 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study aimed to explore the relationship between post-vaccination adverse reactions, decision regret, and willingness to pay (WTP) for the booster dose. An online survey was conducted in Taizhou, China. Questionnaires were completed by 1,085 healthcare workers (HCWs) and 1,054 (97.1%) have received two doses of the COVID-19 vaccine. Mediation analysis method was adopted. Our study presented that post-vaccination adverse reactions in HCWs could decrease their WTP for the booster dose. Of note, HCWs experienced adverse reactions after vaccination would more likely regret their previous vaccination decisions, which, in turn, further reduced their WTP for a booster shot. Decision regret mediated the relationship between adverse post-vaccination reactions and WTP for the booster dose. The findings implied inextricable relationships among post-vaccination adverse reactions, decision regret, and WTP of the booster dose. It suggested that these post-vaccination adverse reactions should be further incorporated into vaccine campaigns to improve vaccine intention and potentially increase willingness to pay for booster doses of COVID-19 vaccine.
Introduction
In January 2020, the World Health Organization declared the new coronavirus strain, SARS-CoV-2, a public health crisis, and the disease was later named coronavirus disease 2019 (COVID-19) and announced a pandemic in March, 2020.Citation1,Citation2 The ongoing COVID-19 pandemic has created a serious public health burden worldwide, putting millions of people at risk.Citation3–5 Based on epidemiological data, droplets from face-to-face contact during conversation, coughing, or sneezing appears to be the most common mechanisms of spread.Citation6 Safe and effective preventive vaccines are potentially useful tools for reducing transmission rates and subsequent infections.Citation7–9 To date, many studies have adopted theory of planned behavior,Citation10–12 protection motivation theory,Citation13 the health belief model,Citation14 and even a cognitive model of empowerment to design standardized instruments to assess the driving factors influencing whether individuals get vaccinated against COVID-19.Citation15–19 In China, vaccines are distributed throughout the public sector; however, in the future, they may be available in the private market. Hence, it is of great importance to assess the acceptance and willingness to pay (WTP) for COVID-19 vaccines.
WTP, defined as the maximum amount of money that people are willing to allocate to a service or health technology, can inform future vaccine demand projections and pricing.Citation20 WTP is a contingent evaluation and includes a hypothetical survey that directly asks individuals the maximum amount they would be willing to pay for the good in question.Citation21,Citation22 Although the COVID-19 vaccine is currently free of cost, a hypothetical scenario is provided for individuals in which the outbreak persists and the vaccine is paid for under the contingent valuation approach.Citation23 Information on people’s willingness to pay for a hypothetical vaccine against the virus could help future price-setting discussions and contribute to decision-making to inform potential pricing for a hypothetical COVID-19 vaccine.
Recently, there have been reports of COVID-19 infection, hospitalization, or even death due to COVID-19 in some people who received their two doses of the vaccine.Citation24,Citation25 In addition, the virus was prone to mutation and vaccine effectiveness decreased over time, which was thought to have contributed to the reemergence of the pandemic.Citation26 Moreover, the effects of inactivated vaccines do not last as long as those of live vaccines that continuously stimulate the immune system.Citation27,Citation28 Hence, timely vaccination with the booster dose to further increase the neutralizing antibody titer in the body can supplement and improve the declining protective efficacy of the vaccine whilst also protecting against further variants that may emerge at any time. Healthcare workers (HCWs) are vulnerable to this highly infectious virus since they are in direct contact with patients with COVID-19.Citation29,Citation30 Recently, many surveys have been conducted on HCWs’ vaccination intentions and concerns regarding vaccination.Citation31–33 Vaccination of HCWs can interrupt the spread of the virus and have beneficial ripple effects in the broader community, which is necessary to implement herd immunization in all the groups that may contribute to COVID-19 transmission. To date, most research on booster vaccination with COVID-19 vaccine has concentrated on the investigation of individuals’ WTP for booster vaccination, with limited attention on the vaccine’s underlying mechanism. Understanding the mechanism of the WTP for the booster dose among HCWs is an essential issue for the promotion of receiving booster vaccination.
Previous studies showed that the most commonly reported side reactions after vaccination included pain at the site of injection, pain in the muscles and bones, general poor feelings, and fever.Citation34,Citation35 Regardless of the vaccine, these adverse reactions affect approximately 1/4 of the population.Citation35 It is of great interest to investigate whether these post-vaccination adverse reactions affect people’s willingness to pay for booster vaccinations. Individuals are usually faced with difficult decisions about their health and may later regret the choices they have made.Citation36–38 Research has found that adverse physical health outcomes are one of the risk factors most frequently reported to be related to decision regret.Citation38 Therefore, in this research, we aimed to study the relationship between post-vaccination adverse reactions, decision regret, and WTP for the booster dose of the COVID-19 vaccine.
Materials and methods
Study design and population
We organized a cross-sectional online investigation on the Wen-Juan-Xing platform (Changsha Ranxing Information Technology Co., Ltd., Hunan, China), which is the largest online survey platform in China. The target population was HCWs in Taizhou, Zhejiang, China. The recruited samples included health professionals, such as doctor and nurse, and administrative support workers, such as janitor and nursing aide. Participants received questionnaires via WeChat or e-mail, and they answered the questionnaire by accessing a Uniform Resource Location (URL) or scanning a Quick Response (QR) code on their mobile phone or computer between August 31 and 8 September 2021. We collected a total of 1,103 questionnaires. A logical check was made on the data. Outliers were eliminated before data analysis. Respondents under 18 years old were excluded and duplicate samples were examined. The time taken to complete the questionnaire was also checked. Those who answered within 120 seconds were excluded. We obtained 1,085 valid questionnaires.
This survey study was approved by the Ethics Committee of Taizhou Hospital, Zhejiang Province, China (Approval number: K20210823). All procedures were conducted in accordance with the guidelines of our institutional Ethics Committee and in compliance with the principles of the Declaration of Helsinki. We did not ask for separate written informed consent since the participation of interviewees in the survey was considered informed consent. Information about all respondents was kept anonymously.
Questionnaires
The main contents of the questionnaire were based on the previous research and included the following four parts.Citation39 First, basic demographic information, including age, sex, education, occupation, professional title, and underlying diseases. Second, vaccination history, such as the COVID-19 vaccination status and post-vaccination adverse reactions. Third, decision regret includes 5 items.Citation40 Each item was answered on a five-point bipolar intensity scale. We evaluated the item statements by circling a number from 1 (strongly agree) to 5 (strongly disagree). Items 2) and 4) were phrased in the negative direction to avoid acquiescence bias. After reversing the scores of these two items, the overall sum score was produced by taking the sum of the five items. Fourth, the willing-to-pay for the booster dose was measured by a question asking whether respondents were willing to receive the booster injection if they have to pay for it. The amount of payment was measured through the question asked what the maximum price they were willing to pay for the booster dose of COVID-19 vaccine.Citation41 The details of the questionnaire were presented in Supplementary Material.
Mediation analysis
Mediation models have been widely utilized to explore the potential mechanism of an independent variable on a response variable, and whether there was a variable that mediated the above relationship.Citation42,Citation43 This could have important policy consequences since mediation analysis played an important role in understanding the potential mechanism whereby the change in one variable caused the change in another. In this study, the exposure () we considered here was post-vaccination adverse reactions (yes or no); the potential mediator (
) was decision regret; and the outcome (
) was WTP for the booster dose of COVID-19 vaccine (yes or no).
We concentrated on the case of a binary outcome () and a continuous mediator (
), and adopted the following three regression models for mediation analysis:
where EquationEquation (1)(1)
(1) described the relation of an independent variable and a response variable (
); EquationEquation (2)
(2)
(2) characterized the relation of an independent variable and a mediator (
); EquationEquation (3)
(3)
(3) summarized the relationship between the independent variable, the mediator, and the response variable (
);
was other baseline covariates;
was the total effect of
on
;
was the effect of
on
;
was the direct effect of
on
;
was the effect of
on
;
,
, and
were the intercept terms;
,
, and
were the residual terms. Here, we considered the Sobel method to test the mediation effect.Citation43
Statistical analysis
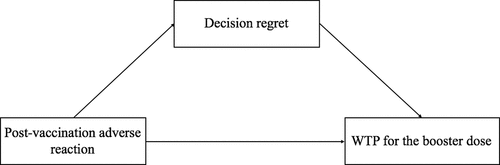
In this study, our main purpose was to explore the relationship between post-vaccination adverse reactions, decision regret, and WTP for the booster dose of COVID-19 vaccine. The framework of the above relationship was characterized in . The exposure we considered here was adverse reactions after vaccination; the potential mediator was the score of decision regret; and the outcome was WTP for the booster dose of COVID-19 vaccine. We conducted mediation analysis based on the above mediation models and adjusted for covariates including age, sex, education, and underlying diseases.
Figure 1. The directed acyclic graph describes the relation among post-vaccination adverse reactions, decision regret, and WTP for the booster dose of COVID-19 vaccine.
Categorical variables of the basic demographic characteristics were presented as counts and percentages. We applied chi-square test to initially identify the possible factors of the outcome. Finally, we adopted the three regression models (i.e., EquationEquations (1(1)
(1) –Equation3
(3)
(3) )) to perform the mediation analysis. Variables considered statistically significant should have a P-value <.05. All statistical analyses were implemented via R software, version 4.1.0 (R Project for Statistical Computing).
Results
Characteristics of the study participants
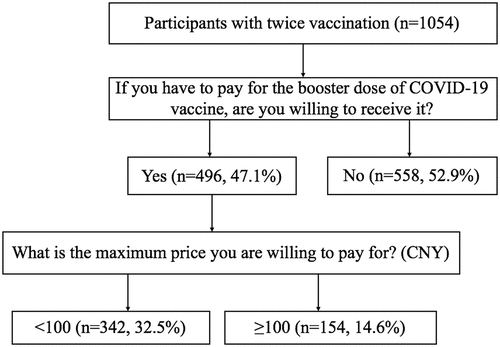
We obtained 1,085 valid questionnaires, and 1,054 (97.1%) have completed their twice COVID-19 vaccinations. presented the process of collecting the willingness to pay and different payment values for the respondents. There were 558 (52.9%) who were not willing to pay for the booster dose. Participants were willing to pay CNY 99 or less (342, 32.5%) and more than CNY 100 (14.6%).
Figure 2. Process of collecting the willingness to pay and different payment values for the respondents.
showed the basic information of the respondents, including post-vaccination adverse reactions, age, sex, education, occupation, professional titles, and underlying disease. Among the study participants, 123 (11.7%) had post-vaccination adverse reactions. The average age of respondents was 34.2 ± 8.5 years old, and most were aged below 40 years old (78.6%). There were 165 (15.7%) males and 889 (84.3%) females. The majority of the respondents were nurses, accounting for 63.6%, and more than half of the participants (67.3%) had undergraduate education levels. The vast majority of respondents had no underlying disease (88.5%).
Table 1. Univariate analysis of factors associated with WTP for the booster dose of COVID-19 vaccine.
A total of 496 (47.1%) respondents were willing to pay for the booster injection. There was a significant difference in WTP between respondents with post-vaccination adverse reactions and those without ( = 12.484, P-value <.001). For participants without adverse reactions after vaccination, 49.1% were willing to pay for the booster dose, while for those with adverse reactions, only 31.7% were willing to pay. Besides, results of univariate analysis illustrated that age, sex, education, occupation, professional titles, and underlying disease had no significant difference in WTP. However, we could see a higher WTP in some subgroups. For example, females had higher WTP than males (i.e., 48.3% > 40.6%). Participants with older age, junior college education levels, nurses, professors, and those who had no underlying diseases also had higher WTP for the booster dose.
Correlations between the main study variables
The correlation coefficients were given in . Post-vaccination adverse reaction had a positive correlation with decision regret (r = 0.14, P-value <.001) and a negative correlation with WTP for the booster dose (r = −0.11, P-value <.001). Decision regret was negatively correlated with WTP for the booster dose (r = −0.21, P-value <.001). To sum up, the results of correlation analysis showed that the pairwise combinations of the above three variables were significant and illustrated that there was a correlation between post-vaccination adverse reaction, decision regret, and WTP for the booster dose.
Table 2. Descriptive statistics and correlations among study variables (n = 1,054).
Testing for the mediation model
The results of the mediation analyses for the relationship between post-vaccination adverse reaction, decision regret, and WTP for the booster dose, adjusting for age, sex, education, and underlying disease were presented in .
Table 3. Testing of the mediating role of decision regret.
Firstly, the effect of post-vaccination adverse reaction on WTP for the booster dose was significant (P-value <.001). Compared with participants who had no adverse reactions after vaccination, those with post-vaccination adverse reactions were less likely to receive the booster dose (OR = 0.48, 95%CI: 0.32 0.72). Hence, post-vaccination adverse reaction was a significant factor affecting the WTP for the booster injection. Secondly, compared with respondents without post-vaccination adverse reactions, those who experienced adverse reactions had higher decision regret scores (B = 1.63, 95%CI: 0.98 2.28). The effect of post-vaccination adverse reaction on decision regret was also significant (P-value <.001).
Thirdly, the impact of decision regret on WTP for the booster dose was also significant after controlling for post-vaccination adverse reactions (OR = 0.89, 95%CI: 0.85 0.92, P-value <.001), which suggested that participants who regretted their previous decisions were less likely to get a booster shot. Finally, the effect of post-vaccination adverse reaction on WTP for the booster dose remained significant (OR = 0.58, 95%CI: 0.38 0.87, P-value <.01). The Sobel test indicated that the mediation effect of decision regret on the relationship between post-vaccination adverse reactions and WTP for the booster dose was significant. This implies that decision regret could significantly mediate the effect of post-vaccination adverse reactions on WTP for the booster dose.
Similarly, we also conducted mediation analysis for willing-to-pay below the price of CNY 100 vs. not willing-to-pay, and willing-to-pay above the price of CNY 100 vs. not willing-to-pay. The estimated direct and indirect associations were summarized in . For willing-to-pay below the price of CNY 100 vs. not willing-to-pay, decision regret had a significant association with both the exposure and the outcome. Similar results could be obtained for willing-to-pay above the price of CNY 100 vs. not willing-to-pay. Therefore, our results further suggested that decision regret significantly mediated the relationship between post-vaccination adverse reactions and WTP for the booster injection.
Table 4. Path analysis coefficients for direct and indirect associations.
Discussion
Clinical implications
The COVID-19 pandemic has severely affected the lives of people around the world. Vaccination has been recognized as an effective method to control and prevent infectious diseases. Considering the potentially lasting impact of the epidemic on humans, we may have to be prepared for ongoing vaccinations. In China, the health care vaccination program is well organized and high rates of vaccination are expected among HCWs. However, fewer studies have investigated the potential mechanisms of the WTP for the booster dose.
This study aimed to explore the relationship between post-vaccination adverse reactions and WTP for the booster dose alongside the potential mechanisms. We focused on HCWs who have received their twice vaccination. In this research, we found that HCWs with post-vaccination adverse reactions had a negative correlation with WTP for the booster dose. Besides, respondents who experienced side reactions after vaccination were more likely to regret their previous vaccination decisions. Furthermore, participants who had higher decision regret scores had less willing to pay for the booster dose. The results showed that regretting the previous decisions could significantly mediate the impact of post-vaccination adverse reactions on WTP for the booster dose. We also conducted mediation analysis for willing-to-pay below the price of CNY 100 vs. not willing-to-pay, and willing-to-pay above the price of CNY 100 vs. not willing-to-pay. Similar results were observed. To the best of our knowledge, this research is one of the few studies on the influence of post-vaccination adverse reactions on WTP for a booster dose.
Several studies have been conducted to investigate the immunogenicity, safety, and efficacy of the booster dose against the pandemic, which provides strong scientific evidence that the booster dose of COVID-19 vaccine can improve the titer and protective range of neutralizing antibodies.Citation44–46 Results of this study showed that only 47.1% of respondents had WTP for the booster shot. Previous studies have presented that, regardless of the vaccine, there were around 1/4 of the vaccinated population affected by post-vaccination side reactions.Citation35 Although all side reactions generally disappear within a week, individuals might regret the choice of vaccination that they made previously. Adverse physical health outcome was one of risk factors most frequently reported to be related to decision regret.Citation38 Both the post-vaccination side reactions and decision regret reduced WTP for the booster dose. Hence, in the further vaccine communication campaigns for booster doses of COVID-19 vaccine, except the information that the vaccine is effective and safe, incorporating adverse reactions after vaccination is also necessary, which might increase the willingness to be vaccinated.
Clinical practice
The current study showed that 47.1% of HCWs were willing to pay for a booster COVID-19 vaccine, and the price accepted by most was below CNY 100. The results reflected the economic value and affordability of future vaccinations. We also found that post-vaccination adverse reaction and decision regret were risk factors of WTP. Notably, decision regret played a mediating role between adverse reaction and WTP. For future vaccine campaigns, post-vaccination adverse reactions should be considered to improve people’s willingness to receive and pay for the booster dose of COVID-19 vaccine. In addition, as decision regret also affected WTP, the government could consider some intervention measures such as decision support tools to prevent or reduce regret.
Methodological consideration
This research is not without limitations and the following aspects require further investigation. Firstly, since only one teaching hospital was considered, the sample may not be representative of the HCWs in China. In addition, we focused on the HCWs who had received two vaccination doses, which may have resulted in selection bias. Secondly, the survey participants were likely to be healthier than the general public, given that they were healthy enough to be employed by a healthcare institution. However, there may be additional differences between HCWs and the general population. Hence, to further identify the role of decision regret in the relationship between adverse reactions after vaccination and willingness to receive the booster dose, the generalization and external validity should be further studied. Thirdly, the online data collection method could potentially lead to over-reporting or under-reporting of WTP for the booster dose. Fourthly, our estimates were conducted at only one-time point; however, decision regret scores of HCWs may change over time. Therefore, the scores may not reflect long-term exposure to various factors. Fifthly, this study found that the adverse reactions and vaccine prices can affect the WTP of vaccination recipients, and further studies should also consider that the number of doses of the vaccine is also an important factor affecting the willingness of vaccination recipients. In addition, although the WTP among different occupations of the participants was not significantly different in this study, annual household income may also be a contributing factor. Hence, it is necessary to consider the income of the population in future research. Further longitudinal and larger sample sizes investigations are essential not only to extrapolate the findings to other regions of China, but also to better understand the causal relationships.
Conclusions
To sum up, our study showed that post-vaccination adverse reactions in HCWs could decrease their WTP for the booster dose of COVID-19 vaccine. Of note, HCWs who experienced side reactions after vaccination were more likely to regret their previous vaccination decisions, which, in turn, further reduced their WTP for booster injections. Generally, side effects after vaccination will disappear within a week; therefore, they should not be a major concern with vaccination. Consequently, post-vaccination adverse reactions should be further incorporated into vaccine campaigns to improve vaccine intention and potentially increase WTP for the booster doses of the COVID-19 vaccine.
Author contributions
CW Luo, WC Jiang, and TH Tung conceived the idea, implemented the method, and drafted the manuscript. CW Luo and WC Jiang were responsible for the coding of the analyses. HX Chen and TH Tung designed the questionnaire. WC Jiang and HX Chen collected the data. All authors edited and approved the final manuscript.
Supplemental Material
Download PDF (96.8 KB)Acknowledgments
We would like to thank participants for their cooperation and support.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2146964
Disclosure statement
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
References
- İ̇pek S, Yurttutan S, Güllü U, Dalkıran T, Acıpayam C, Doğaner A. Is N95 face mask linked to dizziness and headache? Int Arch Occup Environ Health. 2021;94(7):1–8. doi:10.1007/s00420-021-01665-3.
- Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–207. doi:10.1056/NEJMoa2001316.
- Harapan H, Itoh N, Yufika A, Winardi W, Keamg S, Te H, Megawati D, Hayati Z, Wagner AL, Mudatsir M. Coronavirus disease 2019 (COVID-19): a literature review. J Infect Public Health. 2020;13(5):667–73. doi:10.1016/j.jiph.2020.03.019.
- Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–34. doi:10.1016/S1473-3099(20)30120-1.
- Majumder J, Minko T. Recent developments on therapeutic and diagnostic approaches for COVID-19. AAPS J. 2021;23(1):14. doi:10.1208/s12248-020-00532-2.
- Wiersinga W, Rhodes A, Cheng A, Peacock S, Prescott H. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020;324(8):782–93. doi:10.1001/jama.2020.12839.
- Polack F, Thomas S, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. doi:10.1056/NEJMoa2034577.
- Russell F, Greenwood B. Who should be prioritised for COVID-19 vaccination? Hum Vaccin Immunother. 2021;17(5):1317–21. doi:10.1080/21645515.2020.1827882.
- Palacios R, Patiño E, de Oliveira R, Conde M, Batista A, Zeng G, Xin Q, Kallas EG, Flores J, Ockenhouse CF, et al. Double-blind, randomized, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of treating healthcare professionals with the adsorbed COVID-19 (inactivated) vaccine manufactured by Sinovac - PROFISCOV: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):853. doi:10.1186/s13063-020-04775-4.
- Ullah I, Lin C-Y, Malik NI, Wu T-Y, Araban M, Griffiths MD, Pakpour AH. Factors affecting Pakistani young adults’ intentions to uptake COVID-19 vaccination: an extension of the theory of planned behavior. Brain Behav. 2021;11(11):e2370. doi:10.1002/brb3.2370.
- Yahaghi R, Ahmadizade S, Fotuhi R, Taherkhani E, Ranjbaran M, Buchali Z, Jafari R, Zamani N, Shahbazkhania A, Simiari H, et al. Fear of COVID-19 and perceived COVID-19 infectability supplement theory of planned behavior to explain Iranians’ intention to get COVID-19 vaccinated. Vaccines. 2021;9(7):684. doi:10.3390/vaccines9070684.
- Fan C-W, Chen I-H, Ko N-Y, Yen C-F, Lin C-Y, Griffiths MD, Pakpour AH. Extended theory of planned behavior in explaining the intention to COVID-19 vaccination uptake among mainland Chinese university students: an online survey study. Hum Vaccines Immunother. 2021;17(10):3413–20. doi:10.1080/21645515.2021.1933687.
- Huang P-C, Hung C-H, Kuo Y-J, Chen Y-P, Ahorsu DK, Yen C-F, Lin C-Y, Griffiths MD, Pakpour AH. Expanding protection motivation theory to explain willingness of COVID-19 vaccination uptake among Taiwanese university students. Vaccines. 2021;9(9):1046. doi:10.3390/vaccines9091046.
- Wong LP, Alias H, Wong PF, Lee HY, AbuBakar S. The use of the health belief model to assess predictors of intent to receive the COVID-19 vaccine and willingness to pay. Hum Vaccines Immunother. 2020;16(9):2204–14. doi:10.1080/21645515.2020.1790279.
- Fan C-W, Chen J-S, Addo F-M, Adjaottor ES, Amankwaah GB, Yen C-F, Ahorsu DK, Lin C-Y. Examining the validity of the drivers of COVID-19 vaccination acceptance scale using rasch analysis. Expert Rev Vaccines. 2022;21(2):253–60. doi:10.1080/14760584.2022.2011227.
- Yeh Y-C, Chen I-H, Ahorsu DK, Ko N-Y, Chen K-L, Li P-C, Yen C-F, Lin C-Y, Griffiths MD, Pakpour AH. Measurement invariance of the drivers of COVID-19 vaccination acceptance scale: comparison between Taiwanese and mainland Chinese-speaking populations. Vaccines. 2021;9(3):297. doi:10.3390/vaccines9030297.
- Chen I-H, Wu P-L, Yen C-F, Ullah I, Shoib S, Zahid SU, Bashir A, Iqbal N, Addo F-M, Adjaottor ES, et al. Motors of COVID-19 vaccination acceptance scale (MoVac-COVID19S): evidence of measurement invariance across five countries. risk Manag Healthc Policy. 2022;15:435–45. doi:10.2147/RMHP.S351794.
- Pramukti I, Strong C, Chen I-H, Yen C-F, Rifai A, Ibrahim K, Pandin MGR, Subramaniam H, Griffiths MD, Lin C-Y, et al. The motors of COVID-19 vaccination acceptance scale (MoVac-COVID19S): measurement invariant evidence for its nine-item version in Taiwan, Indonesia, and Malaysia. Psychol Res Behav Manag. 2022;15:1617–25. doi:10.2147/PRBM.S363757.
- Chen I-H, Ahorsu DK, Ko N-Y, Yen C-F, Lin C-Y, Griffiths MD, Pakpour AH. Adapting the motors of influenza vaccination acceptance scale into the motors of COVID-19 vaccination acceptance scale: psychometric evaluation among mainland Chinese university students. Vaccine. 2021;39(32):4510–15. doi:10.1016/j.vaccine.2021.06.044.
- He S, Anderson E. Conceptualizing and measuring pathways for how object attachment affects willingness to pay (WTP). Curr Opin Psychol. 2021;39:121–24. doi:10.1016/j.copsyc.2020.09.008.
- Shih H, Chou P, Chen S, Liu J, Lee F, Liu C, Tung T-H. A community-based study of the willingness to pay associated with screening for diabetic retinopathy among type 2 diabetes in Kinmen, Taiwan. J Epidemiol. 2007;17(6):186–93. doi:10.2188/jea.17.186.
- Miller K, Hofstetter R, Krohmer H, Zhang Z. How should consumers’ willingness to pay be measured? An empirical comparison of state-of-the-art approaches. J Mark Res. 2011;48(1):172–84. doi:10.1509/jmkr.48.1.172.
- Dias-Godói I, Tadeu Rocha Sarmento T, Afonso Reis E, Peres Gargano L, Godman B, de Assis Acurcio F, Alvares-Teodoro J, Guerra Júnior AA, Mariano Ruas C. Acceptability and willingness to pay for a hypothetical vaccine against SARS CoV-2 by the Brazilian consumer: a cross-sectional study and the implications. Expert Rev Pharmacoecon Outcomes Res. 2022;22(1):119–29. doi:10.1080/14737167.2021.1931128.
- Vaishya R, Sibal A, Malani A, Prasad K. SARS-CoV-2 infection after COVID-19 immunization in healthcare workers: a retrospective, pilot study. Indian J Med Res. 2021;153(5):550–54. doi:10.4103/ijmr.ijmr_1485_21.
- Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, Mandelboim M, Levin EG, Rubin C, Indenbaum V, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–84. doi:10.1056/NEJMoa2109072.
- Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, Haljasmägi L, Rumm AP, Maruste R, Kärner J, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. doi:10.1016/j.lanepe.2021.100208.
- Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduct Target Ther. 2020;5(1):237. doi:10.1038/s41392-020-00352-y.
- Jalkanen P, Kolehmainen P, Häkkinen H, Huttunen M, Tähtinen P, Lundberg R, Maljanen S, Reinholm A, Tauriainen S, Pakkanen SH, et al. COVID-19 mRNA vaccine induced antibody responses against three SARS-CoV-2 variants. Nat Commun. 2021;12(1):3991. doi:10.1038/s41467-021-24285-4.
- Sabetian G, Moghadami M, Hashemizadeh Fard Haghighi L, Shahriarirad R, Fallahi M, Asmarian N, Moeini YS. COVID-19 infection among healthcare workers: a cross-sectional study in southwest Iran. Virol J. 2021;18(1):58. doi:10.1186/s12985-021-01532-0.
- Zheng L, Wang X, Zhou C, Liu Q, Li S, Sun Q, Wang M, Zhou Q, Wang W. Analysis of the infection status of healthcare workers in Wuhan during the COVID-19 outbreak: a cross-sectional study. Clin Infect Dis. 2020;71(16):2109–13. doi:10.1093/cid/ciaa588.
- Kukreti S, Lu M-Y, Lin Y-H, Strong C, Lin C-Y, Ko N-Y, Chen P-L, Ko W-C. Willingness of Taiwan’s healthcare workers and outpatients to vaccinate against COVID-19 during a period without community outbreaks. Vaccines. 2021;9(3):246. doi:10.3390/vaccines9030246.
- Kotecha IS, Vasavada DA, Kumar P, Nerli LM, Tiwari DS, Parmar DV. Knowledge, attitude, and belief of health-care workers toward COVID-19 vaccine at a tertiary care center in India. Asian J Soc Health Behav. 2022;5(2):63–67. doi:10.4103/shb.shb_20_21.
- Rad MK, Fakhri A, Stein L, Araban M. Health-care staff beliefs and coronavirus disease 2019 vaccinations: a cross-sectional study from Iran. Asian J Soc Health Behav. 2022;5(1):40–46. doi:10.4103/shb.shb_13_22.
- Maruyama A, Sawa T, Teramukai S, Katoh N. Adverse reactions to the first and second doses of Pfizer-BioNtech COVID-19 vaccine among healthcare workers. J Infect Chemother. 2022;28(7):934–42. S1341-321X(22)00094-0. doi:10.1016/j.jiac.2022.03.015.
- Kałucka S, Kusideł E, Głowacka A, Oczoś P, Grzegorczyk-Karolak I. Pre-vaccination stress, post-vaccination adverse reactions, and attitudes towards vaccination after receiving the COVID-19 vaccine among health care workers. Vaccines (Basel). 2022;10(3):401. doi:10.3390/vaccines10030401.
- Zikmund-Fisher B, Couper M, Singer E, Levin C, Fowler FJ, Ziniel S, Ubel PA, Fagerlin A. The DECISIONS study: a nationwide survey of United States adults regarding 9 common medical decisions. Med Decis Making. 2010;30(5 Suppl):20S–34S. doi:10.1177/0272989X09353792.
- O’Connor A, Drake E, Wells G, Tugwell P, Laupacis A, Elmslie T. A survey of the decision-making needs of Canadians faced with complex health decisions. Health Expect. 2003;6(2):97–109. doi:10.1046/j.1369-6513.2003.00215.x.
- Becerra Pérez M, Menear M, Brehaut J, Légaré F. Extent and predictors of decision regret about health care decisions: a systematic review. Med Decis Making. 2016;36(6):777–90. doi:10.1177/0272989X16636113.
- Luo C, Chen H, Tung T. COVID-19 vaccination in China: adverse effects and its impact on health care working decisions on booster dose. Vaccines (Basel). 2022;10(8):1229. doi:10.3390/vaccines10081229.
- Haun M, Schakowski A, Preibsch A, Friederich H, Hartmann M. Assessing decision regret in caregivers of deceased German people with cancer-A psychometric validation of the decision regret scale for caregivers. Health Expect. 2019;22(5):1089–99. doi:10.1111/hex.12941.
- Tung T, Lin X, Chen Y, Zhang M, Zhu J. Willingness-to-pay for a booster dose of inactivated SARS-CoV-2 vaccine in Taizhou, China. Hum Vaccin Immunother. 2022;2099210. doi:10.1080/21645515.2022.2099210.
- Judd C, Kenny D. Process analysis: estimating mediation in treatment evaluations. Eval Rev. 1981;5(5):602–19. doi:10.1177/0193841X8100500502.
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Soc Methodol. 1982;13(13):290–312. doi:10.2307/270723.
- Voysey M, Costa Clemens S, Madhi S, Weckx L, Folegatti P, Aley P, Angus B, Baillie VL, Barnabas SL, Bhorat QE, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdox1 nCov-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881–91. doi:10.1016/S0140-6736(21)00432-3.
- Ramasamy M, Minassian A, Ewer K, Flaxman A, Folegatti P, Owens D, Voysey M, Aley PK, Angus B, Babbage G, et al. Safety and immunogenicity of ChAdox1 nCov-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–93. doi:10.1016/S0140-6736(20)32466-1.
- Yigit M, Ozkaya-Parlakay A, Cosgun Y, Ince Y, Bulut Y, Senel E. Should a third booster dose be scheduled after two doses of CoronaVac? A single-center experience. J Med Virol. 2022;94(1):287–90. doi:10.1002/jmv.27318.
