ABSTRACT
Maternal anti-respiratory syncytial virus (RSV) antibodies protect neonates from RSV disease throughout first weeks of life. Previous studies of maternal immunization in cotton rats showed that a single immunization during pregnancy of RSV-primed dams with virus-like particles (VLPs) assembled with pre-fusion F protein and the wild type G protein boosted their RSV serum antibody concentration and protected pups early in life against RSV challenge. We extended these findings by evaluating responses to RSV infection in litters from two consecutive pregnancies of immunized dams. Using an RSV-primed population of VLP-vaccinated and unvaccinated dams, we defined correlations between dams’ and litters’ RSV neutralizing antibodies (NA); between litters’ NA and protection; and between litter’s NA and their lung expression of selected cytokines, of a first or of a second pregnancy. Lung pathology was also evaluated. We found positive correlation between the NA titers in the dams at delivery and the NA in their first and second litters and negative correlations between the litters’ NA and protection from RSV challenge. Vaccination of dams modulated the mRNA expression for IFNγ and IL-6 and lung pathology in the first and in the second litter at different times after birth, even in the absence of detectable NA. Maternal RSV vaccination enhanced the levels of antibodies transferred to offspring and their protection from challenge. Importantly, maternal vaccination also impacted the immunological and inflammatory response of the offspring’s lungs well into maturity, and after the antiviral effect of maternally transferred NA waned or was no longer detectable.
Introduction
Respiratory syncytial virus (RSV) is a significant cause of morbidity and mortality in the very young and the very old human population.Citation1,Citation2 RSV infection in young children is a major cause of hospitalization and is associated with increased risk ofrecurrent wheezing and asthma later in life.Citation1–5 An antiviral monoclonal antibody directed against RSV F protein, administered prophylactically, is the only preventative approved against RSV and is used in premature or high-risk infants.Citation6 Other antibodies are awaiting FDA approval,Citation7 with the hope of improved efficacy of this therapy. Vaccination has been the focus of RSV investigation for the past 60 years. However, safety has been a paramount obstacle for vaccine development due to the failure of early vaccine trials in the 60s, where two infants died.Citation8–11 Thus, indirect protection of the very young against RSV by maternal vaccination has been the focus of many vaccine developments in the last decade.Citation12 Maternal vaccines are commonly used to protect infants from influenza, tetanus, and pertussis.Citation13,Citation14 It has been reported that RSV maternal antibody (matAb) acquired by the fetus through the placenta or lactation has a protective effect on neonates during the first few weeks of life.Citation15–19 Thus, a goal of the maternal RSV immunization strategy is to increase protective specific matAbs being transferred to neonates to levels that will extend the time of protection against RSV longer after birth. However, the effects of maternal immunization on the responses to RSV in offspring later in the life remain difficult to predict and thus are important to evaluate in animal models.
Our previous studies in the cotton rat model of maternal immunization using a novel virus-like particle (VLP) vaccine candidates assembled with different pre-fusion RSV F proteins along with the RSV G attachment protein showed that maternal vaccination improves protection in young animals (at 4 weeks after birth).Citation20 Here, we show that maternal vaccination also protects litters from lung pathology and cytokine gene expression early on, but not later in life as maternal antibodies in the litter wane. Importantly, we find that maternal vaccination results in an increase in mRNA expression for IL-6 and IFNγ in the lung when RSV infection occurs later in the life of the litters, and when protection from RSV by matAbs wanes or cannot be detected, correlating with the modulation of the litter’s response to RSV in their lung.
Materials and methods
Preparation, characterization, and validation of VLP stocks
VLPs used as immunogens were based on the core M and NP proteins of Newcastle disease virus (NDV) M and NP proteins and contained the RSV F and G glycoproteins.Citation21–23 The RSV proteins were assembled into the VLPs as chimera proteins with the sequences of the ectodomain of RSV F and G glycoproteins fused to the transmembrane and cytoplasmic domains of the NDV F and HN proteins, respectively.
The VLPs were prepared by transfecting avian cells (ELL-0) with cDNAs encoding the NDV M and NP proteins, the G protein chimera, and one of the mutant F chimera proteins, either in the stabilized pre-fusion conformation (DS Cav1),Citation24 with alternative mutations to improve immunogenicity (UC-3 F),Citation25,Citation26 or the with F protein in the post-fusion conformation (post F). VLPs released into the cell supernatant were purified as previously describedCitation27 and the F and G protein contents of purified VLPs was quantified by Western blots and by monoclonal antibody binding to the VLPs as previously described. VLP stocks were adjusted for equivalent levels of F protein.Citation26,Citation28 The pre-fusion or post-fusion conformations of the F protein in the VLPs were validated by assessing the binding of mAbs specific to the pre-fusion form of the F protein to the VLPs as previously reported.Citation28,Citation29
RSV neutralization
RSV was grown in HEp-2 cells, and RSV plaque assays were accomplished on HEp-2 cells as previously described 28. Antibody neutralization assays by a plaque reduction method have been previously described.Citation30 Neutralization titer was defined as log2 of the reciprocal of the dilution of serum that reduced virus titer by 60%.
Animals
Sigmodon hispidus cotton rats were obtained from the inbred colony maintained at Sigmovir Biosystems, Inc. (Rockville, MD). All studies were conducted under applicable laws and guideline and after approval from the Sigmovir Biosystems, Inc.’s Institutional Animal Care and Use Committee. Animals were housed in large polycarbonate cages and fed a standard diet of rodent chow and water ad libitum. The colony was monitored for antibodies to paramyxoviruses and rodent viruses and no such antibodies were found. Female cotton rats were pre-bled before inclusion in the study to rule out the possibility of preexisting antibodies against RSV. All cotton rats born because of breeding during these studies were used for RSV challenge as indicated, and the results used for the litter are geometric means of each parameter obtained from each of its pups.
Real time PCR
Total RNA was extracted from homogenized lung tissue using the RNeasy purification kit (QIAGEN). One µg of total RNA was used to prepare cDNA using QuantiTect Reverse Transcription Kit (Qiagen). For the real-time PCR reactions, the QuantiFast SYBR Green PCR Kit (Qiagen) was used in a final volume of 25 µl, with final primer concentrations of 0.5 µM. Reactions were set up in 96-well trays. Amplifications were performed on a Bio-Rad iCycler for 1 cycle of 95ºC for 3 min, followed by 40 cycles of 95ºC for 10 sec, 60ºC for 10 sec, and 72ºC for 15 sec. The baseline cycles and cycle threshold (Ct) were calculated by the iQ5 software in the PCR Base Line Subtracted Curve Fit mode. Relative quantification of DNA was applied to all samples. The standard curves were developed using serially diluted cDNA sample most enriched in the transcript of interest (e.g., lungs from day 4 post-primary RSV infection for viral transcripts qPCR). The relative expression units were then normalized to the level of β-actin mRNA (“housekeeping gene”) to obtain a ΔCt value for the corresponding sample. The relative 2−ΔΔCt values were plotted. For animal studies, mRNA levels were expressed as the mean ± SEM for all animals in a group. Primer sequences for NS1, forward CACAACAAT GCCAGTGCTACAA, reverse TTAGACCATTAGGTT GAGAGCAATGT; IL-6, forward ATGAAGTTCCTCTCCGCAAGACACT, reverse GACCAGAGGTGATTTTCAGTAGGC; IFNγ, forward CAGATGTCGGGGATCAAAAG, reverse GTTGATGCTTTCCTGGATGG.
Lung histopathology
Lungs were dissected and inflated with 10% neutral buffered formalin to their normal volume, and then immersed in the same fixative solution. Following fixation, the lungs were embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). Four parameters of pulmonary inflammation were evaluated: peribronchiolitis (inflammatory cell infiltration around the bronchioles), perivasculitis (inflammatory cell infiltration around the small blood vessels), interstitial pneumonia (inflammatory cell infiltration and thickening of alveolar walls), and alveolitis (cells within the alveolar spaces). Slides were scored blindly on a 0–4 severity scale. The scores were subsequently converted to a 0–100% histopathology scale.Citation31
Statistical analysis
Statistical analyses (ANOVA) of data were accomplished using Graph Pad Prism 9 software to compare results from litters of vaccinated vs. unvaccinated animals. Pearson correlation coefficients were generated from a total of 60 to 64 pairs of values for the first litters and from 57 to 59 pairs of values of the second litters. Comparisons between litters generated from VLP-vaccinated vs unvaccinated dams were performed using unpaired t-test.
Results
Experimental design
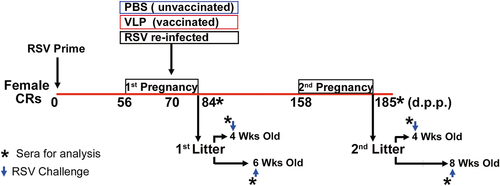
Three-week-old female cotton rats (dams) were tagged, bled, and primed with RSV A/Long intranasally (i.n.) using a dose of 105 PFU/animal in 50 µl. Eight weeks later (d56), females were bled and subsequently paired with RSV naive males 2 weeks older than the females for mating to start the 1st Pregnancy (). At day 70 (two weeks into pregnancy, cotton rats have a length of gestation of 28 days) different groups of dams were immunized with UC-3 F VLPs with 25, 75, 100, or 150 µg total VLP protein/animal (5, 15, 20, or 30 µg F protein), DS-Cav1 F VLPs or post-F VLPs with 100 µg total VLP protein/animal (20 µg F protein), intramuscularly (i.m.). In our previous publication, we showed that UC-3 F VLPs at a dose of 100 µg total VLP protein/animal achieve the best levels of NA in dams and their litters, compared with other doses of the same vaccine or with DS-Cav1 F VLPs or post-F VLPs of the same concentration.Citation20 All dams-litters pairs or litters of vaccinated groups were symbolized by red dots in all the graphs. Control groups were primed females re-exposed to RSV (black dots), or PBS-(mock) vaccinated i.n. during pregnancy (blue dots). All females were bled for serum collection at days 70 and 84 (just before delivery). For analysis and comparison in this study, dams primed, vaccinated with VLPs and their litters were combined in a single cluster (vaccinated), whereas the dams primed and then exposed to RSV, or mock vaccinated during pregnancy were combined in another cluster (unvaccinated) (). Otherwise, animals were treated as a unique population to generate correlations with maternal neutralizing antibodies (NA). Dams delivered pups at approximately day 84. Dams were set again for breeding at day 158 without additional immunization to initiate the 2nd pregnancy. Pups of the 2nd litter were delivered on or about day 185. No vaccination was performed during the second pregnancy. Each litter from a dam with more than one pup was divided in two balanced sex groups, otherwise the pup was randomly assigned to a subgroup. The two subgroups of pups from the 1st litter were bled and challenged with RSV A/Long (105 PFU/animal) at 4 or 6 weeks of age. The two subgroups of pups from the 2nd litter were bled and challenged with the same amount of RSV at 4 and 8 weeks after birth. All pups were sacrificed on day 4 post challenge to measure RSV load (nose and lung viral titers, and lung NS1 gene expression), lung mRNA expression of interferon gamma (IFNγ) and interleukin 6 (IL-6), and lung histopathology. Individual measurements for each parameter for each rat within a litter were performed and their mean was used as the final value for the litter.
Figure 1. Experimental design: Diagram of the protocol to assess correlations and compare lung cytokine mRNA expression and pathology. Groups of 3-week-old female cotton rats (CRs) were primed with RSV (i.n. 105 PFU/animal) (day 0). These animals were then bred twice, once at day 56, and a second time at day 158. Groups of animals (vaccinated) were immunized on day 70 (first pregnancy) only, with UC-3 F VLPs with 25, 75, 100, or 150 µg total VLP protein/animal (5, 15, 20, or 30 µg F protein), DS-Cav1 F VLPs or post-F VLPs with 100 µg total VLP protein/animal (20 µg F protein). Days post-prime (d.p.p.). Control group (unvaccinated) were treated i.m. with PBS, or re-infected intranasally with RSV (RSV re-infected). Offspring of dams were challenged with RSV (i.n. 105 PFU/animal, blue arrow) at 4 or 6 weeks after birth (first pregnancy) or at 4 or 8 weeks after birth (second pregnancy). Asterisks represent serum acquisition to measure RSV neutralizing antibodies.
First litter
Correlations were first drawn between the dams’ NA and the litter’s NA determined prior to RSV challenge. Log2 Dam NA measured prior to delivery in primed mock vaccinated, primed, and RSV exposed, or in primed and VLP-vaccinated cotton rat dams positively correlated with the Log2 Litter NA measured at 4 weeks of age (r = 0.55, p < .0001, and ), and at 6 weeks of age (r = 0.51, p < .0001, ), indicating that the levels of maternal antibodies in the dams before delivery could predict the antibody titers in their litter. However, it was also evident that there was a decrease in the overall titer of the litter NA population at 6 weeks of age, reflecting the decay of their maternal antibodies with time.
Figure 2. Pearson correlations for NA and viral titers in the first pregnancy. Correlation coefficients (r), p (two tail) value, and number of litters used (n) between the NA titers in dams’ serum before delivering and the mean NA titer of their first pregnancy litter measured at 4 (a) and 6 weeks (b) after birth; or between the mean NA titer of each litter and lung (c and d) or nose (e and f) viral titer measured at 4 and 6 weeks after birth, respectively. Blue, red, and black symbols represent pairs from litters of unvaccinated, VLP-vaccinated dams, and RSV re-infected dams, respectively.
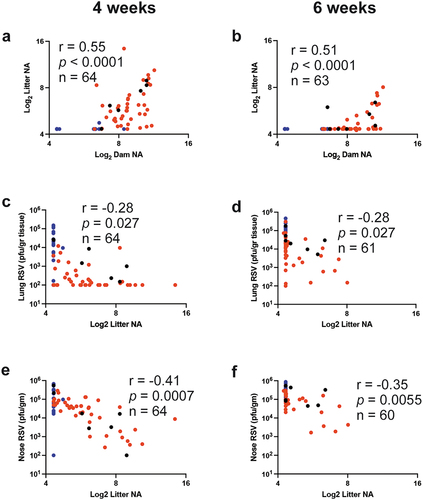
Table 1. Correlation Coefficients for the 1st litter.
After challenging the animals in each litters with RSV when they were 4 (half) or 6 weeks of age (the other half), significant negative correlations were found between the titers of litter NA before RSV challenge and RSV titers in their lungs (r = −0.28, p = .027 for both 4 and 6 weeks at challenge, )) and in their nasal tissue (r = −0.41, p = .0007 for 4 weeks challenged; r = −0.35, p = .0055 for 6 weeks at challenge, . The correlation coefficients between the litter NA and protection were higher in the nose than in the lung at both time points. In addition, as indicated by the detection of mRNA NS1, protection of the lung was significantly higher in litters from dams vaccinated with VLPs or re-infected with RSV during pregnancy ().
Figure 3. Viral quantification and cytokine mRNA expression in lungs of the first pregnancy offspring born from dams unvaccinated or vaccinated during the first pregnancy. qPCR was used for quantification of the lung mRNA expression for the RSV NS1 gene (a and b), and mRNA for cytokines IFNγ (c and d), and IL-6 (e and f) in 4- and 6-week-old offspring sacrificed on day 4 after challenge. Each symbol represents the mean value of the expression of the mRNA in one litter. Significance of differences between the groups was evaluated by one-way ANOVA followed by Tukey post hoc test. ****, p < .0001; *, p < .05. ns, not significant.
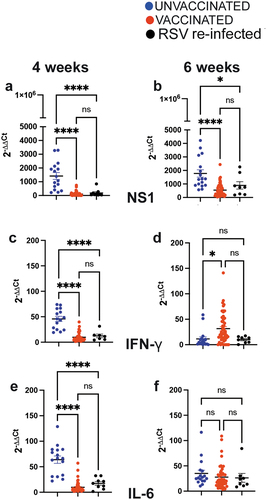
Expression of mRNA for IFNγ and IL-6 was measured in litters from these three clusters. At 4 weeks of age, litters from VLP-vaccinated dams showed significantly reduced expression of IFNγ and IL-6 compared to the cluster of litters from unvaccinated-primed dams (), whereas at 6 weeks, the expression was reversed (more IFNγ mRNA in litters from VLP-vaccinated dams, ), or equalized (for IL-6 mRNA, ). These data indicate that VLP vaccination or RSV re-exposure during pregnancy directly affects the immunological response to RSV in the litter, not only by reducing viral load but also by modulating the lung cytokine response in a time-dependent fashion for 4 and 6 weeks.
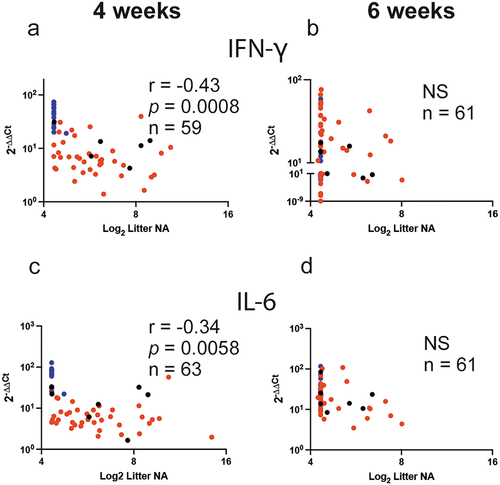
We then drew correlative analysis between the litter NA before challenge either at 4 and 6 weeks of age and the expression levels of mRNA for lung IFNγ and IL-6 at 4 days after their challenge (). We found that there was significant negative correlation between the litter NA and the levels of expression of lung mRNA for IFNγ (r = −0.43, p = .0008) and IL-6 (r = -0.34, p = .0058) only in animals challenged at 4 (), but not at 6 weeks of age (). All together, these data indicate that the dams’ NA define the litters’ NA titers and the expression of the lung mRNA for IFNγ and IL-6 and that enhancing maternal antibodies by VLP vaccination will impact the levels of the RSV-specific lung cytokine response to RSV in a time-dependent fashion.
Figure 4. Pearson correlations for the first pregnancy between NA and the expression of mRNA for IFNγ and IL-6 in the lung. Correlation coefficients (r), p (two tail) value, and number of litters used (n) between the mean NA titer of each litter and the mean expression level of lung mRNA for IFNγ (a and b) and IL-6 (c and d) measured at 4 and 6 weeks after birth, respectively, are shown. Blue, red, and black symbols represent pairs from litters of unvaccinated, VLP-vaccinated dams, and RSV re-infected dams, respectively.
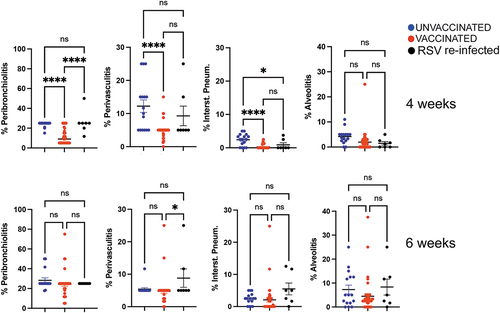
We then compared the lung pathology between the cluster of litters born from VLP-vaccinated dams and the cluster of litters born from primed, mock-vaccinated, or RSV-exposed dams after RSV challenge. VLP-vaccinated dams generated litters that showed significant reduction of lung pathology (peribronchiolitis, perivasculitis, and interstitial pneumonia) at 4 weeks of age (, top) when compared to unvaccinated control, but not at 6 weeks of age (, bottom), indicating a time dependent waning of the protective effect against RSV-induced pathology in the lung of offspring of VLP-vaccinated dams. RSV- re-exposed dams generated litters that showed lower but still significant reduction in interstitial pneumonia, when compared to unvaccinated animals.
Figure 5. Quantification of four lung histological parameters in the first litters from unvaccinated and vaccinated dams during the first pregnancy. Peribronchiolitis, perivasculitis, interstitial pneumonia, and alveolitis were scored blindly on H&E-stained lung slides from pups challenged with RSV and sacrificed on day 4 p.i. Each symbol represents a mean pathology score for each litter. Offspring of the first pregnancy were challenged with RSV i.n. at 4 (top) or 6 (bottom) weeks of age. Significance of differences between the groups was evaluated by one-way ANOVA followed by Tukey post hoc test. ****, p < .0001; *, p < .05; ns, not significant.
Second litter
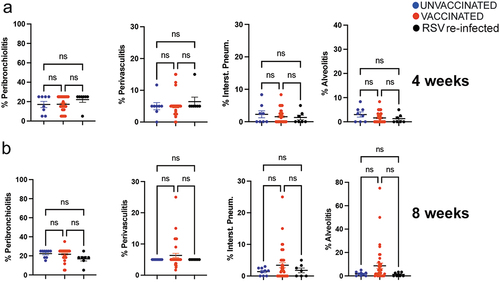
The same groups of primed, VLP-vaccinated dams and primed, unvaccinated or RSV exposed control dams were set for a 2nd pregnancy to analyze the second litter. No vaccination was performed during the 2nd pregnancy, so that all the measurements are the consequence of the vaccination during the 1st pregnancy. Before delivery, dams were bled for quantification of the dams’ NA. Pups in each litter were also bled to determine litters’s NA and challenged at 4 and 8 weeks of age. For the group of the 4-week-old pups, correlations were drawn between dams NA and the 2nd litter NA obtained prior to RSV challenge. Dams’ NA measured prior to delivery in primed and mock-vaccinated, primed, and RSV-exposed, or in primed and VLP vaccinated dams also positively correlated with their litters’ NA measured at 4 weeks of age, (r = 0.66, p < .0001, ). Furthermore, significant negative correlations were found between the 2nd litter NA at 4 weeks of age and the titers of RSV in the lung (), nose (), and the expression of mRNA for IFNγ (), and IL-6 () in the lung (). The correlations for IFNγ and IL-6 were weaker than what was seen for the 1st litter at the same age (compare results in ). Significant protection of the lung was still found in the population of litters from dams primed and vaccinated with VLPs, and in litters from dams RSV-exposed during pregnancy, when compared to those primed but mock-vaccinated (RSV NS1 mRNA, ). In contrast to what was seen with offspring of the first pregnancy, no differences were found when the second pregnancy offspring were challenged at 4 weeks of age and were compared for the expression of IFNγ or IL-6 () or lung pathology ( and Figure S1). However, when the same litter was challenged with RSV at 8 weeks of age, despite the lack of protection, animals born from dams vaccinated with VLPs showed a significant increase in IFNγ and IL-6 compare to whenchallenged at 4 weeks of age and were compared for the expression of and IL-6 mRNA expression (), and an increase (albeit not statistically significant) in interstitial pneumonia and alveolitis in many litters ( and Figure S2). Overall, our data indicate that RSV vaccination during pregnancy influences the overall response to RSV in the litters not only by modulating RSV protection but also the lung immunological response to RSV for two consecutive pregnancies, even at the time when the neutralizing maternal antibodies or protection of the lung are no longer detectable.
Figure 6. Pearson correlations for the second pregnancy. Correlation coefficients (r), p (two tail) value, and number of litters used (n) or comparison between the NA titers in dams’ serum before delivering the second litter and the mean NA of their litter of the second pregnancy (a); between the mean NA titer of each litter and lung (b) or nose (c) viral titers; or between the mean NA titer of each litter and the mean expression of lung mRNA for IFNγ (d) and IL-6 (e). All correlations were performed with samples of animals challenged at 4 weeks of age. Blue, red, and black symbols represent pairs from litters of unvaccinated, VLP-vaccinated dams, and RSV re-infected dams, respectively.
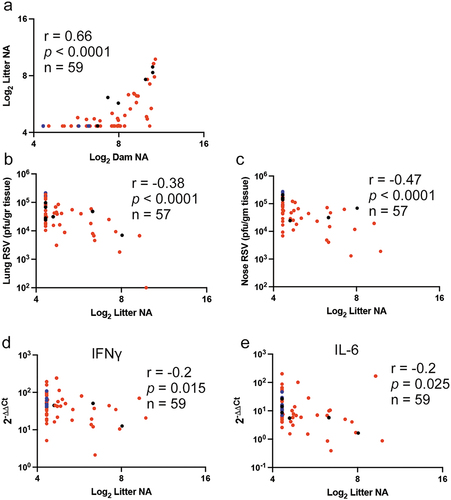
Table 2. Correlation Coefficients for the 2nd litter.
Figure 7. Viral quantification and cytokine mRNA expression in lungs of second pregnancy offspring born from dams unvaccinated, VLP-vaccinated, or RSV re-infected during the first pregnancy. qPCR was used for quantification of the lung mRNA expression for the RSV NS1 gene, and mRNA for cytokines IFNγ, and IL-6, as indicated in 4- (a) and 8- (b) week-old offspring challenged with RSV i.n. and sacrificed on day 4 p.i. Each symbol represents the mean value of the expression of the mRNA in one litter. Significance differences between the groups was evaluated by one-way ANOVA followed by Tukey post hoc test. ****, p < .0001; ***, p < .0005; *, p < .05; ns, not significant.
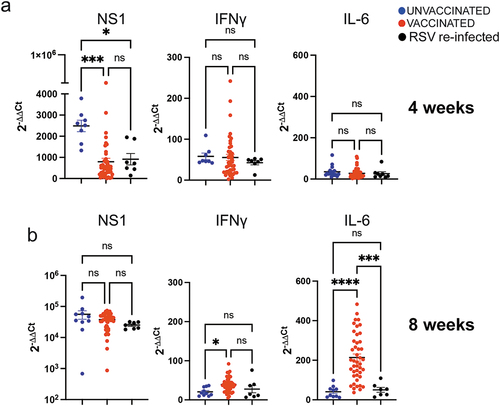
Figure 8. Quantification of four lung histological parameters in the second pregnancy litter from dams unvaccinated or vaccinated during the first pregnancy. Peribronchiolitis, perivasculitis, interstitial pneumonia, and alveolitis were scored blindly on H&E-stained lung slides from pups challenged with RSV and sacrificed on day 4 p.i. Each symbol represents a mean pathology score for each litter. Offspring of the second pregnancy were challenged with RSV i.n. at 4 (top) or 8 (bottom) weeks of age. Significance of differences between the groups was evaluated by one-way ANOVA followed by Tukey post hoc test.
Discussion
Passive transfer of antibodies can enhance protection in the first 2–6 months of the infant’s life.Citation13,Citation14 High efficacy maternal RSV vaccines are projected to have significant benefit for the infant population under 6 months. Several prototype vaccines are currently in phase III clinical trials.Citation12 Protection is especially needed for infants born two months before the peak season of the annual RSV cycleCitation32 and for infants in underdeveloped countries were the burden of RSV disease in higher.Citation33
In clinical trials, the results of maternal vaccination are mostly evaluated with primary and secondary clinical, serological, and safety endpoints early in life (6 month). However, the effects of maternal vaccines later after this period or in subsequent pregnancies are difficult to investigate or premature to predict.
We have performed large studies of maternal RSV vaccination using the cotton rat model.Citation20,Citation26,Citation28,Citation34,Citation35 measuring antibodies in the dams prior to delivery and antibodies in their litters, with subsequent nasal and lung protection upon challenge. Using the cotton rat model of maternal immunization, we first described differences in protection by maternal RSV exposure in two consecutive pregnancies.Citation35 We subsequently showed that vaccination of RSV-primed dams with FI-RSV Lot 100 vaccine does not contribute to vaccine enhanced disease in their pups.Citation34 However, we also showed that pups that received RSV maternal antibodies and were vaccinated with the FI-RSV Lot 100, still developed vaccine enhanced disease,Citation34 replicating previous observations in humans.Citation8–11 By focusing on how to improve vaccine efficacy, we have also followed the decay of maternal antibodies and protection in two consecutive litters after dams were vaccinated with UC-3 FVLP.Citation20 We showed that the UC-3 F VLP vaccine was significantly more efficacious than DS-Cav1 F VLPs or post-F VLPs at the same dose.Citation20 In this study, we extended those results by evaluating the entire population of vaccinated dams that were all primed to mimic RSV seropositivity in the human population and compared to an unvaccinated but primed population of dams or dams re-exposed to RSV during pregnancy, to evaluate the effect of maternal vaccination in their populations of litters during 2 consecutive pregnancies.
We first found that the titers of NA of dams prior to their 1st and 2nd deliveries correlate with the titer of NA found in their litters late in life (at least until 6 weeks of age) and also with the level of protection of litters from RSV replication. However, by comparing these correlations at 4 weeks of age between the two consecutive pregnancies (e.g., comparing ), it became evident that either the efficiency of transfer of NA was higher in the first litter than in the second litter (maternal antibodies at delivery were not significantly different), or that the decay of NA activity was faster in the second litter (e.g., greater number of litters with detectable anti RSV NA from dams having RSV NA < 8 Log2 in the first litter). In addition, although correlation coefficient between litter NA and protection (viral titers in the nose and lung) went up during the second pregnancy, less vaccinated pups achieved measurable levels of NA when compared to the first pregnancy ( vs ). Our previous observations showed an evolution in the species of maternal antibodies transferred occurring between the first and the second litter, when dams were only vaccinated once in the first pregnancy.Citation20 In a recent study on two cohorts of pregnant women from two cities in Kenya, it was shown that the efficiency of maternal transfer of antibodies, measured as cord to maternal titer ratio (CMTR) was reduced when the number of pregnancies was >6, indicating that some mechanisms of antibody transfer become less efficient as the number of viable pregnancies increased.Citation36 Although our results reflect only two pregnancies in cotton rats, our data might reflect a gradual reduction in the efficiency of transfer of antibodies from dams to pups that should be considered for further mechanistic studies, especially due to the impact that this can have on efficacy of human vaccinees.
Importantly, our data showed a stronger association between the litter’s NA and protection of the nose than protection of the lung. This result most likely indicates that the methodology for measuring NA by 60% plaque reduction assay adapts better to correlations in the upper respiratory tract that in the lung since previous analysis using microneutralization assay showed stronger correlations with lung protection (data not shown). Since microneutralization assay has a different range of detection, this observation would suggest that the methodology of NA assay could be adjusted to become more efficient for performing certain correlative analyses.
Our focus in this study was the analysis of the mRNA expression of IFNγ and IL-6 in lungs of pups born from vaccinated dams or dams re-exposed to RSV during pregnancy and subsequently challenged with RSV at different times after birth. We show that the titers of NA in the litter early after birth correlated with protection, with the reduction of the expression of inflammatory cytokines in the lung and decrease in lung pathology, consistent with previous data.Citation26 However, as antibodies wane in the litters and the protection is reduced, those correlations are weaker or no longer can be measured (e.g., at 6 weeks in the first litter and 8 weeks in the second litter). The regulation of cytokine mRNA expression by vaccination, however persists, even with low or undetectable NA in the litter ( and ). These data indicate that the maternal vaccination dictates litters’ responses to late RSV infection even when vaccine protective responses (viral titers or lung histopathology) are no longer measurable. Our preliminary analysis of the effect of serum from pups in an RSV-macrophage activation assay, indicates that even in the absence of detectable NA antibodies against RSV, serum from pups receiving maternal antibodies can modulate viral and inflammatory gene expression (data not shown).
In the lung of young naïve cotton rats, IL-6 mRNA expression is induced early (day 1–2) and decreased by day 4–6 post challenge, whereas mRNA for IFNγ is strongly induced by RSV infection, peaking on day 4 post infection.Citation37 In the context of passive antibody prophylaxis, naïve mice treated with monoclonal antibodies against RSV (motavizumab) show protection from viral replication and reduction of the expression of many inflammatory cytokines, including IL-6 and IFNγ.Citation38 In cotton rat pups from RSV unvaccinated primed dams, an age-dependent expression of mRNA for these two cytokines was found, where the expression was strongly repressed at 1 week of age but was reestablished as the animals became older and their maternal antibodies waned.Citation35 This agrees with our previous results where VLP vaccination extends the inhibition of IL-6 and IFNγ.Citation28 Thus, in cotton rats, the reduction of inflammatory lung cytokine expression during RSV challenge in young animals is seen even in the absence of maternal neutralizing antibodies suggesting that a broader antibody types or specificities are modulating the mechanisms leading to lung pathology.
Overall, our data indicate that the expression of these cytokines is associated with levels of maternal antibodies that are present in the litter at the time of infection () and that these correlations are valid also for a second litter from the same pool of dams (). Importantly, we show that VLP vaccine-induced protection in litters from primed females is significantly stronger than that in litters from unvaccinated primed females (). However, in the first litter, the significant inhibition of IFNγ and IL-6 mRNA expression by VLP-vaccination in the lung of RSV infected animals and reduction of lung pathology was only evident in 4-week-old animals ( and , 4 weeks) and not in 6-week-old animals ( and , 6 weeks). In addition, the first litter from VLP-vaccinated dams challenged at 6 weeks of age showed a reversion in the expression for IFNγ () that was also found for both, IFNγ and IL-6 in the 2nd litter from VLP-vaccinated dams challenged at 8 weeks of age (). This difference in the profile of the lung cytokine expression could be related to differences of the age in the second challenge used for the 1st and 2nd pregnancy (6 vs 8 weeks), or could be related to differences in immunity transferred to offspring during consecutive pregnancies.
IL-6 concentration is enhanced in BAL of infants infected with RSV.Citation39 Children requiring mechanical ventilation for RSV disease had higher levels IL-6Citation40 suggesting a correlation with RSV-induced pathology and clinical severity of disease. However, other studies show an inverse correlation between the concentration of some cytokines (including IL-6 and IFNγ) in nasal washes and the duration of supplemental oxygen therapy, a marker of improvement for RSV-related hospitalizations.Citation41 The immunomodulatory roles of IL-6 and IFNγ in new infections are widely recognized. IL-6 plays a pivotal role in the development of the anti-RSV immune response by stimulating B cells for antibody production and T cell activation, growth, and differentiation. IFNγ is a master modulator for the activation of T cells and T cell proliferation. Mice infected with RSV modified to express IFNγ produce higher titers of antibodies that those infected with wtRSV.Citation42 In the context of the first RSV infection, early IL-6 production regulated the expression of IL-27 by macrophages, which in turn promote the local maturation of regulatory T-cells.Citation43 Recently, a patient with deficient CD14 expression, showed reduced IL-6 production and was prone to recurrent RSV infections.Citation44 Thus, we hypothesize that a reduction of cytokine expression resulting from protection by maternal vaccination early in life, might have a direct impact on the development of newly-formed RSV immune responses.
Furthermore, our results showing increases in the expression of mRNA in the lung for the cytokines IFNγ or IFNγ and IL-6 later in life (for 6-week-old and 8-week-old litters from the 1st and the 2nd pregnancy, respectively) indicates that maternal vaccination has an effect in the litters even when protection has waned. Increases in cytokine expression was strong in animals in the second litter and was paralleled by an increase in lung pathology (alveolitis and interstitial pneumonia) in many of the litters from vaccinated mothers.
Our results show some similarities as well as differences with results published by Eichenger et al. performing maternal vaccination in the mice model of RSV using an RSV F protein in the prefusion conformation and adjuvanted with Advax-SM (a delta inulin-based adjuvant paired with the toll-like receptor 9 agonist, CpG oligodeoxynucleotide)Citation45. In cotton rats, as in the mice model, we found an inverse correlation between IFNγ mRNA expression and neutralizing maternal antibodies early in life of pups (4 weeks). However, later in life (6 or 8 weeks of age) the level of IFNγ mRNA was enhanced in VLP-vaccinated cotton rats. In the mouse model, reduced levels of NA in pups from vaccinated dams was followed by a strong reduction of CD4+ T cells expressing IFNγ in BAL after RSV challenge later in life (day 63 after birth).Citation45 These differences at later times post infection could reflect differences in the setup of the model, vaccine preparation differences, or differences in the compartments analyzed for the expression of IFNγ.
It is well known that high titers of maternal antibodies might inhibit the infant humoral response during infection, as it was reviewed,Citation46 and previously reproduced in our model.Citation34 In addition, maternal antibodies might modulate Th1/Th2 cytokine balance influencing effector T cells.Citation46 Maternal antibody concentrations increased by vaccination have a protective effect early in the life of the infant. However, as the level declines later in infant’s life, the enhanced expression of IFNγ could potentially correlate with a stronger induction of Th1 responses in infants, resulting in more vigorous cellular responses against viral and intracellular pathogens, but potentially leading to an increased lung inflammation with undesirable consequences. Overall, our extended data on RSV maternal immunization in the cotton rat have revealed new results that raise important questions and can form the basis for introducing new endpoints in maternal vaccination clinical trials for RSV.
Acknowledgment
We thank Mr. Charles Smith, Martha Malache, and Ana Rivera for the animal care service.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017 Sep 2;390(10098):946–13. doi:10.1016/S0140-6736(17)30938-8. Epub 2017 Jul 7. PMID: 28689664.
- McLaughlin JM, Khan F, Begier E, Swerdlow DL, Jodar L, Falsey AR. Rates of medically attended RSV among US adults: a systematic review and meta-analysis. Open Forum Infect Dis. 2022 Jun 17;9(7):ofac300. doi:10.1093/ofid/ofac300. PMID: 35873302.
- McGinley JP, Lin GL, Öner D, Golubchik T, O’Connor D, Snape MD, Gruselle O, Langedijk AC, Wildenbeest J, Openshaw P, et al., RESCEU Investigators. Clinical and viral factors associated with disease severity and subsequent wheezing in infants with respiratory syncytial virus infection. J Infect Dis. 2022 Aug 12;226(Suppl 1):S45–54. doi:10.1093/infdis/jiac163. PMID: 35902389.
- van Wijhe M, Johannesen CK, Simonsen L, Jørgensen IM, Fischer TK, Campbell H, Beutels P, Bont L, Pollard A, Openshaw P, et al. A retrospective cohort study on infant respiratory tract infection hospitalizations and recurrent wheeze and asthma risk: impact of respiratory syncytial virus. J Infect Dis. 2022 Aug 12;226(Suppl 1):S55–62. doi:10.1093/infdis/jiac141. PMID: 35426942.
- Wang X, Li Y, Nair H, Campbell H, Wang X, Reeves RM, Li Y, Campbell H, Nair H, van Wijhe M, et al., RESCEU Insvestigators. Time-Varying association between severe respiratory syncytial virus infections and subsequent severe asthma and wheeze and influences of age at the infection. J Infect Dis. 2022 Aug 12;226(Suppl 1):S38–44. doi:10.1093/infdis/jiab308. PMID: 34522963.
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998 Sep;102(3 Pt 1):531–37. doi:10.1542/peds.102.3.531. PMID: 9738173.
- Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA, Muller WJ, Zar HJ, Brooks D, Grenham A, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022 Mar 3;386(9):837–46. doi:10.1056/NEJMoa2110275. PMID: 35235726.
- Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969 Apr;89(4):405–21. doi:10.1093/oxfordjournals.aje.a120954. PMID: 4305197.
- Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969 Apr;89(4):449–63. doi:10.1093/oxfordjournals.aje.a120957. PMID: 4305200.
- Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–48. doi:10.1093/oxfordjournals.aje.a120956. PMID: 4305199.
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–34. doi:10.1093/oxfordjournals.aje.a120955. PMID: 4305198.
- Mazur NI, Terstappen J, Baral R, Bardají A, Beutels P, Buchholz UJ, Cohen C, Crowe JE Jr, Cutland CL, Eckert L, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2022 Aug 8;S1473-3099(22):00291–2. doi:10.1016/S1473-3099(22)00291-2. Epub ahead of print. PMID: 35952703.
- Etti M, Calvert A, Galiza E, Lim S, Khalil A, Le Doare K, Heath PT Maternal vaccination: a review of current evidence and recommendations. Am J Obstet Gynecol. 2022 Apr;226(4):459–74. doi:10.1016/j.ajog.2021.10.041. Epub 2021 Nov 11. PMID: 34774821.
- Chu HY, Englund JA. Maternal immunization. Birth Defects Res. 2017 Mar 15;109(5):379–86. doi:10.1002/bdra.23547. PMID: 28398678.
- Stensballe LG, Fullarton JR, Carbonell-Estrany X, Simões EA Population based external validation of a European predictive model for respiratory syncytial virus hospitalization of premature infants born 33 to 35 weeks of gestational age. Pediatr Infect Dis J. 2010 Apr;29(4):374–76. doi:10.1097/INF.0b013e3181c810da. PMID: 20016397.
- Chu HY, Tielsch J, Katz J, Magaret AS, Khatry S, LeClerq SC, Shrestha L, Kuypers J, Steinhoff MC, Englund JA. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J Clin Virol. 2017 Oct;95:90–95. doi:10.1016/j.jcv.2017.08.017. Epub 2017 Sep 2. PMID: 28903080.
- Ogilvie MM, Vathenen AS, Radford M, Codd J, Key S. Maternal antibody and respiratory syncytial virus infection in infancy. J Med Virol. 1981;7(4):263–71. doi:10.1002/jmv.1890070403. PMID: 7038043.
- Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981 May;98(5):708–15. doi:10.1016/s0022-3476(81)80829-3. PMID: 7229749.
- Lamprecht CL, Krause HE, Mufson MA Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J Infect Dis. 1976 Sep;134(3):211–17. doi:10.1093/infdis/134.3.211. PMID: 977992.
- Blanco JCG, Cullen LM, Kamali A, Sylla FYD, Boukhvalova MS, Morrison TG, Bukreyev A. Evolution of protection after maternal immunization for respiratory syncytial virus in cotton rats. PLoS Pathog. 2021 Dec 23;17(12):e1009856. doi:10.1371/journal.ppat.1009856. PMID: 34941963.
- Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Heaton PM, Fraire AE, Morrison TG Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol. 2010 Jan;84(2):1110–23. doi:10.1128/JVI.01709-09. Epub 2009 Nov 4. PMID: 19889768.
- Morrison TG, Gravel KA, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, Schmidt MR, Morrison TG. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J Virol. 2011 Jan;85(1):366–77. doi:10.1128/JVI.01861-10. Epub 2010 Oct 27. PMID: 20980510.
- McGinnes Cullen L, Schmidt MR, Kenward SA, Woodland RT, Morrison TG, Lyles DS. Murine immune responses to virus-like particle-associated pre- and postfusion forms of the respiratory syncytial virus F protein. J Virol. 2015 Jul;89(13):6835–47. doi:10.1128/JVI.00384-15. Epub 2015 Apr 22. PMID: 25903340.
- McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, Zhou T, Baxa U, Yasuda E, Beaumont T, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013 May 31;340(6136):1113–17. doi:10.1126/science.1234914. Epub 2013 Apr 25. PMID: 23618766.
- Krarup A, Truan D, Furmanova-Hollenstein P, Bogaert L, Bouchier P, Bisschop IJM, Widjojoatmodjo MN, Zahn R, Schuitemaker H, McLellan JS, et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat Commun. 2015 Sep 3;6(1):8143. doi:10.1038/ncomms9143. PMID: 26333350
- Blanco JCG, Fernando LR, Zhang W, Kamali A, Boukhvalova MS, McGinnes-Cullen L, Morrison TG, Dutch RE. Alternative virus-like particle-associated prefusion F proteins as maternal vaccines for respiratory syncytial virus. J Virol. 2019 Nov 13;93(23):e00914–19. doi:10.1128/JVI.00914-19. PMID: 31511382
- McGinnes LW, Morrison TG. Newcastle disease virus-like particles: preparation, purification, quantification, and incorporation of foreign glycoproteins. Curr Protoc Microbiol. 2013 Oct 2;30(1):18.2.1–18.2.21. doi:10.1002/9780471729259.mc1802s30. PMID: 24510891.
- Blanco JCG, Pletneva LM, McGinnes-Cullen L, Otoa RO, Patel MC, Fernando LR, Boukhvalova MS, Morrison TG. Efficacy of a respiratory syncytial virus vaccine candidate in a maternal immunization model. Nat Commun. 2018 May 15;9(1):1904. doi:10.1038/s41467-018-04216-6. PMID: 29765035.
- Cullen LM, Schmidt MR, Torres GM, Capoferri AA, Morrison TG. Comparison of immune responses to different versions of VLP associated stabilized RSV pre-fusion F protein. Vaccines (Basel). 2019 Feb 15;7(1):21. doi:10.3390/vaccines7010021. PMID: 30769923.
- Coates HV, Alling DW, Chanock RM An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am J Epidemiol. 1966 Mar;83(2):299–313. doi:10.1093/oxfordjournals.aje.a120586. PMID: 5933417.
- Prince GA, Prieels JP, Slaoui M, Porter DD. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus). Lab Invest. 1999 Nov;79(11):1385–92. PMID: 10576209.
- Wang X, Li Y, Vazquez Fernandez L, Teirlinck AC, Lehtonen T, van Wijhe M, Stona L, Bangert M, Reeves RM, Bøås H, et al. Respiratory syncytial virus-associated hospital admissions and bed days in children <5 years of age in 7 European countries. J Infect Dis. 2022 Aug 12;226(Suppl 1):S22–28. doi:10.1093/infdis/jiab560. PMID: 35023567.
- Baral R, Li X, Willem L, Antillon M, Vilajeliu A, Jit M, Beutels P, Pecenka C. The impact of maternal RSV vaccine to protect infants in Gavi-supported countries: estimates from two models. Vaccine. 2020 Jul 14;38(33):5139–47. doi:10.1016/j.vaccine.2020.06.036. Epub 2020 Jun 22. PMID: 32586761; PMCID: PMC7342012.
- Blanco JCG, Pletneva LM, Otoa RO, Patel MC, Vogel SN, Boukhvalova MS. Preclinical assessment of safety of maternal vaccination against respiratory syncytial virus (RSV) in cotton rats. Vaccine. 2017 Jul 13;35(32):3951–58. doi:10.1016/j.vaccine.2017.06.009. Epub 2017 Jun 16. PMID: 28624306.
- Blanco JCG, Pletneva LM, Oue RO, Patel MC, Boukhvalova MS. Maternal transfer of RSV immunity in cotton rats vaccinated during pregnancy. Vaccine. 2015 Oct 5;33(41):5371–79. doi:10.1016/j.vaccine.2015.08.071. Epub 2015 Aug 31. PMID: 26335771.
- Nyiro JU, Bukusi E, Mwaengo D, Nyaguara A, Nyawanda B, Otieno N, Bigogo G, Murunga N, Widdowson MA, Verani JR, et al. Efficiency of transplacental transfer of respiratory syncytial virus (RSV) specific antibodies among pregnant women in Kenya. Wellcome Open Res. 2022 Apr 1;7:43. doi:10.12688/wellcomeopenres.17636.2. PMID: 35402734.
- Blanco JC, Richardson JY, Darnell ME, Rowzee A, Pletneva L, Porter DD, Prince GA. Cytokine and chemokine gene expression after primary and secondary respiratory syncytial virus infection in cotton rats. J Infect Dis. 2002 Jun 15;185(12):1780–85. doi:10.1086/340823. Epub 2002 May 31. PMID: 12085325.
- Mejías A, Chávez-Bueno S, Raynor MB, Connolly J, Kiener PA, Jafri HS, Ramilo O. Motavizumab, a neutralizing anti-Respiratory Syncytial Virus (Rsv) monoclonal antibody significantly modifies the local and systemic cytokine responses induced by Rsv in the mouse model. Virol J. 2007 Oct 25;4(1):109. doi:10.1186/1743-422X-4-109. PMID: 17961258.
- Mukherjee S, Lindell DM, Berlin AA, Morris SB, Shanley TP, Hershenson MB, Lukacs NW IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol. 2011 Jul;179(1):248–58. doi:10.1016/j.ajpath.2011.03.003. Epub 2011 May 3. PMID: 21703407.
- Faber TE, Groen H, Welfing M, Jansen KJ, Bont LJ Specific increase in local IL-17 production during recovery from primary RSV bronchiolitis. J Med Virol. 2012 Jul;84(7):1084–88. doi:10.1002/jmv.23291. PMID: 22585726.
- Bennett BL, Garofalo RP, Cron SG, Hosakote YM, Atmar RL, Macias CG, Piedra PA. Immunopathogenesis of respiratory syncytial virus bronchiolitis. J Infect Dis. 2007 May 15;195(10):1532–40. doi:10.1086/515575. Epub 2007 Apr 12. PMID: 17436234.
- Bukreyev A, Whitehead SS, Bukreyeva N, Murphy BR, Collins PL. Interferon gamma expressed by a recombinant respiratory syncytial virus attenuates virus replication in mice without compromising immunogenicity. Proc Natl Acad Sci USA. 1999 Mar 2;96(5):2367–72. doi:10.1073/pnas.96.5.2367. PMID: 10051648.
- Pyle CJ, Uwadiae FI, Swieboda DP, Harker JA, Lopez CB. Early IL-6 signalling promotes IL-27 dependent maturation of regulatory T cells in the lungs and resolution of viral immunopathology. PLoS Pathog. 2017 Sep 27;13(9):e1006640. doi:10.1371/journal.ppat.1006640. PMID: 28953978.
- Besteman SB, Phung E, Raeven HHM, Amatngalim GD, Rumpret M, Crabtree J, Schepp RM, Rodenburg LW, Siemonsma SG, Verleur N, et al. Recurrent respiratory syncytial virus infection in a CD14-Deficient patient. J Infect Dis. 2022 Aug 24;226(2):258–69. doi:10.1093/infdis/jiac114. PMID: 35429403; PMCID: PMC9400420.
- Eichinger KM, Kosanovich JL, Lipp MA, Perkins TN, Petrovsky N, Marshall C, Yondola MA, Empey KM. Maternal immunization with adjuvanted RSV prefusion F protein effectively protects offspring from RSV challenge and alters innate and T cell immunity. Vaccine. 2020 Nov 25;38(50):7885–91. doi:10.1016/j.vaccine.2020.10.065. Epub 2020 Oct 29. PMID: 33129608.
- Orije MRP, Maertens K, Corbière V, Wanlapakorn N, Van Damme P, Leuridan E, Mascart F. The effect of maternal antibodies on the cellular immune response after infant vaccination: a review. Vaccine. 2020 Jan 3;38(1):20–28. doi:10.1016/j.vaccine.2019.10.025. Epub 2019 Oct 28. PMID: 31672332.
