ABSTRACT
Among the potential hazards of HDM immunotherapy (AIT) with HDM allergenic extracts is the possible initiation of de novosensitizations caused by a lack of complementarity between a given HDM vaccine’s content and a patient’s molecular sensitization profile. To investigate whether immunotherapy with HDM extracts affects changes in the profile of sensitizations to allergens contained in the extract and whether neosensitizations occur. Serum samples from patients with HDM allergies (N=63) who received 1 year of treatment with subcutaneous AIT were tested for allergen-specific IgE (sIgE) reactivity to 7 microarrayed HDM allergen molecules (Der p 1, 2,10,11,23; D far 1 and 2) with ImmunoCAP. The HDM non-AIT patients (N=22) who did not receive immunotherapy constituted the study’s control group. The obtained data were analysed at baseline and after 6 and 12 months. In the HDM-AIT group, no neosensitizations after 6 and 12 months of immunotherapy were reported. Conversely, in the HDM non-AIT group, only neosensitizations to Der p 10 were observed. In the study group, sIgE levels against the HDM extract of D. pteronyssinus, D. farinae, rDer p 1, rDer p 2 and Der f 2 decreased after 12 months of AIT (p< .05). SIgE levels against Der f 1, Der p 10, 11 and 23 remained unchanged in the course of 12 months of immunotherapy. In patients with allergic rhinitis with or without concomitant HDM-induced asthma treated with HDM AIT for 12 months, no neosensitizations related to the examined HDM molecules were observed.
Introduction
Allergen immunotherapy (AIT) is the only mechanism-based treatment for IgE-mediated allergic diseases. AIT suppresses the process of allergen-specific inflammation and induces tolerance to specific allergens.Citation1 It is a method of proven short- and long-term efficacy that impedes the progression of milder allergic states into more severe ones. A recent meta-analysis suggested a preventive effect of allergen immunotherapy in asthma development, confirming the association found in previous studies.Citation2 The application of AIT in inhalant allergies is recommended by international medical organizations both for its efficacy and safety.Citation3–7 While AIT is highly appraised, a discussion continues concerning further optimization of both its efficacy and safety, including its immediate and remote effects.
Among the major recommendations for AIT are allergic rhinitis and asthma induced by house dust mites (HDM).Citation4,Citation6,Citation7 HDM is the most frequent perennial allergen responsible for inhalant allergies. Sensitization to HDM and its resultant allergies affect over 500 million people worldwide.Citation8
Among the numerous HDM species, two are essential from the clinical point of view: Dermatophagoides pteronyssinus and Dermatophagoides farinae. Over 90% of HDM allergies result from cosensitization to both coexisting species.Citation8 This model of sensitization lies behind the fact that allergen vaccines for HDM allergic patients usually contain extracts of both these species.
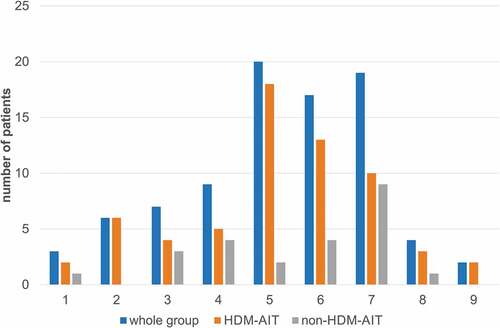
HDM extract contains numerous molecules characterized by high allergenicity. In addition to the major HDM allergens, such as Der p 1, Der p 2 and Der p 23, the extract includes 32 groups of allergens officially registered by the International Union of Immunological Societies (IUIS) Allergen Nomenclature Subcommittee.Citation9,Citation10 Regarding the frequency of their sensitization and their biological properties, the molecules Der p 1, Der p 2 and Der p 23 are of the highest clinical importance.Citation11 They are considered to be major allergens, as each one accounts for the occurrence of sensitization in more than 50% of patients with IgE-dependent HDM allergies. Sensitization to Der p 4, 5, 7, and 21 occurs in less than 40% of patients and to other molecules in less than 10% of patients. Overall, sIgE to Der p 23 occurs more frequently in patients with asthma symptoms than in those with allergic rhinitis. A probable presumption may be made that house dust mite AIT is more effective in patients sensitive to the major extract molecules, but the importance of “broad” vs. “narrow” sensitivity profiles for predicting AIT effects remains to be determined.
The awareness of the variability of allergen composition and content of allergen vaccines has raised a number of questions regarding the practice of AIT. The issue of the efficacy of a vaccine whose content does not match a patient’s individual molecular sensitization profile is one of them. Another one enquires into the possibility of the vaccine inducing “new” sensitizations.
The problem of “new” sensitizations, which might be accounted for using a vaccine that contains allergens to which a given patient has not been sensitized before, remains unresolved. There are studies that suggest the possibility of sensitization to food tropomyosin in the course of HDM AIT.Citation12 Nevertheless, there is no proven clinical evidence that might confirm such claims.Citation13,Citation14 The issue of de novo sensitizations to other HDM molecules, which might undermine the optimal efficacy of AIT, is an ongoing subject of discussion.Citation13–16
The main objective of the present study was to monitor the presumptive variability of molecular sensitization profiles during HDM AIT. Molecular sensitization profiles during the course of a 12-month-long subcutaneous AIT with HDM vaccine based on D. pteronyssinus and D. farinae extract were prospectively assessed. Simultaneously, the temporal development of sensitizations in patients who were not administered HDM AIT was tracked.
Methods
Study design
This is a prospective, observational, real-life, two-center study in which potential changes in the molecular profiles of sensitization to HDMs in adults with moderate to severe symptoms of allergic rhinitis, with or without concurrent asthma caused by HDMs, were analyzed. The patient was included in the study or control group due to a joint decision of the allergist who conducted the treatment and the patient, provided that the patient met all the criteria for participation in the trial.
Patients
All the study patients were sensitized to the natural extract of HDM with clinical relevance as perennial allergic rhinitis with or without asthma; all had positive SPT and positive sIgE levels to HDM extract at concentrations >0.35 kU/L.
The inclusion criteria were as follows: 1. valid indications for and no contraindications against HDM AIT, based on EAACI guidelines, 2. lack of sensitization to other indoor allergens, and 3. HDM non-AIT in the past.
The exclusion criteria were as follows: 1. clinical exacerbation of asthma, uncontrolled asthma, or respiratory infections within 4 weeks prior to study initiation, 2. nasal polyposis, 3. other serious diseases or a chronic unstable disease, and 4. allergy to other inhalant allergens.
Eighty-nine patients were consecutively enrolled in the study based on these criteria. However, 85 patients completed the entire study. Two patients treated with AIT and 2 from the control group did not complete the study (patients discontinued the treatment themselves), and they were not analyzed.
Treatment
Finally, two groups of patients participated in the whole study: the HDM-AIT group (n = 63) that was administered subcutaneous immunotherapy with HDM vaccine Novo Helisen Depot, Allergopharma, Germany (Dermatophagoides pteronyssinus 50% + Dermatophagoides farinae 50%) and the HDM-non AIT group (n = 22) that was not given immunotherapy. SCIT was carried out on a year-round basis according to the manufacturer’s recommendation. The manufacturers claim that the major HDM allergen contents (Dp and Df) antigen Der p 1 and Der f 1 were in the amount of 6.4 μg/mL. Patients received injections weekly at volumes of 0.2, 0.4, and 0.8 mL in vials No. 1 to 2 and 0.1, 0.2, 0.4, 0.6, 0.8, and 1.0 mL in vial No. 3, reaching the maintenance dose of 5000 treatment units (TUs). Then, the maintenance dose was given every 4 weeks. During the 12 months of treatment, patients received a mean cumulative dose of extract in therapeutic units of 64,700 ± 1800 TU. In both study groups, symptomatic treatment was conducted whenever symptoms of allergy occurred and included: antihistamine drugs, corticosteroid nasal sprays, inhaled steroids, beta-2 mimetics, and antileukotriene drugs.
Diagnostic procedures
A careful examination of the eyes, ears, nose, and throat was performed in all the study patients. The severity of perennial allergic rhinitis was assessed using the Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines.Citation7
Asthma diagnosis
Asthma was diagnosed based on the GINA criteria.Citation17 The treatment, daily symptoms, and episodes of asthma exacerbation and coincidences with asthmatic symptoms and HDM natural exposure (heating season) were analyzed. Controlled asthma was confirmed if the ACQ score was below 0.7, and it was checked one week before the patients’ inclusion in the study. All patients had a positive lung function reversibility test, defined as an increase of ≥12% in FEV1 within 10 to 40 minutes, following 4 inhalations of salbutamol aerosol (400 mcg).
sIge assay
Serum-specific IgE levels to D. pteronyssinus, D. farinae, and to rDer p 1, rDer p 2, r Der p 10, rDer p 11, rDer p 23 were determined by Immuno CAP (ThermoFisher Scientific, Uppsala, Sweden) following the manufacturer’s instructions. In both groups, sIgE levels against HDM extract, including D. pteronyssinus, D. farinae and 6 other molecules (Der p 1, 2, 10, 11, 23; at the start of the study and after 6 and 12 months), were assayed. Vaccination was carried out according to EAACI recommendations and the manufacturer’s instructions. Values are expressed in kU/L.
New sensitizations
A new sensitization was defined as an increase in IgE levels against HDM extract or a molecule from <0.15 kU/L to ≥0.15 kU/L. An assessment of the occurrence of new sensitizations was performed in the 6th and 12th months of the study.
The study was approved by the local bioethics committees of the Medical University of the Medical University of Silesia, Poland (KNW-1-131/N/9/K). All patients signed an informed consent form.
Statistical analysis
The data obtained in the course of the study were collected and systematized using the tools of the Excel 2016 spreadsheet. Statistical analysis was performed with Statistica 13.3 PL package (TIBCO Software Inc. 2017 http://statistica.io.). Due to the rejection by the W Shapiro‒Wilk test of the hypothesis about the normality of the distribution of the studied variables, nonparametric tests were used in the analysis. The following tests were used: the Friedman ANOVA test with the post hoc test (Duna test) for dependent variables (time course) and the Mann‒Whitney U test and the Kruskal‒Wallis rank ANOVA test with the multiple comparison test for independent variables (between groups). For the entire statistical study, a borderline p value was adopted to reject the null hypothesis of .05. The analysis of the distributions of variables was performed with the use of multipartition tables in conjunction with the Pearson’s Chi 2 and Chi 2 NW tests that were applied according to the calculated expected numbers.
Results
Demographic data
Demographic data are presented in . The results of the serological tests of the study group are given in .
Table 1. Demographic data of the subjects included in the study – cohort HDM-AIT and HDM non-AIT.
Table 2. Frequency of sensitization to specific HDM molecules in the study group and the control group.
Molecular reactivity patterns of HDM allergic patients and allergen-specific IgE levels at baseline
In both groups, sensitization to Der p 2 (91.7%) was the most frequent, while sensitization to Der p 10 (14.1%) was the rarest. No significant differences occurred with regard to the frequency of sensitizations to specific HDM molecules between the HDM-AIT group and the control group (). Likewise, there were no differences in the number of sensitizations suffered by patients in the study group and the control group ().
IgE reactivity to HDM allergens in patients with HDM allergies at baseline and after 12 months of HDM immunotherapy
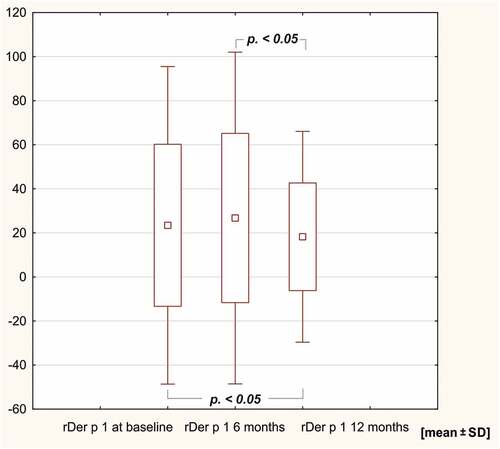
In the study group, sIgE levels against the HDM extract of Dermatophagoides pteronyssinus, rDer p 1, rDer p 2, D far 2 and Dermatophagoides farinae decreased after 12 months of AIT (p < .05). SIgE levels against Der f 1, Der p 10, 11 and 23 remained unchanged in the course of 12 months of immunotherapy ( and ).
Figure 2. sIge levels against the extract of Dermatophagoides pteronyssinus after 6 and 12 months in the HDM AIT study group.
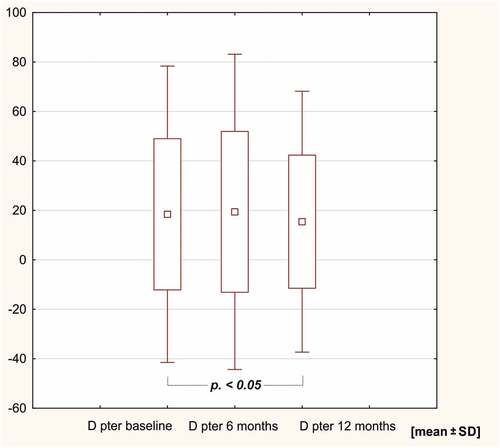
Figure 3. sIge levels against the extract of Dermatophagoides farinae after 6 and 12 months in the HDM AIT study group.
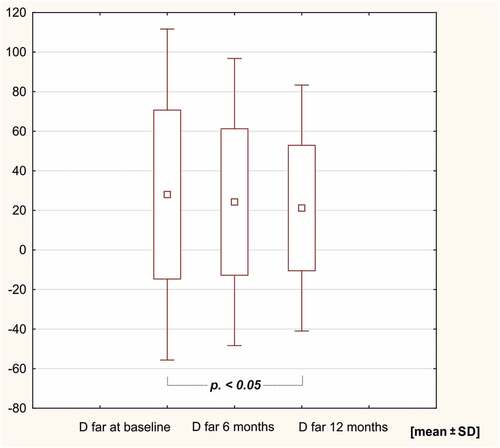
Figure 5. sIge levels against rDer p 2 and rD far 2 after 6 and 12 months of HDM AIT (HDM-AIT study group).
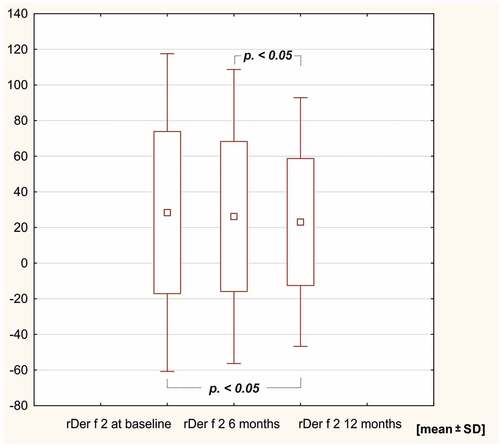
Table 3. sIge levels against HDM extract and specific HDM molecules at baseline, after 6 months and after 12 months of study.
In contrast, during 12 months of observation, patients in the control group did not feature any changes in IgE reactivity against HDM extracts or Der p and Der f molecules.
Neosensitizations during immunotherapy in the HDM-AIT group and the control non-HDM-AIT group
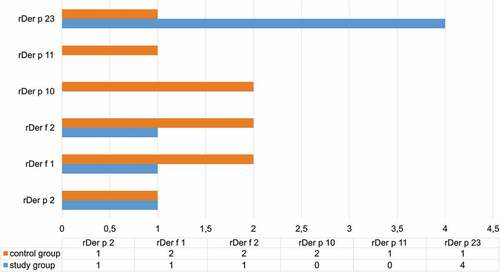
In the HDM-AIT group, neosensitizations were reported during the 12-month observation period, although their prevalence was not higher than in the control, non-AIT group.
However, the non-HDM-AIT control group featured more frequent neosensitizations against Der p 10 i Der f 2 than the HDM-AIT group ().
Discussion
Allergen immunotherapy is the only kind of therapy that modifies the course of allergy, and is thus an immensely valuable instrument in treating allergy. While numerous studies have cast light on its major hazards, i.e., occurrences of systemic allergic reactions and dangerous but infrequent cases of anaphylaxis, our study addresses another risk linked to immunotherapy with allergen extracts, namely, induction of “new sensitizations,” that is, sensitizations to the elements of the allergen extract to which the patient had not developed sIgE antibodies before AIT. Since vaccines used in AIT are manufactured from natural allergen sources, they contain many allergenic elements in unknown quantities, incompatible with the molecular sensitization profiles of patients treated with them. A question then arises whether patients given HDM extract immunotherapy develop “new sensitizations” other than their documented sensitizations from before AIT. Our study indicates that the frequency of the occurrences of “new sensitizations” does not differ between the HDM AIT group and the non-AIT group. By extension, our results do not confirm the thesis according to which “new sensitizations” are induced due to the use of an HDM vaccine whose content is not complementary to individual molecular sensitization profiles. In our opinion, the infrequently observed fluctuations of sIgE levels around the positivity threshold in both investigated groups reflect natural alterations of sIgE levels against particular molecules that result from changing exposition factors and/or individual ontogenetic reactivity.
Our study did not show the occurrence of new sensitizations in the study group of patients with HDM allergic rhinitis, with or without concomitant HDM-induced asthma, who were administered a 12-month-long treatment of AIT with HDM extract, regardless of the fact that most of the patients experienced essential clinical improvement as a result of AIT.
We observed no more sIgE in HDM-AIT patients in relation to “new” HDM particles, namely, molecules to which particular patients had not been sensitized before they were given HDM-AIT.
By extension, our results do not confirm the thesis according to which “new sensitizations” are induced due to the use of an HDM vaccine whose content is not complementary to individual molecular sensitization profiles. In our opinion, the infrequently observed fluctuations of sIgE levels around the positivity threshold in both investigated groups reflect natural alterations of sIgE levels against particular molecules, which result from changing exposition factors and/or individual ontogenetic reactivity.
Similar conclusions were drawn by other authors who investigated the problem of neosensitization in the course of 12 months-long AIT with HDM extract.Citation13–15–Citation18 In agreement with our findings, those authors did not report significant changes in the profiles of molecular sensitizations against major and minor molecules during subcutaneous Citation15,Citation16 and sublinqual Citation13,Citation14 immunotherapy. Running counter to both the abovementioned and our own observations is the paper of Gellrich et al., regarding the development of neosensitizations in the cohort of 51 patients subject to SCIT with different groups of allergens for over 12 months (Fagales (N = 18), Poaceae (N = 19) and HDM (N = 14)), which suggests that such occurrences may take place.Citation19
An objective and decisive comparison between the said studies is made difficult by the fact that in each particular study varied molecules are examined, and the authors adopt varying cutoff points for classifying results as positive or negative (in our case 0.15 kU/L).
The cutoff value for most immunological tests used to assess sIgE in serum is traditionally assumed to be 0.35 kU/L, although new diagnostic tests detect much lower sIgE concentrations, with the detection limit set at 0.1 kU/L. Clinical pertinence of low IgE concentrations (0.1–0.35 kU/L) is not decisively proven, except for, among others, cases of anaphylaxis to Hymenoptera venom in patients with mastocytosis in whom also low detected values of sIgE (<0.35 kUA/L) are clinically meaningful.Citation20 In our study, we adopted a lower cutoff point to broaden the range of sIgE levels that might potentially mark neosensitizations while disregarding their clinical relevance.
Justified criticism may be accepted regarding the lack of sensitizations to some of the minor molecules, such as Der p 10 and 11, which might result from their low/insufficient representation in the allergen extract used in AIT.Citation21 However, such a critical argument does not apply to molecules that are standardized in commercially available allergen vaccines, such as Der p1 and Der p 2.
Theoretically, it is also possible that the immunogenicity of minor HDM molecules in the extract, unlike the immunogenicity of the major molecules Der p 2 and Der p 1, is very low, and hence they do not stimulate new sensitizations. There are authors who adopted this way of thinking, interpreting the lack of increase of IgG4 against Der p 5, 7, 21, while noting a significant increase in IgG4 against Der p 1 and 2 during HDM SCIT.Citation15,Citation22 However, the thesis is challenged by Potapova et al., who reported a significant rise in IgG and IgG4 against Der p 1, 2, 23 as well as Der p 4, 15, 18 and 37 during HDM SLIT.Citation14 Finally, it might be possible that neosensitizations in the course of AIT develop over a period of time longer than 12 months, which was raised by Gellich et al.Citation19 This possibility was considered, and the authors of the present study continued observation of the study group. However, this investigation was conducted with unmatched stored sera in which the controls were not necessarily selected by the same criteria.
Overall, the baseline reactivity of sIgE as well as the molecular sensitization profile regarding the major and minor HDM allergens examined in our study were coincident with the profiles analyzed in other studies.Citation14 Similar to other authors, we determined dominant sensitizations to three molecules Der p 2, Der p 1 and Der p 23, whereby sensitizations to the last one were in the lower range of the frequency of such occurrences, which may be accounted for by geographical specificity of synchronization to Der p 23 and/or by low numbers of patients with allergic rhinitis and concomitant asthma in whom sensitization to Der p 23 is more frequent.Citation11
In the course of HDM AIT conducted in our study, after 12 months of observation, changes in sIgE reactivity were noticed, including a decrease in sIgE levels against HDM extract and against molecules rDer p 1 and 2. A similar dynamic of the changes in sIgE levels after a year of AIT has been reported by other authors []. Potapova et al. noted the same effect in a group of patients who were administered HDM SLIT.Citation14 In her study, concentrations of sIgE against most molecules decreased after 12 months of AIT. The author postulates that this phenomenon may result from an increase in IgG4 reactivity and the resultant competitive binding of IgE via IgG. In turn, in the group of patients who were not administered HDM AIT, an increase in sIgE levels for some molecules was noticed.Citation14,Citation23 This might be explained by natural changes in the molecular sensitization profiles under the influence of environmental exposure as well as by the fact that sIgE concentrations were assessed as positive/negative for a lower cutoff point adopted at 0,15 kU/L.
In our opinion, the present study has advantages, although it is not free from a few limitations. On the upside, it involves a prospective assessment of parallel groups, an analysis of sIgE reactivity against major and minor HDM allergens and is based on a substantial study cohort.
As far as its limitations are concerned, a much longer time perspective would be greatly favorable, and so would be a broader panel of minor HDM molecules. Finally, the study does not provide answers regarding the clinical pertinence of sIgE alterations.
Conclusions
In a group of patients with allergic rhinitis and with or without concomitant HDM-induced asthma treated with HDM AIT for 12 months, we did not observe neosensitizations related to the examined HDM molecules.
Abbreviations
| AIT | = | allergen immunotherapy |
| Der p | = | Dermatophagoides pteronyssinus |
| Der f | = | Dermatophagoides farinae |
| rDer p | = | recombinant Dermatophagoides pteronyssinus |
| SCIT | = | subcutaneous immunotherapy |
| HDM | = | house dust mite |
| sIgE | = | allergen |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Dhami S, Nurmatov U, Arasi S, Khan T, Asaria M, Zaman H, Agarwal A, Netuveli G, Roberts G, Pfaar O, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72(11):1597–8.
- Farraia M, Paciência I, Castro Mendes F, Cavaleiro Rufo J, Shamji M, Agache I, Moreira A. Allergen immunotherapy for asthma prevention: a systematic review and meta-analysis of randomized and non-randomized controlled studies. Allergy. 2022;77:1719–35.
- Shamji MH, Kappen JH, Akdis M, Jensen-Jarolim E, Knol EF, Kleine-Tebbe J, Bohle B, Chaker AM, Till SJ, Valenta R, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI position paper. Allergy. 2017;72:1156–73.
- Roberts G, Pfaar O, Akdis CA, Pfaar OEA, Ansotegui IJ, Durham SR, Gerth van Wijk R, Halken S, Larenas-Linnemann D, Pawankar R, et al. EAACI guidelines on allergen immunotherapy. Allergic Rhinoconjunctivitis Allergy. 2018;73:765–98.
- Pfaar O, Bousquet J, Durham SR, Jörg Kleine-Tebbe J, Larché M, Roberts G, Shamji MH, Gerth van Wijk R. One hundred and ten years of allergen immunotherapy: a journey from empiric observation to evidence. Allergy. 2021;77:454–68.
- Agache I, Lau S, Akdis CA, Smolinska S, Bonini M, Cavkaytar O, Flood B, Gajdanowicz P, Izuhara K, Kalayci O, et al. EAACI guidelines on allergen immunotherapy: house dust mite- driven allergic asthma. Allergy. 2019;74:855–73.
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, Van Weel C, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63:8–160.
- Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010;16:321–28.
- WHO/IUIS Allergen Nomenclature Sub-Committee. Allergen nomenclature; [accessed 2021 Oct 1]. http://www.allergen.org.
- King TP, Hoffman D, Lowenstein H, Marsh DG, Platts-Mills TA, Thomas W. Allergen nomenclature. Allergy. 1995;50:765–74.
- Weghofer M, Thomas WR, Kronqvist M, Mari A, Purohit A, Pauli G, Horak F, Grönlund H, van Hage M, Valenta R, et al. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest. 2008;38(12):959–65.
- Zhao L, Zhang Y, Zhang S, Zhang L, Lan F. The effect of immunotherapy on cross-reactivity between house dust mite and other allergens in house dust mite -sensitized patients with allergic rhinitis. Expert Rev Clin Immunol. 2021 Sep;17(9):969–75.
- Baron-Bodo V, Batard T, Nguyen H, Fréreux M, Horiot S, Harwanegg C, Bergmann KC, de Beaumont O, Moingeon P. Absence of IgE neosensitization in house dust mite allergic patients following sublingual immunotherapy. Clin Exp Allergy. 2012;42:1510–18.
- Potapova E, Bordas- Le Floch V, Vrtala T, Huang S, Canonica H-J, Valenta GW, Matricardi R, Mascarell PM, Schlederer L. Molecular reactivity profiling upon immunotherapy with a 300 IR sublingual house dust mite tablet reveals marked humoral changes towards major allergens. Allergy. 2022;10:1–12.
- Chen KW, Zieglmayer P, Zieglmayer R, Lemell P, Horak F, Bunu CP, Valenta R, Vrtala S. Selection of house dust mite-allergic patients by molecular diagnosis may enhance success of specific immunotherapy. J Allergy Clin Immunol. 2019;143:e1212.
- Rodríguez-Domínguez A, Berings M, Rohrbach A, Huang H-J, Curin M, Gevaert P, Matricardi PM, Valenta R, Vrtala S. Molecular profiling of allergen-specific antibody responses may enhance success of specific immunotherapy. J Allergy Clin Immunol. 2020;146:1097–108.
- The 2022 update of the Global Strategy for Asthma Management and Prevention; 2022. [ accessed 2022 Jul]. www.ginasthma.org/gina-reports/.
- Lupinek C, Marth K, Niederberger V, Valenta R. Analysis of serum IgE reactivity profiles with microarrayed allergens indicates absence of de novo IgE sensitizations in adults. J Allergy Clin Immunol. 2012;130:1418–20 e1414.
- Gellrich D, Eder K, Högerle C, Becker S, Canis M, Gröger M. De Novo sensitization during subcutaneous allergen specific immunotherapy - an analysis of 51 cases of SCIT and 33 symptomatically treated controls. Sci Rep. 2020;10:5048.
- Sturm GJ, Varga EM, Roberts G, Becker S, Canis M, Gröger M. EAACI guidelines on allergen immunotherapy: hymenoptera venom allergy. Allergy. 2018 Apr;73(4):744–64.
- Casset A, Mari A, Purohit A, Resch Y, Weghofer M, Ferrara R, Thomas WR, Alessandri C, Chen K-W, de Blay F, et al. Varying allergen composition and content affects the in vivo allergenic activity of commercial Dermatophagoides pteronyssinus extracts. Int Arch Allergy Immunol. 2012;159:253–62.
- Caraballo L, Valenta R, Acevedo N, Zakzuk J. Are the terms major and minor allergens useful for precision allergology? Front Immunol. 2021;12:651500.
- Niederberger, V, Neubauer, A, Gevaert, P, Zidarn, M, Worm, M, and Aberer, M. 2018. Safety and efficacy of immunotherapy with the recombinant B cell epitope-based grass pollen vaccine, BM32. J Allergy Clin Immunol. 142: 497–509.