016/j.intimp.2020.106463.
- Wang G, Joel M, Yuan J, Wang J, Cai X, Ocansey D, Yan Y, Qian H, Zhang X, Xu W, et al. Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease by inhibiting ERK phosphorylation in neutrophils. Inflammopharmacology. 2020;28(2):603–16. doi:10.1007/s10787-019-00683-5.
- Jiang D, Muschhammer J, Qi Y, Kügler A, de Vries JC, Saffarzadeh M, Sindrilaru A, Beken SV, Wlaschek M, Kluth MA, et al. Suppression of neutrophil-mediated tissue damage—a novel skill of mesenchymal stem cells. Stem Cells. 2016;34(9):2393–406. doi:10.1002/stem.2417.
- Requena J, Alvarez-Palomo AB, Codina-Pascual M, Delgado-Morales R, Moran S, Esteller M, Sal M, Juan M, Boronat Barado A, Consiglio A, et al. Global proteomic and methylome analysis in human induced pluripotent stem cells reveals overexpression of a human TLR3 affecting proper innate immune response signaling. Stem Cells. 2019;37(4):476–88. doi:10.1002/stem.2966.
- Hackel A, Aksamit A, Bruderek K, Lang S, Brandau S. TNF-α and IL-1β sensitize human MSC for IFN-γ signaling and enhance neutrophil recruitment. Eur J Immunol. 2021;51(2):319–30. doi:10.1002/eji.201948336.
- Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE(2)-dependent mechanism. Sci Rep. 2016;6(1):38308. doi:10.1038/srep38308.
- Jin L, Deng Z, Zhang J, Yang C, Liu J, Han W, Ye P, Si Y, Chen G. Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J Transl Med. 2019;17(1):251. doi:10.1186/s12967-019-1999-8.
- Stone Ii R, Natesan S, Kowalczewski CJ, Mangum LH, Clay NE, Clohessy RM, Carlsson AH, Tassin DH, Chan RK, Rizzo JA, et al. Advancements in regenerative strategies through the continuum of burn care. Front Pharmacol. 2018;9:672. doi:10.3389/fphar.2018.00672.
- Sierra-Sánchez Á, Montero-Vilchez T, Quiñones-Vico MI, Sanchez-Diaz M, Arias-Santiago S. Current advanced therapies based on human mesenchymal stem cells for skin diseases. Front Cell Dev Biol. 2021;9:643125. doi:10.3389/fcell.2021.643125.
- Wu P, Zhang B, Shi H, Qian H, Xu W. MSC-exosome: a novel cell-free therapy for cutaneous regeneration. Cytotherapy. 2018;20(3):291–301. doi:10.1016/j.jcyt.2017.11.002.
- Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35(4):851–58. doi:10.1002/stem.2575.
- Shen Z, Huang W, Liu J, Tian J, Wang S, Rui K. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front Immunol. 2021;12:749192. doi:10.3389/fimmu.2021.749192.
- Cho KA, Cha JE, Kim J, Kim YH, Ryu KH, Woo SY. Mesenchymal stem cell-derived exosomes attenuate TLR7-mediated mast cell activation. Tissue Eng Regen Med. 2022;19(1):117–29. doi:10.1007/s13770-021-00395-4.
- Liu J, Kuwabara A, Kamio Y, Hu S, Park J, Hashimoto T, Lee JW. Human mesenchymal stem cell-derived microvesicles prevent the rupture of intracranial aneurysm in part by suppression of mast cell activation via a PGE2-dependent mechanism. Stem Cells. 2016;34(12):2943–55. doi:10.1002/stem.2448.
- Bogatcheva NV, Coleman ME. Conditioned medium of mesenchymal stromal cells: a new class of therapeutics. Biochemistry (Mosc). 2019;84(11):1375–89. doi:10.1134/S0006297919110129.
- Montero-Vilchez T, Sierra-Sánchez Á, Sanchez-Diaz M, Quiñones-Vico MI, Sanabria-de-la-Torre R, Martinez-Lopez A, Arias-Santiago S. Mesenchymal stromal cell-conditioned medium for skin diseases: a systematic review. Front Cell Dev Biol. 2021;9:654210. doi:10.3389/fcell.2021.654210.
- Griffiths C, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021;397(10281):1301–15. doi:10.1016/S0140-6736(20)32549-6.
- Elmets CA, Leonardi CL, Davis D, Gelfand JM, Lichten J, Mehta NN, Armstrong AW, Connor C, Cordoro KM, Elewski BE, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–113. doi:10.1016/j.jaad.2018.11.058.
- Jørgensen KK, Olsen IC, Goll GL, Lorentzen M, Bolstad N, Haavardsholm EA, Lundin K, Mørk C, Jahnsen J, Kvien TK. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389(10086):2304–16. doi:10.1016/S0140-6736(17)30068-5.
- van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z, Werth VP, Gordon RM, Zhou B, Hsu B, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. 2018;392(10155):1330–39. doi:10.1016/S0140-6736(18)32167-6.
- McInnes IB, Behrens F, Mease PJ, Kavanaugh A, Ritchlin C, Nash P, Masmitja JG, Goupille P, Korotaeva T, Gottlieb AB, et al. Secukinumab versus adalimumab for treatment of active psoriatic arthritis (EXCEED): a double-blind, parallel-group, randomised, active-controlled, phase 3b trial. Lancet. 2020;395(10235):1496–505. doi:10.1016/S0140-6736(20)30564-X.
- Reich K, Armstrong AW, Langley RG, Flavin S, Randazzo B, Li S, Hsu MC, Branigan P, Blauvelt A. Guselkumab versus secukinumab for the treatment of moderate-to-severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet. 2019;394(10201):831–39. doi:10.1016/S0140-6736(19)31773-8.
- Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–60. doi:10.1001/jama.2020.4006.
- Ovejero-Benito MC, Muñoz-Aceituno E, Reolid A, Saiz-Rodríguez M, Abad-Santos F, Daudén E. Pharmacogenetics and pharmacogenomics in moderate-to-severe psoriasis. Am J Clin Dermatol. 2018;19(2):209–22. doi:10.1007/s40257-017-0322-9.
- Sah SK, Park KH, Yun CO, Kang KS, Kim TY. Effects of human mesenchymal stem cells transduced with superoxide dismutase on imiquimod-induced psoriasis-like skin inflammation in mice. Antioxid Redox Signal. 2016;24(5):233–48. doi:10.1089/ars.2015.6368.
- Lee YS, Sah SK, Lee JH, Seo KW, Kang KS, Kim TY. Human umbilical cord blood-derived mesenchymal stem cells ameliorate psoriasis-like skin inflammation in mice. Biochem Biophys Rep. 2017;9:281–88. doi:10.1016/j.bbrep.2016.10.002.
- Chen M, Peng J, Xie Q, Xiao N, Su X, Mei H, Lu Y, Zhou J, Dai Y, Wang S, et al. Mesenchymal stem cells alleviate moderate-to-severe psoriasis by reducing the production of type I interferon (IFN-I) by plasmacytoid dendritic cells (pDCs). Stem Cells Int. 2019;2019:6961052. doi:10.1155/2019/6961052.
- Zhang B, Lai RC, Sim WK, Choo A, Lane EB, Lim SK. Topical application of mesenchymal stem cell exosomes alleviates the imiquimod induced psoriasis-like inflammation. Int J Mol Sci. 2021;22(2):720. doi:10.3390/ijms22020720.
- Ahn H, Lee SY, Jung WJ, Pi J, Lee KH. Psoriasis treatment using minimally manipulated umbilical cord-derived mesenchymal stem cells: a case report. World J Clin Cases. 2021;9(23):6798–803. doi:10.12998/wjcc.v9.i23.6798.
- Chen H, Niu JW, Ning HM, Pan X, Li XB, Li Y, Wang DH, Hu LD, Sheng HX, Xu M, et al. Treatment of psoriasis with mesenchymal stem cells. Am J Med. 2016;129(3):e13–14. doi:10.1016/j.amjmed.2015.11.001.
- Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390(10103):1685–99. doi:10.1016/S0140-6736(17)30933-9.
- Sierra-Sepúlveda A, Esquinca-González A, Benavides-Suárez SA, Sordo-Lima DE, Caballero-Islas AE, Cabral-Castañeda AR, Rodríguez-Reyna TS. Systemic sclerosis pathogenesis and emerging therapies, beyond the fibroblast. Biomed Res Int. 2019;2019:4569826. doi:10.1155/2019/4569826.
- Farina A, Cirone M, York M, Lenna S, Padilla C, Mclaughlin S, Faggioni A, Lafyatis R, Trojanowska M, Farina GA. Epstein-Barr virus infection induces aberrant TLR activation pathway and fibroblast-myofibroblast conversion in scleroderma. J Invest Dermatol. 2014;134(4):954–64. doi:10.1038/jid.2013.423.
- Yoshizaki A. Pathogenic roles of B lymphocytes in systemic sclerosis. Immunol Lett. 2018;195:76–82. doi:10.1016/j.imlet.2018.01.002.
- Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, Distler O, Clements P, Cutolo M, Czirjak L, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. 2017;76(8):1327–39. doi:10.1136/annrheumdis-2016-209909.
- Agostini E, De Luca G, Bruni C, Bartoli F, Tofani L, Campochiaro C, Pacini G, Moggi-Pignone A, Guiducci S, Bellando-Randone S, et al. Intravenous immunoglobulins reduce skin thickness in systemic sclerosis: evidence from systematic literature review and from real life experience. Autoimmun Rev. 2021;20(12):102981. doi:10.1016/j.autrev.2021.102981.
- Khaki M, Salmanian AH, Abtahi H, Ganji A, Mosayebi G. Mesenchymal stem cells differentiate to endothelial cells using recombinant vascular endothelial growth factor -A. Rep Biochem Mol Biol. 2018;6:144–50.
- Zhang S, Chen L, Zhang G, Zhang B. Umbilical cord-matrix stem cells induce the functional restoration of vascular endothelial cells and enhance skin wound healing in diabetic mice via the polarized macrophages. Stem Cell Res Ther. 2020;11(1):39. doi:10.1186/s13287-020-1561-x.
- Wang Z, Li S, Wang Y, Zhang X, Chen L, Sun D. GDNF enhances the anti-inflammatory effect of human adipose-derived mesenchymal stem cell-based therapy in renal interstitial fibrosis. Stem Cell Res. 2019;41:101605. doi:10.1016/j.scr.2019.101605.
- Chen X, Wu Y, Wang Y, Chen L, Zheng W, Zhou S, Xu H, Li Y, Yuan L, Xiang C. Human menstrual blood-derived stem cells mitigate bleomycin-induced pulmonary fibrosis through anti-apoptosis and anti-inflammatory effects. Stem Cell Res Ther. 2020;11(1):477. doi:10.1186/s13287-020-01926-x.
- Wang Z, Sun D. Adipose-derived mesenchymal stem cells: a new tool for the treatment of renal fibrosis. Stem Cells Dev. 2018;27(20):1406–11. doi:10.1089/scd.2017.0304.
- Minteer DM, Marra KG, Rubin JP. Adipose stem cells: biology, safety, regulation, and regenerative potential. Clin Plast Surg. 2015;42(2):169–79. doi:10.1016/j.cps.2014.12.007.
- Del Papa N, Di Luca G, Andracco R, Zaccara E, Maglione W, Pignataro F, Minniti A, Vitali C. Regional grafting of autologous adipose tissue is effective in inducing prompt healing of indolent digital ulcers in patients with systemic sclerosis: results of a monocentric randomized controlled study. Arthritis Res Ther. 2019;21(1):7. doi:10.1186/s13075-018-1792-8.
- Maria AT, Toupet K, Maumus M, Fonteneau G, Le Quellec A, Jorgensen C, Guilpain P, Noël D. Human adipose mesenchymal stem cells as potent anti-fibrosis therapy for systemic sclerosis. J Autoimmun. 2016;70:31–39. doi:10.1016/j.jaut.2016.03.013.
- Rubio GA, Elliot SJ, Wikramanayake TC, Xia X, Pereira-Simon S, Thaller SR, Glinos GD, Jozic I, Hirt P, Pastar I, et al. Mesenchymal stromal cells prevent bleomycin-induced lung and skin fibrosis in aged mice and restore wound healing. J Cell Physiol. 2018;233(8):5503–12. doi:10.1002/jcp.26418.
- Gheisari M, Ahmadzadeh A, Nobari N, Iranmanesh B, Mozafari N. Autologous fat grafting in the treatment of facial scleroderma. Dermatol Res Pract. 2018;2018:6568016. doi:10.1155/2018/6568016.
- Granel B, Daumas A, Jouve E, Harlé JR, Nguyen PS, Chabannon C, Colavolpe N, Reynier JC, Truillet R, Mallet S, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis. 2015;74(12):2175–82. doi:10.1136/annrheumdis-2014-205681.
- Marangoni RG, Korman BD, Wei J, Wood TA, Graham LV, Whitfield ML, Scherer PE, Tourtellotte WG, Varga J. Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors. Arthritis Rheumatol. 2015;67(4):1062–73. doi:10.1002/art.38990.
- Taki Z, Gostjeva E, Thilly W, Yaseen B, Lopez H, Mirza M, Hassuji Z, Vigneswaran S, Ahmed Abdi B, Hart A, et al. Pathogenic activation of mesenchymal stem cells is induced by the disease microenvironment in systemic sclerosis. Arthritis Rheumatol. 2020;72(8):1361–74. doi:10.1002/art.41267.
- Guiducci S, Porta F, Saccardi R, Guidi S, Ibba-Manneschi L, Manetti M, Mazzanti B, Dal Pozzo S, Milia AF, Bellando-Randone S, et al. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: a case report. Ann Intern Med. 2010;153(10):650–54. doi:10.7326/0003-4819-153-10-201011160-00007.
- Fonteneau G, Bony C, Goulabchand R, Maria A, Le Quellec A, Rivière S, Jorgensen C, Guilpain P, Noël D. Serum-mediated oxidative stress from systemic sclerosis patients affects mesenchymal stem cell function. Front Immunol. 2017;8:988. doi:10.3389/fimmu.2017.00988.
- DeWane ME, Waldman R, Lu J. Dermatomyositis: clinical features and pathogenesis. J Am Acad Dermatol. 2020;82(2):267–81. doi:10.1016/j.jaad.2019.06.1309.
- Aussy A, Boyer O, Cordel N. Dermatomyositis and immune-mediated necrotizing myopathies: a window on autoimmunity and cancer. Front Immunol. 2017;8:992. doi:10.3389/fimmu.2017.00992.
- Miller FW, Cooper RG, Vencovský J, Rider LG, Danko K, Wedderburn LR, Lundberg IE, Pachman LM, Reed AM, Ytterberg SR, et al. Genome-wide association study of dermatomyositis reveals genetic overlap with other autoimmune disorders. Arthritis Rheum. 2013;65(12):3239–47. doi:10.1002/art.38137.
- Miyashiro D, Arnone M, Ferreira PS, Romiti R, Sanches JA. Extensive cutaneous involvement by dermatomyositis: report of six cases and review of the literature. Autoimmun Rev. 2020;19(12):102680. doi:10.1016/j.autrev.2020.102680.
- Callen JP. Dermatomyositis. Lancet. 2000;355(9197):53–57. doi:10.1016/S0140-6736(99)05157-0.
- Wang D, Zhang H, Cao M, Tang Y, Liang J, Feng X, Wang H, Hua B, Liu B, Sun L. Efficacy of allogeneic mesenchymal stem cell transplantation in patients with drug-resistant polymyositis and dermatomyositis. Ann Rheum Dis. 2011;70(7):1285–88. doi:10.1136/ard.2010.141804.
- Zhu J, Su G, Lai J, Dong B, Kang M, Li S, Zhou Z, Wu F. Long-term follow-up of autologous hematopoietic stem cell transplantation for refractory juvenile dermatomyositis: a case-series study. Pediatr Rheumatol Online J. 2018;16(1):72. doi:10.1186/s12969-018-0284-3.
- Bawany F, Tbakhi B, Mendler JH, Richardson CT, Bennett JM, Aljitawi OS. Remission of anti-TIF1γ dermatomyositis after allogeneic hematopoietic stem cell transplant for myelodysplastic syndrome. Blood Adv. 2020;4(22):5698–701. doi:10.1182/bloodadvances.2020003104.
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–60. doi:10.1016/S0140-6736(20)31286-1.
- Beck LA, Thaçi D, Hamilton JD, Graham NM, Bieber T, Rocklin R, Ming JE, Ren H, Kao R, Simpson E, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–39. doi:10.1056/NEJMoa1314768.
- Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, Simpson EL, Papp KA, Hong HC, Rubel D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389(10086):2287–303. doi:10.1016/S0140-6736(17)31191-1.
- Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour JP, Spelman L, Katoh N, Saeki H, Poulin Y, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. 2021;184(3):437–49. doi:10.1111/bjd.19574.
- Guttman-Yassky E, Thaçi D, Pangan AL, Hong HC, Papp KA, Reich K, Beck LA, Mohamed MF, Othman AA, Anderson JK, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–84. doi:10.1016/j.jaci.2019.11.025.
- Weidinger S, Schreiber S. Abrocitinib for atopic dermatitis: a step forward. Lancet. 2020;396(10246):215–17. doi:10.1016/S0140-6736(20)31284-8.
- Simpson EL, Lacour JP, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, King BA, Thyssen JP, Silverberg JI, Bieber T, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–55. doi:10.1111/bjd.18898.
- Kim HS, Yun JW, Shin TH, Lee SH, Lee BC, Yu KR, Seo Y, Lee S, Kang TW, Choi SW, et al. Human umbilical cord blood mesenchymal stem cell-derived PGE2 and TGF-β1 alleviate atopic dermatitis by reducing mast cell degranulation. Stem Cells. 2015;33(4):1254–66. doi:10.1002/stem.1913.
- Orciani M, Campanati A, Caffarini M, Ganzetti G, Consales V, Lucarini G, Offidani A, Di Primio R. T helper (Th)1, Th17 and Th2 imbalance in mesenchymal stem cells of adult patients with atopic dermatitis: at the origin of the problem. Br J Dermatol. 2017;176(6):1569–76. doi:10.1111/bjd.15078.
- Kim M, Lee SH, Kim Y, Kwon Y, Park Y, Lee HK, Jung HS, Jeoung D. Human adipose tissue-derived mesenchymal stem cells attenuate atopic dermatitis by regulating the expression of MIP-2, miR-122a-SOCS1 Axis, and Th1/Th2 Responses. Front Pharmacol. 2018;9:1175. doi:10.3389/fphar.2018.01175.
- Ryu B, Baek J, Kim H, Lee JH, Kim J, Jeong YH, Lee SG, Kang KR, Oh MS, Kim EY, et al. Anti-inflammatory effects of M-MSCs in DNCB-induced atopic dermatitis mice. Biomedicines. 2020;8(10):439. doi:10.3390/biomedicines8100439.
- Na K, Yoo HS, Zhang YX, Choi MS, Lee K, Yi TG, Song SU, Jeon MS. Bone marrow-derived clonal mesenchymal stem cells inhibit ovalbumin-induced atopic dermatitis. Cell Death Dis. 2014;5(7):e1345. doi:10.1038/cddis.2014.299.
- Shin KO, Ha DH, Kim JO, Crumrine DA, Meyer JM, Wakefield JS, Lee Y, Kim B, Kim S, Kim HK, et al. Exosomes from human adipose tissue-derived mesenchymal stem cells promote epidermal barrier repair by inducing de novo synthesis of ceramides in atopic dermatitis. Cells. 2020;9(3):680. doi:10.3390/cells9030680.
- Song JY, Kang HJ, Ju HM, Park A, Park H, Hong JS, Kim CJ, Shim JY, Yu J, Choi J. Umbilical cord-derived mesenchymal stem cell extracts ameliorate atopic dermatitis in mice by reducing the T cell responses. Sci Rep. 2019;9(1):6623. doi:10.1038/s41598-019-42964-7.
- Lee BC, Kim JJ, Lee JY, Kang I, Shin N, Lee SE, Choi SW, Cho JY, Kim HS, Kang KS. Disease-specific primed human adult stem cells effectively ameliorate experimental atopic dermatitis in mice. Theranostics. 2019;9(12):3608–21. doi:10.7150/thno.32945.
- Pierce LM, Kurata WE. Priming with toll-like receptor 3 agonist poly(I:C) enhances content of innate immune defense proteins but not MicroRNAs in human mesenchymal stem cell-derived extracellular vesicles. Front Cell Dev Biol. 2021;9:676356. doi:10.3389/fcell.2021.676356.
- Kim HS, Lee JH, Roh KH, Jun HJ, Kang KS, Kim TY. Clinical trial of human umbilical cord blood-derived stem cells for the treatment of moderate-to-severe atopic dermatitis: phase I/IIa studies. Stem Cells. 2017;35(1):248–55. doi:10.1002/stem.2401.
- Shin HT, Lee SH, Yoon HS, Heo JH, Lee SB, Byun JW, Shin J, Cho YK, Chung E, Jeon MS, et al. Long-term efficacy and safety of intravenous injection of clonal mesenchymal stem cells derived from bone marrow in five adults with moderate to severe atopic dermatitis. J Dermatol. 2021;48(8):1236–42. doi:10.1111/1346-8138.15928.
