ABSTRACT
Pemphigus is a rare autoimmune disease and has the potential to be fatal without treatment. Pemphigus erythematosus (PE) is a benign type of pemphigus foliaceus. Glucocorticoids and immunosuppressive agents are primary therapeutic modalities in pemphigus erythematosus, which may lead to considerable side effects. There is a growing need for new pemphigus therapies with fewer adverse effects. Dupilumab is a humanized monoclonal IgG4 antibody that inhibits the signaling of interleukin-4 (IL-4) and interleukin-13 (IL-13) and has been applied for atopic dermatitis and asthma. Recently, dupilumab was thought to be beneficial in aggressive refractory pemphigus vulgaris. We report two cases: a 39-year-old male and a 59-year-old woman diagnosed with PE with atypical clinical features. With dupilumab, patients’ skin lesions significantly improved, and suitable maintenance glucocorticosteroid doses were reached. In conclusion, we reported the short-term effectiveness and safety of dupilumab in two cases of atypical generalized PE. As an adjunct, such a biologic agent is expected to be efficacious in pemphigus erythematosus.
Introduction
Pemphigus is a group of rare and severe autoimmune diseases clinically characterized by widespread erosion and blistering of the skin and mucous membranes.Citation1 It is caused by pathogenic autoantibodies that attack two desmosomal adhesion proteins: desmoglein 3 (Dsg 3) and desmoglein 1 (Dsg 1). Depending on the clinical features, pemphigus can be categorized into four subtypes: vulgaris, vegetans, foliaceus, and erythematosus. Pemphigus vulgaris (PV) is the most common and severe clinical variation.Citation2 A benign variation of pemphigus foliaceus is known as pemphigus erythematosus (PE). PE mainly involves photodistributed areas such as the face, scalp, back, and seborrheic areas of the upper trunk, which resemble cutaneous lupus erythematosus.Citation1 Systemic glucocorticoids, with or without immunosuppressive medications, are used to treat severe PE. However, some patients may inadequately respond to conventional therapy, and others can not afford long-term use of these drugs due to their severe side effects, such as the high risk of infections. Therefore, there is still a need for novel pemphigus treatment. Targeting interleukin-4 (IL-4) and interleukin-13 (IL-13) has been shown to be beneficial in pemphigus.Citation3
Dupilumab is a biologic agent targeting the alpha subunit of interleukin‐4 receptor (IL‐4 Rα) fortreating atopic dermatitis and asthma. Atthe moment, two undergoing clinical trials (NCT04776694 and NCT04206553) are being conducted to evaluate the effect of dupilumab on bullous pemphigoid.Citation4,Citation5 This report described two PE patients whose symptoms were relieved after dupilumab was added to conventional therapy.
Patient presentation
Case 1
A 39-year-old man had extensive blistering and ulceration on his face, trunk, abdomen, and extremities. Physical examination indicated blistering skin lesions all over the body with positive Nikolsky’s symptoms (). He was diagnosed with PE based on typical clinical features, histopathological findings, and direct immunofluorescence (). The blood eosinophil count and the serum IgE level were within the normal range.
Figure 1. The clinical photographs, Pathological sections, and direct immunofluorescence of patients in Case 1. (a) the clinical photograph taken on admission to the hospital, three weeks after admission, and seven month follow-up (left to right). (b) Histopathological image of the lesions: intraepithelial cleavage with detached keratinocytes primarily localized just under the stratum corneum. (c) Intercellular deposition of immunoglobulin G (IgG) and complement component (C3) by direct immunofluorescence microscopy: spinosum intercellular deposition of complement IgG and C3.
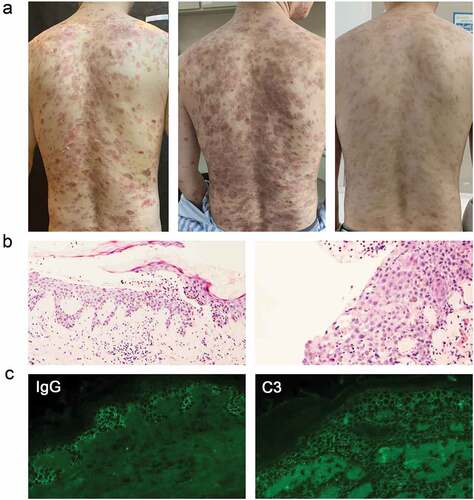
He was given 40 mg of methylprednisolone daily, but it was ineffective, along with intense itching and worsened condition. In addition to having a sleep disorder, the patient’s blood IgE level was rising (329 IU/ml; normal range <165 IU/ml). He refused to increase glucocorticoid dosage. Thus, dupilumab (600 mg subcutaneously once a week) was added to his treatment regimen after being informed about the drug’s mechanism and potential risks.
After three weeks of this combined therapy, the symptoms subsided with a relief of pruritus. Pemphigus disease area index (PDAI) total activity scores decreased from 41 to 0, with blood IgE levels decreased to 234 IU/ml. The patient was discharged and continued a long-term maintenance regimen of dupilumab and low-dose methylprednisolone. After ten months of observation, the patient’s condition was managed with low-dose glucocorticoid (methylprednisolone 4 mg/day) and maintained dupilumab therapy (300 mg every four weeks).
Case 2
A 59-year-old woman presented with widespread erosions affecting her face, trunk, abdomen, and limbs without mucosal involvement for more than ten years (). She was diagnosed with pemphigus and treated with 12-16 mg methylprednisolone daily. However, she suffered from severe osteoporosis which was possibly a side effect of glucocorticoid. To decrease the glucocorticoid dosage, adjuvant drugs (doxycycline, mycophenolate mofetil, and methotrexate) were added, but her condition had progressed rapidly, which leaving her face, trunk, abdomen, and extremities covered in erosions. Typical clinical symptoms and pathological results all contributed to the diagnosis of PE (). With her informed consent, a moderate dose glucocorticoid (methylprednisolone 16 mg daily) and dupilumab (600 mg subcutaneously initially and 300 mg subcutaneously every week) were administered.
Figure 2. The clinical photographs, Pathological sections, and direct immunofluorescence of patients in Case 2. (a) the clinical photograph taken on admission to the hospital, four weeks after admission, seven weeks after admission, and five month follow-up (left to right). (b) Histopathological image of the lesions: subcorneal split directly below the stratum corneum. (c) Intercellular deposition of immunoglobulin G (IgG) and complement component (C3) by direct immunofluorescence microscopy: spinosum intercellular deposition of complement IgG and C3.
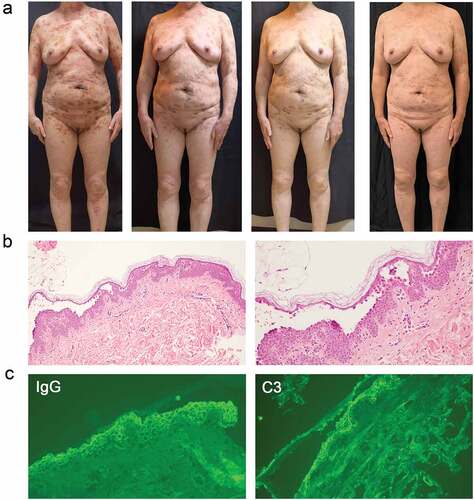
After receiving this combined treatment for four weeks, the symptom subsided. The PDAI total activity scores dropped from 50 to 6 when she was discharged. The patient has been followed up for five months. Her condition had under reasonable control, with 8 mg methylprednisolone daily and 300 mg dupilumab every two weeks.
Discussion
Pemphigus is a group of severe chronic autoimmune blistering diseases. Pemphigus erythematosus is a form of pemphigus foliaceus that typically develops in seborrheic areas. Although these two patients had extensive blisters and erythematous erosions, their pathological results supported the diagnosis of PE. Thus, the two patients were diagnosed with generalized and atypical pemphigus erythematosus.
The most crucial element in treating pemphigus is systemic glucocorticoids with or without immunosuppressants.Citation6 Some patients cannot tolerate the usage of glucocorticoids for a prolonged period due to their severe side effects. Intravenous infusion of gamma globulin, plasmapheresis, and rituximab could be selected as adjuvant medications. Currently, more research is needed to explore effective and safe therapies.
The T helper 2 (Th2) pathway is crucial in pemphigus. The production of anti-Dsg IgG and illness severity linked to Th2 activity. IgG4 and IgE subtypes make up the majority of pathogenic autoantibodies. Dsg 3 specific IgG4 and IgE correlate with active disease potently stimulated by IL-4 and IL-13.Citation7–9 IL-4 induces Th2 cell proliferation, promotes isotype shift to IgG4 and IgE, and stimulates eosinophils, mast cell proliferation, and degranulation. The potential benefits of IL-4 and IL-13 targeting in pemphigus have already been demonstrated.Citation3,Citation9,Citation10 In one case report, a patient with severe PV and tuberculosis was treated with dupilumab in combination therapy.Citation10
Dupilumab targets both IL-4 and IL-13, which can effectively manage pruritus. It has been used to treat atopic dermatitis, starting with a subcutaneous dose of 600 mg and then 300 mg every two weeks.Citation9 Weekly treatment may be necessaryfor eosinophilic esophagitis and bullous pemphigoid. In our cases, dupilumab was administered to the patients in two different doses, 600 mg for the initial and 300 mg every two weeks or four weeks, depending on the severity of their conditions.
In the first case, the patient’s condition was effectively managed by low-dose glucocorticoid (methylprednisolone 4 mg/day) and dupilumab (300 mg every four weeks) after ten months follow-up, without any prominent side effects. The second patient’s condition was well controlled owing to 8 mg glucocorticoid daily and 300 mg dupilumab every two weeks after five month of follow-up. Due to these cases, dupilumab might be helpful in collaboration with glucocorticoids to treat PE and control the progression of the disease.
Neither patient experienced any notable adverse effects. The patient was willing to utilize dupilumab despite the relatively high cost, considering its safety and efficacy. In order to verify this standpoint, more instances and clinical practice are still required.
Author contributions
All authors included in the study contributed to the research on the topic, manuscript writing, and patient management. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Ethics statement
The studies involving human participants were reviewed and approved by the Sir Run-Run Shaw Hospital Ethical Committee. The patients provided their written informed consent to participate in this study. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet. 2019;394(10201):882–4. doi:10.1016/S0140-6736(19)31778-7.
- Egami S, Yamagami J, Amagai M. Autoimmune bullous skin diseases, pemphigus and pemphigoid. J Allergy Clin Immunol. 2020;145(4):1031–47. doi:10.1016/j.jaci.2020.02.013.
- Tavakolpour S, Tavakolpour V. Interleukin 4 inhibition as a potential therapeutic in pemphigus. Cytokine. 2016;77:189–95. doi:10.1016/j.cyto.2015.09.017.
- Abdat R, Waldman RA, de Bedout V, Czernik A, Mcleod M, King B, Gordon S, Ahmed R, Nichols A, Rothe M, et al. Dupilumab as a novel therapy for bullous pemphigoid: a multicenter case series. J Am Acad Dermatol. 2020;83(1):46–52. doi:10.1016/j.jaad.2020.01.089.
- Kaye A, Gordon SC, Deverapalli SC, Her MJ, Rosmarin D. Dupilumab for the treatment of Recalcitrant Bullous Pemphigoid. JAMA Dermatol. 2018;154(10):1225–26. doi:10.1001/jamadermatol.2018.2526.
- Harman KE, Brown D, Exton LS, Groves RW, Hampton PJ, Mohd MM, Setterfield JF, Yesudian PD, McHenry PM, Gibbon K, et al. British Association of Dermatologists’ guidelines for the management of pemphigus vulgaris 2017. Br J Dermatol. 2017;177(5):1170–201. doi:10.1111/bjd.15930.
- Funakoshi T, Lunardon L, Ellebrecht CT, Nagler AR, O’Leary CE, Payne AS. Enrichment of total serum IgG4 in patients with pemphigus. Br J Dermatol. 2012;167(6):1245–53. doi:10.1111/j.1365-2133.2012.11144.x.
- Nagel A, Lang A, Engel D, Podstawa E, Hunzelmann N, de Pita O, Borradori L, Uter W, Hertl M. Clinical activity of pemphigus vulgaris relates to IgE autoantibodies against desmoglein 3. Clin Immunol. 2010;134(3):320–30. doi:10.1016/j.clim.2009.11.006.
- Gudjonsson JE, Kabashima K, Eyerich K. Mechanisms of skin autoimmunity: cellular and soluble immune components of the skin. J Allergy Clin Immunol. 2020;146(1):8–16. doi:10.1016/j.jaci.2020.05.009.
- Chen S, Zhan S, Hua C, Tang Y, Cheng H. A novel combined use of dupilumab for treatment of aggressive refractory pemphigus vulgaris complicated with pulmonary tuberculosis: a case report and the RNA-seq analysis. Front Immunol. 2022;13:825796. doi:10.3389/fimmu.2022.825796.
