ABSTRACT
Bacterial antibiotic resistance is a public health issue. It means that drugs become ineffective, infections persist and have a huge impact on the health of patients and their spreading increases. To address a complex threat such as bacterial antibiotic resistance different and integrated approaches are needed including discovery of new antibiotics, improvement of diagnostics tools and improvement of antibiotic stewardship. Absolutely relevant are prevention of infections as well as decrease in the use of antibiotics. Vaccines are an important tool in the fight against bacterial antibiotic resistance and can help prevent it in several ways. Indeed, vaccines are highly effective in preventing diseases that might otherwise require the use of antibiotics to treat symptoms and associated complications. Preventing infections through vaccination helps reduce the need for and widespread and inappropriate use of antibiotics, including for secondary bacterial infections.
Background
Antimicrobial resistance (AMR) occurs when bacteria, fungi, viruses and parasites become resistant to antimicrobial drugs and become “super microorganisms” (superbugs).
Bacterial antibiotic resistance, as well as AMR, implies that drugs are ineffective, infections persist and have a huge impact on the health of patients with a significant increase in their morbidity.Citation1
The World Health Organization (WHO) has recently reiterated that antibiotic resistance represents a significant global threat and that without timely intervention, common diseases will become no longer curable. Bacterial antibiotic resistance will also lead to a dramatic increase in health costs, impacting on food supply and livelihoods and contributing to increasing levels of poverty and inequality.Citation2
The most recent data estimate that around 5 million antibiotic resistance-related deaths occurred in 2019, including 1.27 million deaths attributable to antibiotic-resistant bacteria. Globally, lower respiratory tract infections were the most frequent among those associated with antibiotic resistance (with over 1.5 million deaths); the impact of bacterial antibiotic resistance was highest in western sub-Saharan Africa. The six major pathogens bringing resistance traits to antibiotics are Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. Methicillin-resistant Staphylococcus aureus caused over 100,000 deaths in 2019.Citation3
These data therefore seem to confirm what was previously observed. In particular, the Organization for Economic Cooperation and Development (OECD-OECD) reported that in 2017 about 17% of bacterial infections in OECD countries were resistant to antibiotics. The OECD estimated that bacterial antibiotic resistance could have caused an estimated 2.4 million deaths in Europe, North America and Australia over the period 2015–2020 and that, over the same period, it would have a huge economic impact on health services in these countries. Noteworthy, nearly 75% of deaths due to antibiotic-resistant superbugs could be avoided at low cost (2USD person/year) by investing in simple measures such as hand washing and a more prudent prescription of antibiotics.Citation4
It is estimated that 10 million deaths due to multi-drug resistant bacteria will occur every year after 2050; this is equivalent to the number of people currently dying from cancer each year.Citation5
An impact assessment of bacterial antibiotic resistance was recently conducted in Europe, and it was shown that there were 671,689 infections with antibiotic-resistant bacteria in 2015, corresponding to an incidence of 131 infections per 100,000 population and an attributable mortality of 6.44 deaths per 100,000 inhabitants. The overall burden of disease is usually assessed using the disability-adjusted life year (DALY); this time-based measure combines years of life lost due to premature mortality (YLLs) and years of life lost due to time lived in states of less than full health (or years of healthy life lost due to disability: YLDs). The overall DALY rate of all types of infections with antibiotic-resistant bacteria was 170 per 100,000 inhabitants, similar to the overall impact of HIV, influenza, and tuberculosis in the same year in Europe. The impact was higher in infants (<1 year of age) and in people >65 years of age, increased compared to what was recorded in 2007 and particularly high in Italy and Greece.Citation6
The latest report published by the European Center for Disease Prevention and Control (ECDC) confirms that bacterial antibiotic resistance is a serious challenge. EARS-Net (European antimicrobial resistance surveillance system) data estimate that more than 670,000 infections occur each year due to antibiotic-resistant bacteria and that about 33,000 people die as a direct consequence of these infections; the economic impact for European health systems can be quantified at around 1.1 billion euros. ECDC concludes that public health action to tackle antibiotic resistance remains insufficient and that further investment in public health interventions is urgently needed to address this issue. It has been estimated that antibiotic management programs, increased hygiene, media campaigns and the use of rapid diagnostic tests have the potential to prevent approximately 27,000 deaths each year in Europe and would save around € 1.4 billion annually.Citation7
Bacterial antibiotic resistance is a complex topic to address () as it recognizes various causes including the increased use of antibiotics (including their inappropriate use) in both human and veterinary medicine, the use of antibiotics in animal husbandry and agriculture, the spread of health care-related infections caused by antibiotic-resistant microorganisms (and limited control of these infections), increased morbidity of resistant strains due to increased travel and international travel.Citation8
Figure 1. Causes and routes of transmission of antibiotic resistant bacteria (modified fromCitation8).
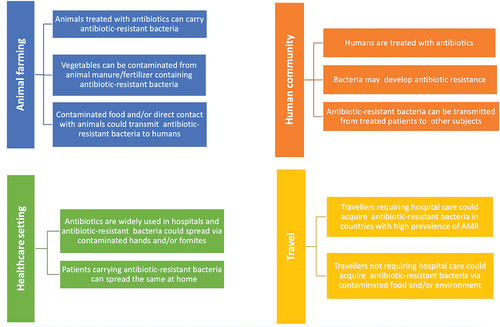
In 2017, the European Union (EU) developed the new Action Plan to combat antibiotic resistance, based on a “One Health” approach that considers the health of humans, animals, and the environment in an integrated way. “One Health” is an approach to the design and implementation of programs, policies, legislation, and research in which multiple sectors communicate and work together to achieve better public health outcomes. Food safety, zoonosis control, and the fight against antibiotic resistance are areas of work where a One Health approach is particularly relevant.Citation9
The development of new preventive vaccines was and is part of this specific area.Citation10 This document highlights that vaccines have proven to be fundamental (and also beneficial from a pharmacoeconomic perspective) in preventing the onset and spread of infectious diseases. Vaccines also have considerable potential in terms of reducing the incidence of bacterial antibiotic resistance.
Role of immunization in the fight against bacterial antibiotic resistanc
A “vaccine” is defined as a preparation containing live and attenuated or killed microorganisms, or parts of them or their modified products, capable of stimulating an active response by the immune system. The goal of immunization is to make the vaccinated person no longer susceptible to sickness in case of contact with the microorganism. The extensive application of vaccines has made it possible to control, and in some cases eliminate or eradicate, many infectious diseases that significantly impacted public health both in terms of morbidity and lethality.Citation11
Vaccines are important tools in the fight against bacterial antibiotic resistance and can help prevent it in several ways. Indeed, established vaccines are highly effective in preventing diseases that might otherwise require the use of antibiotics (and/or other antimicrobial agents) to treat symptoms and associated complications. Preventing infections through vaccination helps reduce the need for and widespread and inappropriate use of antibiotics, including for secondary bacterial infections. For example, conjugate vaccines for Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae not only prevent life-threatening diseases, such as pneumonia, meningitis, and sepsis, especially in young children, but also reduce the need for antibiotics for treatment and hence the potential for the development of antibiotic-resistant strains of Hib and other bacteria.
The consumption of antibiotics is a key factor in resistance to them. Antibiotics are often inappropriately prescribed for viral infections, such as colds and flu, so vaccines against these viral infections also reduce the use of antibiotics and reduce secondary infections that may also be resistant to antibiotics.Citation12
The WHO Immunization Agenda 2030 highlights that vaccination can contribute to the control of bacterial antibiotic resistance. In particular, it identifies some priority actions that include increasing the use of available vaccines to maximize the impact on bacterial antibiotic resistance, the development of new vaccines that can contribute to the prevention and control of bacterial antibiotic resistance and the expansion and sharing of related knowledge on the impact of vaccines on this issue. Obviously, the fight against bacterial antibiotic resistance cannot be based exclusively on immunization, but requires the optimization of preventive aspects, antibiotic stewardship as well as the search for new therapeutic options.
Vaccines in the fight against bacterial antibiotic resistance
In this context, it is necessary to consider the direct impact of vaccines, the herd immunity induced by them and the lack of availability of effective antibiotics. As for the direct impact, the use of vaccines implies a reduction in the incidence of infections, a reduced use of antibiotics and therefore fewer opportunities to have AMR. Herd immunity induced by vaccines leads to the reduction of infections even in non-vaccinated subjects and therefore also a reduced use of antibiotics.
Finally, the development of new vaccines can be the operational response to the unavailability of effective antibiotics for a specific pathogen.Citation13
In relation to the characteristics of the different pathogens, the potential impact of vaccines on AMR results from one or more of the following factors:
reduction of the individual risk of infection of vaccinated subjects
prevention of transmission
decrease in bacterial carriage
decrease in the size of the pathogen population in the host
decrease in the incidence of vaccine-preventable diseases
reduction of the need for assistance/treatment and therefore of the possible exposure to resistant pathogens.Citation14
The potential of vaccination as a tool against antibiotic resistance has long been recognized and is receiving renewed attention. Reductions in antibiotic use and bacterial resistance have been observed after the introduction of vaccines against Streptococcus pneumoniae and Haemophilus influenzae type b and numerous research has highlighted the role of vaccines as a key measure to reduce the demand/use of antibiotics and thus to combat bacterial resistance.Citation15
By limiting the evaluation to vaccines used in humans, the reduction of bacterial antibiotic resistance by immunization can be achieved by exploiting one or more of the following mechanisms.Citation16
The first of these is the prevention of infections caused by resistant pathogens; in this way, the direct and indirect effect of vaccination is exploited (for example conjugated Hib vaccine).
A second option is called the “bystander effect.” Based on this effect, any vaccine involving changes in antibiotic use could potentially impact bacterial antibiotic resistance in unvaccinated people due to reduced selection pressure.
Besides, a vaccine could have effects related to the severity of infections. Vaccines that reduce the risk of symptomatic infection without reducing the risk of carriage/asymptomatic infection can lead to reductions in the proportion of infections treated with antibiotics and therefore a reduction in selective pressure for resistant phenotypes. This phenomenon has been observed in the case of malaria; in high prevalence areas, the level of immunity in the population is particularly high, there are more asymptomatic infections, these are not identified and treated and therefore there is a lower selection of drug resistance.
Another possible effect is related to the selection of serotypes. This selection occurs when a pathogen population is composed of multiple competing subtypes and the used vaccine only acts on a subset of these. If the subtypes included in the vaccine are more likely to be resistant, overall resistance may decrease; this phenomenon has been observed with the use of the pneumococcal conjugate vaccine.Citation17
Interspecific effects are possible as well. Bacteria and viruses interact in complex ways, and some viral infections are known to correlate with an increased risk of bacterial superinfections (e.g.: influenza and secondary bacterial pneumonia). Vaccination against one pathogen could therefore reduce the transmission of another, leading to a decrease in both resistant and sensitive phenotypes. Finally, the possible selective targeting effect should be considered. In this case, if a resistant strain of a given pathogen is transmitted preferentially in the health sector (for example in hospitals where the use of antibiotics is high), while a sensitive strain is transmitted better in the community, acting on the hospital population with a vaccine would result in an increased overall effect on the resistant strain, leading to a decline in resistance both in the hospital and in the community.
Other intervention options to combat bacterial antibiotic resistance by vaccination are also currently being explored. In particular, the potential of the technologies available to produce new generation vaccines against antibiotic-resistant pathogens such as reverse vaccinology, structural vaccinology, generalized modules for membrane antigens (GMMA), bioconjugation and use of adjuvants are being evaluated.Citation18
Impact of some vaccines on bacterial antibiotic resistance
Bacterial vaccines
Haemophilus influenzae type b (Hib)
As previously anticipated, disease prevention by vaccination reduces both the use of antibiotics and antibiotic resistance. In this sense, the Haemophilus influenzae type b (Hib) conjugate vaccine has been a success because its extensive use has made it possible to reduce the use of antibiotics and bacterial antibiotic resistance. Prior to the introduction of effective conjugate vaccines, Hib disease in children <5 years of age ranged from 3.5 to 601 per 100,000 in many countries around the world. A steady increase in resistance of Hib to beta-lactams has been observed since the early 1970s, mediated by expression of beta-lactamase and/or modified penicillin-binding proteins. A global surveillance study in 1999–2000 found that 16.6% of all Hib strains in the world were beta-lactamase positive with wide variations between countries. This picture changed dramatically after the introduction of the Hib conjugate vaccine. Cases of the disease significantly decreased after the introduction of routine use of conjugated Hib vaccines also leading to a significant decrease in beta-lactamase positive strains.Citation15
In the USA, since the introduction of the polysaccharide vaccine and conjugate vaccines in 1985 and 1990, the incidence of invasive Hib disease in children <5 years of age has decreased by 99%, resulting in <1 case/100,000. Surveillance of invasive H. influenzae type b disease has shown low rates of invasive disease in children <5 years of age; between 2010 and 2017, the average incidence was 0.15 per 100,000. In the post-vaccine era, the epidemiology of invasive H. influenzae type b disease in the United States has changed and the majority of invasive disease in all age groups is now caused by untypable H. influenzae.Citation19
The most up-to-date data indicate that >90% of countries worldwide have implemented Hib conjugate vaccination in their national immunization programs and, although the kinetics of the immune response vary with the type of Hib vaccine and program used, a high control of Hib disease was observed in all contexts. Elimination of the disease can be achieved with a further global increase in vaccination coverage.Citation20
These assessments are confirmed in some recently published studies referring to the impact of vaccination in countries such as Portugal and Ireland or in a review on the molecular epidemiology of Hib.Citation21-23
Streptococcus pneumoniae
The introduction of conjugate vaccines to prevent pneumococcal disease has significantly changed the epidemiology of this important disease. Data from the USA show that in the 1990s, before the introduction of the 7-valent pneumococcal conjugate vaccine (PCV7), about 63,000 cases of invasive pneumococcal disease caused by both vaccine and non-vaccine serotypes were recorded. An increase in S. pneumoniae resistant to 3 or more drug classes was also recorded in the pre-vaccination period. Similarly to what observed with the use of conjugated Hib vaccines, the pneumococcal polysaccharide conjugated vaccines were found to be highly effective against invasive pneumococcal disease (IPD) in the target population of the vaccine intervention (children <5 years). There was not only a decline in the incidence of cases but also a significant reduction in bacterial colonization in US children with the onset of herd immunity in adults as well. Switching with polyvalent conjugate vaccines (e.g., PCV13) has further increased the direct and indirect effect of vaccination. The introduction of vaccination with conjugate vaccines has correlated with a reduction in bacterial antibiotic resistance. In the USA, between 2009 and 2013, rates of non-susceptible IPD to antibiotics caused by serotypes included in PCV13, but not PCV7, decreased by 97% among children <5 years of age and by 64% among adults aged over 65 years.Citation24
The 13-valent pneumococcal conjugate vaccine (PCV13) has been shown to tackle bacterial antibiotic resistance. Data available in the United States, Europe, and Africa show that the introduction of PCV has led to a reduction in the use of antibiotics and a decrease in episodes of invasive disease caused by resistant S. pneumoniae. A 2016 study estimated that universal PCV coverage could avoid 11.4 million days per year of antibiotic use in children <5 years of age.Citation25 Very recently, a study illustrated the advantages in terms of reducing antibiotic resistance deriving from pneumococcal vaccination in China, including the reduction of treatment failures and large cost savings, underlining the value of vaccination with PCV13.Citation26
Pertussis
Pertussis is a highly contagious respiratory disease caused by the bacterium Bordetella pertussis. Pertussis is not usually fatal for adults but is potentially dangerous for unvaccinated infants, children and in high-risk groups. Vaccination, like the disease, does not provide permanent protection as it wanes over time. The reduction of natural immunity in adulthood makes people susceptible to pertussis and allows for B. pertussis reservoirs that can transmit the infection to the unvaccinated population (infants) or susceptible individuals.Citation27
In adults, pertussis presents atypically without the classic symptoms (inspiratory screaming, post-tussive vomiting, paroxysms, apnea) making the diagnosis of pertussis difficult as it manifests as a prolonged cough of lesser severity in those who were previously vaccinated during childhood.Citation28,Citation29
Despite European Union recommendations that emphasizes the need for laboratory testing to confirm a suspected case of pertussis, the diagnosis of pertussis is most of the times based on clinical signs and symptoms as laboratory confirmation is missing as it requests a strict protocol to follow.Citation30
Non-specific clinical signs of respiratory tract disease are widespread in the adult population, especially in winter. The clinical presentation of pertussis in adults is similar to that of other respiratory diseases such as influenza or para-influenza syndromes, the common cold, acute laryngotracheitis, bronchitis, etc. Their common symptoms include malaise, rhinorrhea, cough and fever.Citation28,Citation29,Citation31,Citation32 ()
Figure 2. Differential diagnosis of pertussis and other respiratory diseases (modified fromCitation27,Citation29-33.
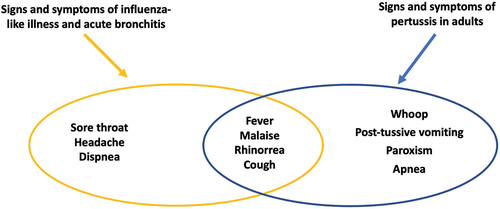
Pertussis, with its atypical presentation in adults, most probably contributes to increasing the overall number of patients with respiratory pathologies and therefore contributes to the prescription of antibiotics. A study from UK documented an increase of antibiotic prescriptions in patients with asthma and pertussis compared with those with asthma only.Citation33
Since the clinical presentation of pertussis is compatible with flu syndromes, colds and acute bronchitis, failure to control the disease, especially in adults, contributes to generating antibiotic prescriptions that could be avoided.
A survey of Italian experts was recently published to record their opinion on the role of vaccinations against antibiotic resistance. Antibiotic resistance was a priority concern in the daily professional activity of 82.4% of interviewees. Overall, 47.1% of the same believed that all immunizations included in the Italian vaccination calendar have a role in the fight against antimicrobial resistance. In addition, 92.2% of respondents believed that vaccination against pertussis for all individuals (healthy or with clinical conditions at risk) could help tackle bacterial antibiotic resistance.Citation34
All healthcare providers should be aware that recommending existing vaccinations for adults, including vaccination against pertussis (dTpa), in order to increase the overall health of the population also contributes to combating antibiotic resistance.
Viral vaccines
Influenza
Viral vaccines are also effective in reducing bacterial antibiotic resistance. The main mechanisms involved in achieving this result are represented on one hand by the prevention of viral infections of the respiratory tract and the related inappropriate prescriptions of antibiotics and on the other hand by the prevention of secondary bacterial infections and the related prescription of antibiotics.Citation15,Citation35
In the case of viral infections, prevention by vaccination does not decrease the risk of infection with antibiotic-resistant pathogens and therefore does not have a direct effect on bacterial antibiotic resistance. In this context, vaccination makes it possible to obtain an indirect effect represented by the reduced possibility of fever and secondary infections resulting from the reduced risk of the target infection of the vaccination itself.Citation36
The above effect is well documented for influenza vaccination. Influenza increases the risk of secondary bacterial infections (e.g., pneumonia, otitis media) that require antibiotic treatment. Besides, influenza has a major impact on health services and families each winter in temperate countries and correlates with over-prescribing of antibiotics in children. This infectious disease is in fact one of the main causes of fever for which there is a certain propensity for an inappropriate prescription of antibiotics and the same increases the risk of superinfections from microorganisms potentially resistant to antibiotics. Some of the influenza clinical complications (as primary viral pneumonia and/or worsening of clinical conditions) are of non-bacterial origin, others (as secondary pneumonia, otitis media, sinusitis and bronchitis) are the result of bacterial superinfections.Citation37,Citation38
In 2000, the Ontario region of Canada started a universal influenza immunization program offering free vaccines to anyone aged >6 months. Universal influenza immunization was associated with a 64% decrease in influenza-associated respiratory antibiotic prescriptions (incremental decrease of 10 per 1,000 population). The introduction of the program was associated with reductions of greater than 39%, 42%, 55% and 59% in influenza-associated mortality, hospitalizations, emergency room access and medical visits, respectively.Citation39
It has been shown that there is a close temporal link between the incidence of influenza disease and the use of antibacterials, and therefore immunization implies a reduction in the incidence of influenza and reduction of antibiotic prescriptions. In general, about 30–50% of antibiotic prescriptions in outpatient care are inappropriate; many of these inappropriately treated infections are influenza-related and could be prevented with immunization.Citation40
A study conducted in 3 areas of the USA estimated the incidence of physician-assisted influenza cases and cases avoided with vaccination for the influenza seasons from 2013/14 to 2015/16. The incidence of influenza with medical assistance was between 14 and 54 per 1,000 population while the cases avoided ranged from 9 (2014/15 season) to 28 per 1,000 (2013/14 season) indicating that the vaccination schedule involved significant reductions in outpatient visits for influenza, even in years when vaccine was not well matched to the dominant circulating influenza strain. It has been shown that on average, vaccinating 1,000 people avoided 13.9 outpatient visits due to influenza; in practice, 1 outpatient visit was avoided for every 72 immunized subjects.Citation41
The assessment of the impact of influenza vaccination in the 2016/2017 season in the USA showed that vaccination coverage rates ranged from 33% (Nevada) to 52% (Rhode Island), while antibiotic use rates ranged from 125 (Alaska) to 377 prescriptions per 1,000 population (West Virginia). In particular, vaccination coverage rates were highly correlated with reduced prescription rates; a 1% increase in influenza vaccination rate was significantly associated with 1.40 fewer antibiotic prescriptions per 1,000 population. The increased vaccination coverage rate in the pediatric population (ages 0–18 years) had the strongest effect, followed by that observed in the elderly (>65 years).Citation42
The potential impact of influenza vaccination on antibiotic use has also been assessed in Africa. It is estimated that the direct impact of vaccination could avoid more than 390 prescriptions per 100,000 population per year by using a 50% effective influenza vaccine with 30% coverage in adults >65 years of age in South Africa or in children aged between 2 and 5 years in Senegal. Across Africa, simply by reducing the number of severe acute respiratory infections, the use of a vaccine with the same characteristics could avoid at least 24,000 antibiotic prescriptions per year if administered to children <5 years of age.Citation43
Given the annual recurrence of influenza epidemics and the high frequency of influenza illness, the potential impact of the influenza vaccine on bacterial antibiotic resistance is high as reported by numerous studies.Citation44-46
In principle, a synergistic effect exerted by the pneumococcal and influenza vaccines can be postulated. A meta-analysis showed that the additional preventive effects of the concomitant vaccination (influenza and pneumococcal vaccines) compared to influenza vaccination alone for pneumonia and death were 15% and 19%, respectively. Compared to pneumococcal vaccination alone, concomitant influenza and pneumococcal vaccination resulted in a 24% reduction in pneumonia and a 28% reduction in death; when compared with placebo or no vaccination, the efficacy of concomitant vaccination was 29% for pneumonia, 38% for death, 35% for influenza, and 18% for hospitalization.Citation47
Varicella
Varicella is often associated with secondary bacterial infections; vaccination in these cases would reduce viral infections and the consequent inappropriate use of antibiotics associated with it.Citation18
In the specific case of varicella, the impact of vaccination would therefore represent an indirect effect, that is, by preventing the infectious disease, a decreased possibility of fever and secondary bacterial infections is also obtained.
In Belgium, a substantial burden of varicella in a primary care setting has been assessed, highlighting high rates of varicella-related complications as well as of antibiotic use. In this context universal varicella vaccination would reduce both incidence and clinical and economic burden of varicella.Citation48
The impact of universal varicella vaccination on the use and cost of antibiotics and antivirals for varicella management has been evaluated in the USA pointing out that high vaccination coverage rates would imply a significant reduction in annual antibiotic and antiviral use.Citation49
Rotavirus
Rotavirus disease is very common in infants and young children, even it can involve older children and adults as well. It generally causes a severe diarrheal disease and dehydration, requiring hospitalization.
Nowadays, a specific treatment for rotavirus disease is not available and any used medicine aims at reducing symptoms. However, some experts have pointed out that a significant value could be attributed to some vaccines targeting viral pathogens when considering reduction in antibiotic use including rotavirus vaccines.Citation14
Two live-attenuated, oral vaccines has been introduced into the national immunization programs of several countries showing a significant impact on morbidity and mortality and an adequate tolerability and safety profile.Citation50 Very recently, a retrospective cohort study of US children evaluated antibiotic prescriptions associated with acute gastroenteritis (AGE) showing that prescriptions were significantly lower in children with complete vaccination against rotavirus in respect to unimmunized children.Citation51 Noteworthy, antibiotics in viral diseases are not effective and their prescription should be avoided unless a bacterial complication has been ruled out.Citation52
Respiratory syncytial virus (RSV)
RSV is an important cause of disease in infants, young children, the elderly and adults with preexisting pathological conditions. Most children have RSV infection by 2 years of age and in newborns this pathogen is the most common cause of acute lower respiratory tract infections. Noteworthy, the immune response after natural infection is incomplete and is not long-lasting; for this reason, RSV can reinfect people throughout their lives. Older people are at increased risk for serious infections and associated complications due to age-related decline in immunity while adults with chronic heart or lung disease or a weakened immune system are at increased risk of severe RSV infection.Citation53
The inappropriate antibiotic prescribing during respiratory tract infections is quite common in the pediatric age and has been assessed in two different studies conducted in Israel and in Scotland, respectively.Citation54,Citation55 Similar results have been reported in studies conducted on adults. In particular, the impact on medical resource utilization among adults has been quite similar or even greater than that of influenza and implies often inappropriate antibiotic use.Citation56,Citation57
Nowadays, research is aimed at identifying safe and effective preventive means against RSV infection that provide lasting and sustainable protection. The development of vaccines or monoclonal antibodies (mAbs) against RSV has been included among the priorities of the World Health Organization (WHO) and today many vaccines and mAbs are in the clinical trial phase with the perspective of their availability in the short to medium term. New vaccines and mAbs would help to hinder also the inappropriate use of antibiotics during RSV disease.Citation58
In addition to the above examples, other vaccines already in use have the potential to impact on bacterial antibiotic resistance. In particular, data or ongoing evaluations are available on the potential impact on bacterial antibiotic resistance by oral cholera vaccine and the typhoid conjugate vaccine.
In the future, vaccines for Clostridium difficile, Salmonella enterica (serovars Typhi and Paratyphi A), non-typhoid invasive Salmonella, Shigella, Streptococcus type A, Mycobacterium tuberculosis, are under development and evaluation, opening also interesting perspectives for the prevention of bacterial antibiotic resistance.Citation18,Citation59
Conclusions
Vaccines and antibiotics have made it possible to achieve enormous results against various infectious diseases and to save millions of lives. As antibiotic resistance has become a public health problem, eroding the value of antibiotics, this issue needs to be addressed by identifying effective intervention strategies. Several vaccines have shown their ability to reduce inappropriate antibiotics prescriptions and achieving high immunization coverage rates should be considered a priority also for antibiotic stewardship.Citation60
WHO considers it essential that the quality and safety of antibiotics and their appropriate use in humans and animals be ensured, infection prevention and control improved, the supply system for medicines strengthened and financial coverage against high costs of treating drug-resistant infections guaranteed.Citation61
It is therefore essential to program synergistic action at multiple levels promoting the development of multi-sector national action plans, strengthening national systems and improving awareness and behavioral change to combat antibiotic resistance.
Vaccines, together with other possible interventions, have the potential to play an important role due to their direct and indirect effect in the fight against bacterial antibiotic resistance.
Acknowledgments
The Author wishes to acknowledge the scientific contribution of Federico Marchetti to this review.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO. Antimicrobial resistance. Fact sheets. 2021 Nov 17. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
- WHO. WHO strategic priorities on antimicrobial resistance. Preserving antimicrobials for today and tomorrow. WHO; 2021. https://apps.who.int/iris/bitstream/handle/10665/351719/9789240041387-eng.pdf?sequence=1&isAllowed=y.
- Murray CJ, Ikuta KS, Sharara F, Swetschinski L, Robles Aguilar G, Gray A, Han C, Bisignano C, Rao P, Wool E, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–8. doi:10.1016/S0140-6736(21)02724-0.
- OECD. Stemming the superbug tide: just a few dollars more. In: OECD health policy studies. Paris: OECD Publishing; 2018. doi:10.1787/9789264307599-en.
- Tagliabue A, Rappuoli R. Changing priorities in vaccinology: antibiotic resistance moving to the top. Front Immunol. 2018;9:1068. doi:10.3389/fimmu.2018.01068.
- Cassini A, Högberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. doi:10.1016/S1473-3099(18)30605-4.
- European Centre for Disease Prevention and Control. Antimicrobial resistance in the EU/EEA (EARS-Net) - Annual Epidemiological Report 2020. Stockholm: ECDC; 2022. https://www.ecdc.europa.eu/sites/default/files/documents/AER-EARS-Net-2020.pdf.
- ECDC. Antibiotic resistance: how does antibiotic resistance spread? Infographic. [accessed 2014 Nov 18]. https://www.ecdc.europa.eu/en/publications-data/antibiotic-resistance-how-does-antibiotic-resistance-spread.
- WHO. Quadripartite Memorandum of Understanding (MoU) signed for a new era of One Health collaboration. 2022 Apr 22. https://www.who.int/news/item/29-04-2022-quadripartite-memorandum-of-understanding-mou-signed-for-a-new-era-of-one-health-collaboration.
- European Commission. Communication from the Commission to the Council and the European Parliament “A European One Health Action Plan against antimicrobial resistance (AMR)”. 2017 Jun 29. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52017DC0339&from=IT.
- Pezzotti P, Bellino S, Prestinaci F, Iacchini S, Lucaroni F, Camoni L, Barbieri MM, Ricciardi W, Stefanelli P, Rezza G. The impact of immunization programs on 10 vaccine preventable diseases in Italy: 1900–2015. Vaccine. 2018;36(11):1435–43. doi:10.1016/j.vaccine.2018.01.065.
- WHO. Leveraging vaccines to reduce antibiotic use and prevent antimicrobial resistance: an action framework. World Health; 2020.
- Alghamdi S. The role of vaccines in combating antimicrobial resistance (AMR) bacteria. Saudi J Biol Sci. 2021;28(12):7505–10. doi:10.1016/j.sjbs.2021.08.054.
- Malarski M, Hasso-Agopsowicz M, Soble A, Mok W, Mathewson S, Vekemans J. Vaccine impact on antimicrobial resistance to inform Gavi, the Vaccine Alliance’s 2018 vaccine investment strategy: report from an expert survey. F1000Res. 2019;8:1685. doi:10.12688/f1000research.20100.1.
- Jansen KU, Anderson AS. The role of vaccines in fighting antimicrobial resistance (AMR). Hum Vaccin Immunother. 2018;14(9):2142–49. doi:10.1080/21645515.2018.1476814.
- Jit M, Cooper B. The role of vaccines in combating antimicrobial resistance. In: North J, Anderson M, Cecchini M, and Mossialos E, editors. Challenges to tackling antimicrobial resistance: economic and policy responses (European Observatory on Health Systems and Policies). Cambridge: Cambridge University Press; 2020. p. 181–206. doi:10.1017/9781108864121.009.
- Andrejko K, Ratnasiri B, Hausdorff WP, Laxminarayan R, Lewnard JA. Antimicrobial resistance in paediatric Streptococcus pneumoniae isolates amid global implementation of pneumococcal conjugate vaccines: a systematic review and meta-regression analysis. Lancet Microbe. 2021;2(9):e450–60. PMID: 34485957; PMCID: PMC8410609. doi:10.1016/S2666-5247(21)00064-1.
- Micoli F, Bagnoli F, Rappuoli R, Serruto D. The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol. 2021;19(5):287–302. doi:10.1038/s41579-020-00506-3.
- Oliver SE. Chapter 2: Haemophilus influenzae invasive disease. In: VPD Surveillance Manual. [ accessed 2019 Oct 25]. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt02-hib.html.
- Slack M, Esposito S, Haas H, Mihalyi A, Nissen M, Mukherjee P, Harrington L. Haemophilus influenzae type b disease in the era of conjugate vaccines: critical factors for successful eradication. Expert Rev Vaccines. 2020;19(10):903–17. doi:10.1080/14760584.2020.1825948.
- Heliodoro CIM, Bettencourt CR, Bajanca-Lavado MP, Portuguese Group for the Study of Haemophilus influenzae invasive infection. Molecular epidemiology of invasive Haemophilus influenzae disease in Portugal: an update of the post-vaccine period, 2011–2018. Eur J Clin Microbiol Infect Dis. 2020;39(8):1471–80. doi:10.1007/s10096-020-03865-0.
- McElligott M, Meyler K, Bennett D, Mulhall R, Drew RJ, Cunney R. Epidemiology of Haemophilus influenzae in the Republic of Ireland, 2010–2018. Eur J Clin Microbiol Infect Dis. 2020;39(12):2335–44. doi:10.1007/s10096-020-03971-z.
- Wen S, Feng D, Chen D, Yang L, Xu Z. Molecular epidemiology and evolution of Haemophilus influenzae. Infect Genet Evol. 2020;80:104205. doi:10.1016/j.meegid.2020.104205.
- Gierke R, McGee L, Beall B, Pilishvili T. Chapter 11: pneumococcal. In: VPD surveillance manual. [accessed 2020 Jun 29]. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt11-pneumo.html.
- Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, Davies S. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–75. doi:10.1016/S0140-6736(15)00474-2.
- Lu EY, Chen HH, Zhao H, Ozawa S. Health and economic impact of the pneumococcal conjugate vaccine in hindering antimicrobial resistance in China. Proc Natl Acad Sci USA. 2021;118(13):e2004933118. doi:10.1073/pnas.2004933118.
- Blasi F, Bonanni P, Braido F, Gabutti G, Marchetti F, Centanni S. The unmet need for pertussis prevention in patients with chronic obstructive pulmonary disease in the Italian context. Hum Vaccin Immunother. 2020;16(2):340–48. doi:10.1080/21645515.2019.1652517.
- Lee GM, Lett S, Schauer S, LeBaron C, Murphy TV, Rusinak D, Lieu TA, Massachusetts Pertussis Study Group. Societal costs and morbidity of pertussis in adolescents and adults. Clin Infect Dis. 2004;39(11):1572–80. doi:10.1086/425006.
- Moore A, Harnden A, Grant CC, Patel S, Irwin RS, Altman KW, Azoulay E, Barker AF, Bolser DC, Birring SS, CHEST Expert Cough Panel. Clinically diagnosing pertussis-associated cough in adults and children: CHEST Guideline and Expert Panel Report. Chest. 2019;155(1):147–54. doi:10.1016/j.chest.2018.09.027.
- Centers for Disease Control and Prevention (CDC). Diagnosis confirmation. 2019. [ accesso 2022 Gennaio]. https://www.cdc.gov/pertussis/clinical/diagnostic-testing/diagnosis-confirmation.html.
- Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560–65. PMID: 27929206.
- Centers for Disease Control and Prevention (CDC). Cold vs Flu. https://www.cdc.gov/flu/symptoms/coldflu.htm.
- Bhavsar A, Aris E, Harrington L, Simeone JC, Ramond A, Lambrelli D, Papi A, Boulet LP, Meszaros K, Jamet N, et al. Burden of pertussis in individuals with a diagnosis of asthma: a retrospective database study in England. J Asthma Allergy. 2022;15:35–51. doi:10.2147/JAA.S335960.
- Marchetti F, Prato R, Viale P. Survey among Italian experts on existing vaccines’ role in limiting antibiotic resistance. Human Vaccin Immunother. 2021;17(11):4283–90. doi:10.1080/21645515.2021.1969853.
- Buchy P, Ascioglu S, Buisson Y, Datta S, Nissen M, Tambyah PA, Vong S. Impact of vaccines on antimicrobial resistance. Int J Infect Dis. 2020;90:188–96. doi:10.1016/j.ijid.2019.10.005.
- Klugman KP, Black S. Impact of existing vaccines in reducing antibiotic resistance: primary and secondary effects. Proc Natl Acad Sci USA. 2018;115(51):12896–901. doi:10.1073/pnas.1721095115.
- Giacchetta I, Primieri C, Cavalieri R, Domnich A, de Waure C. The burden of seasonal influenza in Italy: a systematic review of influenza-related complications, hospitalizations, and mortality. Influenza Other Respir Viruses. 2022;16(2):351–65. doi:10.1111/irv.12925.
- Grohskopf LA, Alyanak E, Broder KR, Blanton LH, Fry AM, Jernigan DB, Atmar RL. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2020–21 influenza season. MMWR Recomm Rep. 2020;69(8):1–24. doi:10.15585/mmwr.rr6908a1.
- Kwong JC, Maaten S, Upshur RE, Patrick DM, Marra F. The effect of universal influenza immunization on antibiotic prescriptions: an ecological study. Clin Infect Dis. 2009;49(5):750–56. doi:10.1086/605087.
- Piltcher OB, Kosugi EM, Sakano E, Mion O, Testa JRG, Romano FR, Santos MCJ, Di Francesco RC, Mitre EI, Bezerra TFP, et al. How to avoid the inappropriate use of antibiotics in upper respiratory tract infections? A position statement from an expert panel. Braz J Otorhinolaryngol. 2018;84(3):265–79. doi:10.1016/j.bjorl.2018.02.001.
- Jackson ML, Phillips CH, Benoit J, Jackson LA, Gaglani M, Murthy K, McLean HQ, Belongia EA, Malosh R, Zimmerman R, et al. Burden of medically attended influenza infection and cases averted by vaccination – United States, 2013/14 through 2015/16 influenza seasons. Vaccine. 2018;36(4):467–72. doi:10.1016/j.vaccine.2017.12.014.
- Klein EY, Schueller E, Tseng KK, Morgan DJ, Laxminarayan R, Nandi A. The impact of influenza vaccination on antibiotic use in the United States, 2010-2017. Open Forum Infect Dis. 2020;7(7):ofaa223. PMID: 32665959; PMCID: PMC7336555. doi:10.1093/ofid/ofaa223.
- Knight GM, Clarkson M, de Silva TI. Potential impact of influenza vaccine roll-out on antibiotic use in Africa. J Antimicrob Chemother. 2018;73(8):2197–200. doi:10.1093/jac/dky172.
- Esposito S, Principi N, European Society of Clinical Microbiology Infectious Diseases (ESCMID) Vaccine Study Group (EVASG). Influenza vaccination and prevention of antimicrobial resistance. Expert Rev Vaccines. 2018;17(10):881–88. doi:10.1080/14760584.2018.1525298.
- Muller-Pebody B, Sinnathamby MA, Warburton F, Rooney G, Andrews N, Whitaker H, Henderson KL, Tsang C, Hopkins S, Pebody RG. Impact of the childhood influenza vaccine programme on antibiotic prescribing rates in primary care in England. Vaccine. 2021;39(45):6622–27. doi:10.1016/j.vaccine.2021.09.069.
- Barchitta M, Maugeri A, Vinci R, Agodi A. The inverse relationship between influenza vaccination and antimicrobial resistance: an ecological analysis of Italian data. Vaccines (Basel). 2022;10(4):554. doi:10.3390/vaccines10040554.
- Yin M, Huang L, Zhang Y, Yu N, Xu X, Liang Y, Ni J. Effectiveness and safety of dual influenza and pneumococcal vaccination versus separate administration or no vaccination in older adults: a meta-analysis. Expert Rev Vaccines. 2018;17(7):653–63. doi:10.1080/14760584.2018.1495077.
- Vandenhaute J, Tsakeu E, Chevalier P, Pawaskar M, Benčina G, Vertriest J. Assessing the use of antibiotics and the burden of varicella in Belgium using a retrospective GP database analysis. BMC Infect Dis. 2021;21(1):1150. doi:10.1186/s12879-021-06848-4.
- Pawaskar M, Fergie J, Harley C, Samant S, Veeranki P, Diaz O, Conway JH. Impact of universal varicella vaccination on the use and cost of antibiotics and antivirals for varicella management in the United States. PLoS One. 2022;17(6):e0269916. doi:10.1371/journal.pone.0269916.
- Hall EW, Tippett A, Fridkin S, Anderson EJ, Lopman B, Benkeser D, Baker JM. Association between rotavirus vaccination and antibiotic prescribing among commercially insured US children, 2007-2018. Open Forum Infect Dis. 2022;9(7):ofac276. doi:10.1093/ofid/ofac276.
- Burke RM, Tate JE, Kirkwood CD, Steele AD, Parashar UD. Current and new rotavirus vaccines. Curr Opin Infect Dis. 2019 Oct;32(5):435–44. doi:10.1097/QCO.0000000000000572.
- CDC. Rotavirus. https://www.cdc.gov/rotavirus/index.html.
- Nam HH, Ison MG. Respiratory syncytial virus. Semin Respir Crit Care Med. 2021;42(6):788–99. Epub 2021 Dec 16. PMID: 34918321. doi:10.1055/s-0041-1736182.
- Obolski U, Kassem E, Na’Amnih W, Tannous S, Kagan V, Muhsen K. Unnecessary antibiotic treatment of children hospitalised with respiratory syncytial virus (RSV) bronchiolitis: risk factors and prescription patterns. J Glob Antimicrob Resist. 2021;27:303–08. doi:10.1016/j.jgar.2021.10.015.
- Fitzpatrick T, Malcolm W, McMenamin J, Reynolds A, Guttmann A, Hardelid P. Community-based antibiotic prescribing attributable to Respiratory Syncytial Virus and other common respiratory viruses in young children: a population-based time-series study of Scottish children. Clin Infect Dis. 2021;72(12):2144–53. doi:10.1093/cid/ciaa403.
- Hartnett J, Donga P, Ispas G, Vandendijck Y, Anderson D, House S, Suner S. Risk factors and medical resource utilization in US adults hospitalized with influenza or respiratory syncytial virus in the hospitalized acute respiratory tract infection study. Influenza Other Respir Viruses. 2022;16(5):906–15. doi:10.1111/irv.12994.
- Walsh E, Lee N, Sander I, Stolper R, Zakar J, Wyffels V, Myers D, Fleischhackl R. RSV-associated hospitalization in adults in the USA: a retrospective chart review investigating burden, management strategies, and outcomes. Health Sci Rep. 2022;5(3):e556. doi:10.1002/hsr2.556.
- Mazur NI, Terstappen J, Baral R, Bardají A, Beutels P, Buchholz UJ, Cohen C, Crowe JE Jr, Cutland CL, Eckert L, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2022;S1473-3099(22):00291–2. doi:10.1016/S1473-3099(22)00291-2.
- Jansen KU, Gruber WC, Simon R, Wassil J, Anderson AS. The impact of human vaccines on bacterial antimicrobial resistance. A review. Environ Chem Lett. 2021;19(6):4031–62. doi:10.1007/s10311-021-01274-z.
- Doherty TM, Hausdorff WP, Kristinsson KG. Effect of vaccination on the use of antimicrobial agents: a systematic literature review. Ann Med. 2020;52(6):283–99. Epub 2020 Jun 29. PMID: 32597236; PMCID: PMC7880080. doi:10.1080/07853890.2020.1782460. .
- WHO. Global action plan on antimicrobial resistance. 2015. https://www.who.int/publications/i/item/9789241509763.
