ABSTRACT
Messenger RNA (mRNA)-based vaccine platforms used for the development of mRNA-1273 and BNT162b2 have provided a robust adaptable approach to offer protection against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, as variants of concern (VoCs), such as omicron and associated sub-variants, emerge, boosting strategies must also adapt to keep pace with the changing landscape. Heterologous vaccination regimens involving the administration of booster vaccines different than the primary vaccination series offer a practical, effective, and safe approach to continue to reduce the global burden of coronavirus disease 2019 (COVID-19). To understand the immunogenicity, effectiveness, and safety of heterologous mRNA-based vaccination strategies, relevant clinical and real-world observational studies were identified and summarized. Overall, heterologous boosting strategies with mRNA-based vaccines that are currently available and those in development will play an important global role in protecting individuals from COVID-19 caused by emerging VoCs.
Introduction
Strategies for robust protection against COVID-19
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, the development of safe and effective vaccines to prevent severe COVID-19 disease has been at the forefront of prophylactic strategies.Citation1–5 Over the course of the pandemic, multiple vaccines based on different scientific platforms have been authorized for primary vaccination schedules globally.Citation6 With the emergence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) variants of concern (VoCs), the focus has gradually shifted from primary vaccination approaches to vaccine boosting for those who have already completed the primary series.Citation7–14
Evaluating evidence of specific heterologous regimens, combined with considerations, such as those outlined in , will help guide future COVID-19 boosting approaches. As with heterologous primary series, “mix and match” or heterologous booster strategies, whereby booster vaccines are different than the primary vaccines, could simplify the implementation of mass vaccination programs and supply management within clinics/healthcare centers. These strategies also allow healthcare providers to select the most effective booster combination and schedule for their patients based on the latest evidence and the vaccine(s) available at the time of booster administration. Heterologous boosting strategies not only serve to help mitigate issues with regional vaccine supply and storage but also may expand booster selections based on real-world effectiveness against circulating VoCs and those that may emerge in the future. Together with the heightened need to protect those most vulnerable to severe COVID-19 disease, such as immunocompromised populations and older adults, booster strategies play a key role in global health outcomes. These considerations, combined with the evaluation of available clinical and real-world observational data, serve to aid in the decision-making process of selecting vaccine boosters.Citation14–33
Table 1. Considerations for COVID-19 boosting strategiesCitation14–17.
Other key considerations for the development of future vaccines against COVID-19 include the ability of a scientific platform to derive safe and highly effective booster strategies and the delivery of a technology that can rapidly be adapted to target future VoCs. As such, the development of the messenger RNA (mRNA)-based vaccine platform provides a robust and adaptable approach against SARS-CoV-2 and VoCs. In this review, we describe the current scientific literature within the context of heterologous vaccine regimens and the associated implications for emerging heterologous mRNA-based booster strategies.
The COVID-19 vaccine landscape
Several COVID-19 vaccines have been developed and evaluated in clinical trials within the United States and Europe, including the mRNA-based vaccines mRNA-1273 (SpikevaxTM; Moderna, Inc., Cambridge, MA, USA) and BNT162b2 (Comirnaty®; Pfizer Inc, New York, NY, USA; BioNTech Manufacturing GmbH, Mainz, Germany), the adenoviral vector–based vaccines Ad26.COV2.S (Janssen COVID-19 vaccine or JCovden; Johnson & Johnson, New Brunswick, NJ, USA) ChAd-Ox1.S (VaxzevriaTM; AstraZeneca, Luton, UK) and the recombinant protein-based adjuvanted vaccine NVX-CoV2373 (NuvaxovidTM; Novavax US, Gaithersburg, MD, USA) (summarized in ).Citation6, Citation34–42 Based on demonstrated efficacy in clinical trials, the US Food and Drug Administration (FDA) first granted emergency use authorization (EUA) for mRNA-1273 (2-dose primary vaccination series) in adults aged ≥18 years and BNT162b2 (2-dose primary vaccination series) in individuals aged ≥16 years in December 2020.Citation37,Citation43,Citation44 Both mRNA-1273 and BNT162b2 subsequently received full authorization from the FDA for the prevention of COVID-19 in individuals in these age groups.Citation45,Citation46 The EUA for mRNA-1273 and BNT162b2 has now been extended to children aged ≥6 months.Citation47 Similarly, the FDA granted an EUA for NVX-CoV2373 (2-dose primary vaccination series, adjuvanted vaccine) in individuals aged ≥12 years and limited EUA of Ad26.COV2.S (1-dose primary vaccination series) in adults ≥18 years for whom other authorized or approved COVID-19 vaccines are not accessible or clinically appropriate or who elect to receive Ad26.COV2.S because they would otherwise not receive a COVID-19 vaccine.Citation39,Citation48–52 Likewise, mRNA-1273, BNT162b2, Ad26.COV2.S, ChAd-Ox1.S and NVX-CoV2373 have gained authorization for use from the European Medicines Agency (EMA) and in multiple countries worldwide.Citation35,Citation39,Citation41,Citation53–55
Table 2. A summary of the COVID-19 vaccine landscape as of 21 September 2022.
Widespread COVID-19 vaccination campaigns have facilitated an estimated 12.23 billion administered vaccine doses globally as of mid-July 2022.Citation18 Overall, 40 COVID-19 vaccines have been authorized worldwide, including those mentioned previously as well as inactivated virus whole-cell vaccines BBIBP-CorV (Covilo; Sinopharm, Beijing, China), BBV152 (Covaxin®; Bharat Biotech, Turakapally, India), and CoronaVac (Sinovac; Beijing, China); the non-replication viral vector Sputnik V (Gamaleya; Moscow, Russia), Ad5-nCoV (Convideia; CanSinoBio, Tianjin, China); and protein subunit-based vaccine COVOVAXTM (Serum Institute of India, Novovax formulation, Pune, India) (summarized in ).Citation6,Citation15 As of the same date, approximately 67% of the vaccine-eligible population has been fully vaccinated (received the primary vaccination-series) in the United States, among whom the majority received mRNA-based vaccines (38% received mRNA-1273 and 59% received BNT162b2).Citation56 Globally, vaccines type has varied from country to country, with mRNA-based vaccine doses contributing to a large proportion of all primary and boosters doses.Citation18
Considerations for vaccine boosting
Clinical and real-world observational studies suggest that mRNA-based COVID-19 vaccines are highly effective against symptomatic and severe COVID-19.Citation57–61 However, as the COVID-19 disease landscape continues to evolve, studies indicate a waning of natural and vaccine-elicited immunity over time.Citation62–64 Additionally, the emergence of SARS-CoV-2 variants, such as B.1.617.2 (delta) and B.1.1.529 (omicron), with subvariants BA.1, BA.2, BA.3, BA.4 and BA.5, has raised concerns of increased transmissibility and evasion of both natural and vaccine-induced immunity.Citation8–10,Citation13,Citation16,Citation57 Specifically, although the initial real-world observational studies confirmed that a 2-dose mRNA-1273 vaccination schedule is highly effective against SARS-CoV-2 variant infection as well as severe COVID-19 outcomes (i.e., hospitalization and death),Citation57,Citation62,Citation65 more recent studies indicate that there is a moderate decline in mRNA-based vaccine effectiveness (VE) against infection with the delta variant and the more significant omicron variant and subvariants.Citation66,Citation67
As continued protection of populations against severe outcomes from COVID-19 is crucial to help mitigate the healthcare and economic impacts of the disease, booster vaccination lowers the risk of breakthrough disease as a result of SARS-CoV-2 antigenic variation and waning of natural and/or vaccine-induced immunity.Citation8–10,Citation13,Citation16,Citation64 Accordingly, stakeholders, including advisory groups, regulatory authorities, and policymakers, have begun to examine the implications of periodic or seasonal booster vaccine administration, with an emphasis on the individuals most at risk of infection (such as the elderly, immunocompromised individuals, and front-line healthcare workers) and those who received their primary dosing earliest in the vaccine rollout.Citation14,Citation57–64,Citation68–70 It is imperative that the decision of when and which vaccine booster regimen(s) to choose to be made based on clinical evidence.Citation71–75
Initial booster studies focused on homologous approaches toward boosting (i.e., the same vaccine is administered for the primary series and booster), and several studies have focused on evaluating the safety and immunogenicity of such booster strategies;Citation4,Citation71–76 the current knowledge regarding COVID-19 homologous booster strategies is reviewed elsewhere.Citation77–80 Notably, for mRNA-based COVID-19 vaccines, evidence suggests that homologous booster vaccination after the 2-dose primary series can increase neutralizing antibody titers against wild‐type (WT) SARS-CoV-2 and the delta variant compared with titers following the completion of the primary vaccination series.Citation4 Regarding safety, local and systemic adverse events were similar following dose 2 and the booster dose.Citation81 Based on such evidence, homologous booster regimens with mRNA-1273 and BNT162b2 have been authorized in the United States and Europe.Citation35,Citation82,Citation83 Additionally, for adenoviral vector-based vaccines, a second Ad26.COV2.S dose is also authorized as a homologous booster in the United States.Citation50
Heterologous primary and booster vaccination regimens
As introduced earlier, a practical strategy to expand COVID-19 vaccine coverage is to consider “mixing and matching” vaccines in a heterologous vaccination schedule.Citation84,Citation85 More specifically, these heterologous schedules can include regimens of vaccines from different platforms (e.g., Ad26.COV2.S and mRNA-1273) or different vaccines from the same platform (e.g., mRNA-1273 and BNT162b2). If such strategies can elicit tolerability and immunogenicity profiles similar to those of homologous vaccination regimens, they offer several advantages, including potentially optimizing the breadth and longevity of protection against COVID-19 attained with currently available vaccines, providing protection against emerging VoCs with vaccines in development and simplifying the logistics of booster vaccine administration (). Heterologous vaccination strategies are also being investigated to evaluate immune response across a range of diseases, including Ebola, HIV, malaria, tuberculosis, and influenza.Citation86,Citation87
In both the United States and Europe, heterologous boosting with any available COVID-19 vaccine is currently authorized,Citation17,Citation84 with the decision-making of which booster to receive left to individuals and their healthcare providers. Consequently, additional evidence regarding specific booster regimens following a primary vaccination series is needed to make better decisions regarding the optimal heterologous booster strategies for COVID-19 vaccination. In the United States, an mRNA-based COVID-19 vaccine booster is recommended after completion of any COVID-19 primary vaccination series (mRNA-1273 for individuals aged ≥6 years; BNT162b2 for individuals aged ≥5 years).Citation88 Additionally, a second mRNA-based COVID-19 vaccine booster is recommended for adults aged ≥50 years and for individuals with certain kinds of immunocompromise (aged ≥18 years with mRNA-1273; aged ≥12 years with BNT162b2).Citation17,Citation85 Together with the increased importance of protecting those individuals most at risk of developing severe COVID-19 disease and the current global availability of various authorized primary-booster vaccine permutations, guidance on optimal choices should be based on evidence from clinical studies and real-world data that explore heterologous vaccination regimens for both the primary series and booster(s).
To address the need for guidance on optimal vaccine choices, we provide a brief overview of recent studies meeting selection criteria (see Methods for article selection criteria; ) that examined heterologous mRNA-based 2 or 3-dose vaccination schedules of different vaccines, including ≥1 mRNA-based vaccine (mRNA-1273 or BNT162b2), in the context of supporting future booster regimen choices; of note, we discuss data pertaining to the currently authorized COVID-19 primary vaccines in the United States or Europe paired with mRNA-based boosting vaccines.Citation17,Citation84 No correlate of protection has been established, but since protection against SARS-CoV-2 is likely to be associated with humoral and cellular immune responses,Citation89–92 we first compared the immunogenicity data of regimens of different mRNA-based vaccines and then summarized vaccine reactogenicity and safety.
Table 3. PubMed literature search criteria and results.
Materials and methods
A literature search using PubMed was conducted to capture clinical trials and observational studies of COVID-19 vaccination regimens (). The search was limited to English language articles published between 1 January 2021 and 29 June 2022, which generated 595 articles. Exclusion criteria were used to remove articles from the list, including articles describing studies with non-mRNA-based vaccines only; real-world observational studies that included data outside of the December 2021 to June 2022 time period (to capture the latest circulating VoCs); small participant populations for observational studies (~<100,000 individuals, to capture a large-enough real environment); and articles that were not peer-reviewed. If a study evaluated both an mRNA vaccine and a non-mRNA vaccine as a booster, it was included. Exceptions were made to the selection criteria if a study was not yet peer-reviewed but otherwise met the inclusion criteria laid out in . Using these criteria, 16 articles that identified 15 studies (two articles described the same study) were selected for inclusion in this review, including eight articles describing clinical trials and seven reporting observational studies. Further three clinical studies (that matched the inclusion criteria) and one observational study were identified outside of the PubMed search based on knowledge of mRNA-based vaccines in development, pipelines, and related articles.
Briefly, the 11 clinical studies include 1) the National Institute of Health Division of Microbiology and Infectious Diseases (NIH DMID) study in the United States evaluating heterologous booster regimens with mRNA-1273, BNT162b2, or Ad26.COV2.S;Citation26,Citation32 2) the COV-BOOST study in the United Kingdom assessing heterologous boost combinations with BNT162b2 or ChAd-Ox1.S;Citation21 3) a COV-BOOST extension study;Citation93 4) the Com-COV2 study in the United Kingdom assessing heterologous primary vaccination series with mRNA-1273, BNT162b2, ChAd-Ox1.S or NVX-CoV2373;Citation20 5) the ARNCOMBI study in France comparing heterologous primary vaccination series with two doses of different vaccines to homologous dosing with mRNA-1273 and BNT162b2;Citation94 6) the SWITCH trial in the Netherlands evaluating Ad26.COV2.S as a primary vaccine with Ad26.COV2.S, mRNA-1273, or BNT162b2 as the second dose;Citation95 7) the HKSH study in Hong Kong assessing boosting with CoronaVac or BNT162b2;Citation96 8) the PRIBIVAC trial in Singapore evaluating heterologous or homologous boosting with mRNA-1273 or BNT162b2;Citation97 and 9–11) three studies investigating VoC targeting monovalent or bivalent vaccines.Citation98,Citation99 The study details are shown in . In addition, eight observational real-world studies assessing outcomes following heterologous regimens with mRNA-1273 or BNT162b2 were also selected ().Citation23,Citation25,Citation100–104 Studies were conducted in adults ≥18 years of age.
Table 4. Recent clinical trials evaluating heterologous vaccine regimens (2, 3, or 4 doses)*.
Table 5. Recent real-world observational studies evaluating heterologous vaccine regimens (2 or 3 doses)*.
Summary of results
Immunogenicity of heterologous primary regimens
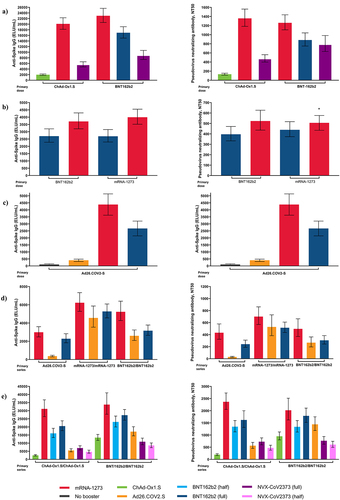
Heterologous 2-dose primary regimens have been identified as a valid tool to expand COVID-19 vaccination recommendations and may provide useful information about the ability to mix and match booster doses. As such, the Com-COV2 study showed that a heterologous primary vaccination series with a first dose of ChAd-Ox1.S or BNT162b2 followed by a second dose of mRNA-1273 was non-inferior to the homologous 2-dose primary schedule with ChAd-Ox1.S or BNT162b2.Citation20 At 28 days following the second dose, anti-spike immunoglobulin G (IgG) was highest for ChAd-Ox1.S/mRNA-1273 or BNT162b2/mRNA-1273 heterologous combinations compared with homologous primary combinations.Citation20 Notably, among the currently authorized vaccines assessed, the ChAd-Ox1.S/mRNA-1273 schedule showed the highest cellular response ().Citation20 Similar to the Com-COV2 study, results from the recent ARNCOMBI study found that heterologous BTN162b2/mRNA-1273 was non-inferior to homologous BNT162b2 or mRNA-1273 vaccine regimens ().Citation94 Results from the SWITCH trial in individuals who received Ad26.COV2.S as their primary vaccine dose showed that S-specific antibody binding was the highest among individuals who received mRNA-1273 as dose 2 followed by BNT162b2 as dose 2 () and a post hoc analysis also found that mRNA-1273 as dose 2 induced higher antibody binding compared with BNT162b2 as dose 2.Citation95
Figure 1. Immunogenicity outcomes by priming vaccine series and booster from five COVID-19 clinical trials including prime-boosting 2-dose regimens in: a) Com-COV2, b) ARNCOMBI, and c) SWITCH; and prime boosting 3-dose regimens in: d) NIH DMID and e) COV-BOOST. Response to booster is shown as bars.
Immunogenicity of heterologous booster regimens
Humoral and cellular immunogenicity data from heterologous booster studies are important in determining the best protective strategies against COVID-19 disease. Data from the phase 1/2 NIH DMID trial showed that at 29 days following boost, anti-spike IgG was highest in individuals boosted with mRNA-1273 (i.e. received mRNA-1273 for dose 3 after either a primary vaccination series of mRNA-1273, BNT192b2, or Ad26.COV2.S) when compared with other homologous or heterologous 3-dose regimens ().Citation26,Citation32 Likewise, pseudovirus antibody neutralization was highest following boosting with mRNA-1273 (for both homologous and heterologous regimen) compared with the other regimens evaluated ().Citation26 Evaluation of cellular immune response found that all three vaccines used for the primary vaccination series (mRNA-1273, BNT192b2, or Ad26.COV2.S) induced CD4+ T-cell responses, and similar to the kinetics of natural infection, persisted in the majority of individuals for up to 6 months.Citation26 Comparatively, a more durable CD8+ T-cell response was shown in participants who received a primary regimen of Ad26.COV2.S or mRNA vaccine with Ad26.COV2.S as a heterologous booster.Citation26
In the COV-BOOST study, anti-spike IgG levels were highest 28 days following boost among participants who received a heterologous boost with mRNA-1273 after a primary 2-dose series of ChAd-Ox1.S or BNT162b2 compared with a homologous booster vaccination ().Citation21 Notably, all vaccines given as the booster dose in this study induced substantial cellular immune responses in participants primed with two doses of ChAd-Ox1.S.Citation21 The mRNA vaccines and Ad26.COV2.S also showed increased T-cell responses when administered after a 2-dose primary vaccination series of ChAd-Ox1.S or BNT162b2, with ChAd-Ox1.S followed by an mRNA-1273 heterologous booster evoking the strongest T-cell response.Citation21 Further data from a sub-trial extension arm of COV-BOOST showed that administration of a fourth-dose of an mRNA-based vaccine to substantially increase both humoral and cellular immunity for approximately 7 months following the third-dose. Anti-spike IgG levels at Day 14 following the fourth dose were higher than those at Day 28 after the third dose for both the BNT162b2 and mRNA-1273 homologous and heterologous schedules.Citation93
Overall, the clinical studies discussed here provide evidence to suggest that heterologous primary and booster regimens typically produce immune responses that are similar to or better than homologous schedules.Citation20,Citation21,Citation32 Additionally, the studies suggest that a vaccination regimen including mRNA-based COVID-19 vaccines, notably mRNA-1273, may provide optimal immune responses against SARS-CoV-2 compared with other heterologous primary or booster vaccine regimens and that combined with an adenoviral vector-based vaccine primary regimen yields a more durable cellular immune response.Citation26
Heterologous boosting against emerging variants of concern
As SARS-CoV-2 VoCs continue to emerge within the COVID-19 landscape, vaccination strategies must be able to rapidly target them. Indeed, results from current clinical and real-world observational studies suggest that a heterologous boost regimen may serve as an effective approach.Citation21,Citation100,Citation104,Citation105
Currently, the majority of the published heterologous vaccine clinical trial data is from general populations of healthy adults. As more data are gathered, regimens may also later be tailored against specific circulating VoCs and specific populations most at risk of COVID-19 infection. Interim results from the randomized PRIBIVAC study evaluating homologous and heterologous boosting with mRNA-based vaccines found heterologous boosting with mRNA-1273 induced stronger neutralization capacity compared with homologous BNT162b2 against VoCs (including omicron) among older individuals aged ≥60 years (84% and 73%, p = .0073, respectively).Citation97
As the SARS-CoV-2 virus continues to evolve, booster vaccines are needed to provide protection against emerging VoCs. Results from the COV-BOOST clinical study found that heterologous boosting with mRNA vaccines generated humoral and cellular immune responses against VoCs strains comparable to those of the WT strains.Citation21 A ChAd-Ox1.S/ChAd-Ox1.S primary vaccination series plus an mRNA-1273 booster regimen had a similar neutralization capacity against the delta strain as against the WT (50% neutralizing antibody titer [N50] WT strain, geometric mean titer [GMR]: 26.98; 95% confidence interval [CI], 21.88–33.26 vs N50 delta, GMR: 27.17; 95% CI, 20.81–35.47).Citation21 Comparatively, for a ChAd-Ox1.S/ChAd-Ox1.S primary vaccination series plus BNT162b2 booster, neutralization antibody titers against the delta strain trended lower compared with WT strains (N50 WT, GMR: 21.58; 95% CI, 16.93–27.51 vs N50 delta, GMR: 14.43; 95% CI, 10.97–18.98).Citation21 Cellular immune responses associated with both of these mRNA vaccines were similar for the beta and delta strains compared with WT strains, with the strongest response against the delta strain observed among individuals who received vaccinations of ChAd-Ox1.S/ChAd-Ox1.S plus an mRNA-1273 booster.Citation21
Similarly, an observational study in Germany in adults assessing BNT162b2, mRNA-1273, and ChAd-Ox1.S heterologous primary and booster combinations in both COVID-19–naive and – convalescent individuals showed that humoral and cellular responses develop significantly faster with mRNA vaccines compared with ChAd-Ox1.S.Citation104 Additionally, the study demonstrated that compared with ChAd-Ox1.S, mRNA vaccines produce higher neutralizing antibody titers against SARS-CoV-2 VoCs, including the alpha, beta, and gamma variants.Citation104 The highest neutralization capacity against VoCs was induced by the heterologous ChAd-Ox1.S/mRNA-1273 regimen (87% for alpha, 85% for beta, and 71% for gamma), followed by ChAd-Ox1.S/BNT162b2 (82% for alpha, 70% for beta, and 55% for gamma).Citation104
Studies have also investigated the effectiveness of heterologous booster schedules against SARS-CoV-2 variants (). A large retrospective nationwide Swedish cohort study in adults assessed both heterologous boosting (a ChAd-Ox1.S primary vaccination series followed by BNT162b2 or a ChAd-Ox1.S primary vaccination series followed by mRNA-1273) and homologous boosting with ChAd-Ox1.S after ChAd-Ox1.S priming with a single dose.Citation100 In this study, heterologous ChAd-Ox1.S/mRNA-1273 and ChAd-Ox1.S/BNT162b2 vaccinations were associated with 79% and 67% VE against symptomatic COVID-19, respectively, including against disease caused by the delta variant, the dominant variant during the study period.Citation100 When examining all heterologous schedules combined, VE against symptomatic COVID-19 was significantly higher (68%, 95% CI, 61–74; p < .001) compared with the homologous ChAd-Ox1.S/ChAd-Ox1.S schedule (50%, 95% CI, 41–58; p < .001).Citation100
Real-world observational studies have provided snapshots of current vaccination strategies during times when the delta and omicron variants were dominant (). Results from the nationwide prospective FONASA study in Chile (during delta variant dominance) in which the primary regimen comprised 2-dose CoronaVac, indicated that heterologous boosting with BNT162b2 or ChAd-Ox1.S had superior VE across all clinical outcomes evaluated (symptomatic COVID-19, hospitalization, and admission to an intensive care unit) compared with homologous CoronaVac vaccination (three doses).Citation25 Data from a test-negative study in England found that boosting with mRNA-based vaccines offered protection against the delta variant, with respect to mild and severe COVID-19 disease; however, protection against symptomatic disease waned after 10 weeks.Citation102 Another test-negative study found that after a primary vaccination with either ChAd-Ox1.S or BNT162b2, boosting with either BNT162b2 or mRNA-1273 provided a substantial increase in protection against symptomatic disease, which again waned over a 5-to 9-week period; VE was substantially lower against omicron compared with the delta variant.Citation23
The increased immune evasion and hyper-transmissibility of omicron and the reported waning of VE following boosting further highlights the need to stay up-to-date with optimizing vaccination strategies.Citation66,Citation106 Indeed, mRNA-based vaccine boosters have been shown to be more protective compared with receiving only a primary mRNA-based vaccine series, especially noting the significantly higher difference in terms of 3- versus 2-dose protection against the omicron variant.Citation101,Citation107,Citation108 During omicron dominance, the VISION network study in the United States compared boosting with Ad26.COV2.S and mRNA-based vaccines with respect to VE against emergency department and urgent care visits. The study found that VE was highest with mRNA-based vaccine boosting (79% for an Ad26.COV2.S/mRNA-based vaccine regimen, 83% for a 3-dose mRNA-based vaccine regimen, and 54% for a homologous Ad26.COV2.S/Ad26.COV2.S regimen).Citation24 Similarly, VE against hospitalization was highest with mRNA-based vaccine boosting (78% for an Ad26.COV2.S single dose primary vaccination series followed by an mRNA-based booster, 90% for a 3-dose mRNA-based vaccine regimen, and 67% for an Ad26.COV2.S/Ad26.COV2.S regimen).Citation24 Another study based on data collected during omicron dominance indicated that the VE of mRNA-based vaccine boosting (mRNA-1273 or BNT162b2) was moderately effective in preventing infection (51%); the highest VE against infection with an mRNA-based booster (dose 3) was observed in individuals primed with 2-dose ChAd-Ox1.S (59%) and 2-dose mRNA-1273 (55%), followed by 2-dose BNT162b2 (50%) and Ad26.COV2.S (48%).Citation103
Overall, these real-world observational studies, in conjunction with the clinical data, suggest that mRNA-based heterologous boosting strategies may be successful against emerging variants. While most available data were collected during the dominant circulation of the delta, omicron, and subvariants BA.1 and BA.2, 2 doses of an mRNA-based vaccine regimen followed by a booster have also shown evidence of cross-neutralization against the BA.4/5 variant and omicron subvariant BA.2.12.1.Citation109 This suggests incremental clinical protection gains may still be obtained with the current boosting strategies. Additional data relating to the most recent omicron subvariants, BA.4/5, and future variants, will further inform such recommendations.Citation101,Citation108,Citation110,Citation111
Safety and reactogenicity of heterologous vaccine regimens
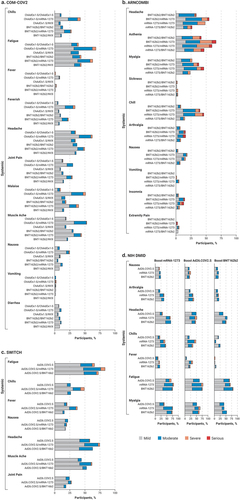
In addition to being able to elicit a strong immunogenic response, heterologous booster vaccination regimens also need to demonstrate an acceptable safety profile that is comparable to that of the primary vaccination series. Although the studies summarized here were not powered to detect reactogenicity differences and were limited in size, overall results showed that adverse events were short-lived. Among the studies reviewed, results from the phase 1/2 NIH DMID study demonstrated that both homologous and heterologous booster vaccinations combinations were well‐tolerated and that reactogenicity events such as injection site pain, malaise, headache, and myalgia within a 7-day period following booster administration were similar to those reported for the primary vaccination series ().Citation26,Citation32 Among the many booster permutations evaluated in the COV-BOOST study, three vaccines showed high reactogenicity, which included mRNA-1273 after a ChAd-Ox1.S or BNT162b2 2-dose primary vaccination series and ChAd-Ox1.S or Ad26.COV2.S after a BNT162b2 2-dose primary vaccination series.Citation21 Although not powered to compare regimens, mRNA-1273 appeared more reactogenic in terms of reported pain and redness at the injection site and fatigue compared with BNT162b2 within the 7-day period following booster administeration.Citation21 Participants who received an mRNA-based vaccine or Ad26.COV2.S after two doses of ChAd-Ox1.S demonstrated more frequent systemic and local adverse events within the 7-day period post-vaccination compared with individuals receiving other vaccine combinations.Citation21 Overall, serious adverse events were infrequent and were largely similar between vaccine and control groups.Citation21 A sub-trial extension arm of COV-BOOST conducted during the time period in which omicron was a dominant strain found that a second booster (dose 4 of the regimen) of BNT162b2 (30 µg) or mRNA-1273 (50 µg) was well tolerated, with short-lived reactogenicity events similar to the primary vaccination series (dose 2) and the first booster (dose 3), including pain, malaise, headache, myalgia, and fatigue experienced within the 7-day period following the booster dose.Citation93
Figure 2. Solicited local reactions within 0 to 7 days of injection in four clinical trials evaluating heterologous vaccine regimens. a) Com-COV2, b) ARNCOMBI, c) SWITCH, and d) NIH DMID.
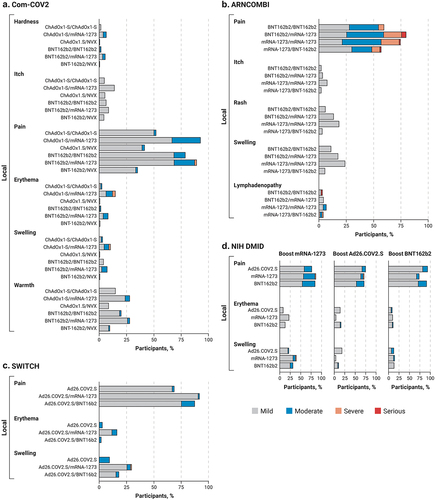
Figure 3. Solicited systemic reactions within 0 to 7 days of injection in four clinical trials evaluating heterologous vaccine regimens. a) Com-COV2, b) ARNCOMBI, c) SWITCH, and d) NIH DMID. Vaccine dosing regimens are as described per .
In Com-COV2, heterologous dose 2 with mRNA-1273 showed increased systemic reactogenicity compared with the homologous primary vaccination series within the 7-day period following dose 2, including feeling feverish (although with no increase in temperature); having chills, muscle aches, and general malaise; and reporting headache ().Citation20 Additionally, results suggest increased reactogenicity for the heterologous over the homologous mRNA primary vaccination series vaccine schedule (BNT162b2/mRNA-1273 vs BNT162b2/BNT162b2). However, this effect may be due to the mixing of different mRNA-based vaccines or due to the higher dosage of mRNA-1273.Citation20 In all, mRNA-based heterologous regimens produced transient local and systemic adverse events, with acceptable tolerability profiles, especially in the context of the benefits associated with higher immunogenicity and T-cell response.Citation20 In the ARNCOMBI study, although dose 2 with mRNA-1273 was associated with a higher rate of local and systemic adverse events (pain at the injections site, headache, myalgia, and joint pain) compared with dose 2 with BNT162b2 (p < .0001), overall, all homologous and heterologous primary vaccination regimens were well tolerated.Citation94
The safety and reactogenic profiles of mRNA-based boost-ers paired with various primary regimens administered worldwide have also been investigated. In the HKSH study, a 2-dose BBIBP-CorV or a 2-dose CoronaVac primary vaccination series, followed by a BNT162b2 booster, was reported to be safe, with adverse events similar to those reported for other heterologous mRNA-based vaccine regimens.Citation96
Although not described within publications from this search, rare cases of myocarditis and pericarditis have been reported after the administration of mRNA-1273 or BNT-162b2.Citation112,Citation113 In particular, rates of myocarditis in mRNA-1273 vaccine recipients were not higher than expected for the general population, with the exception of males aged 18–24 years.
Emerging mRNA-based vaccine boosters in development
As described previously, heterologous boosting with mRNA-based boosters elicits strong immune responses to WT SARS-CoV-2 infection and provided additional protection against VoCs such as omicron and its variants. To further counter emerging VoCs, booster vaccines targeting a specific variant and/or variants may provide better coverage and help equip healthcare professionals to prevent future outbreaks and avoid the associated impact on healthcare systems. The mRNA-based vaccine platform as a technology also offers a fast, efficient, and flexible solution to adapt to the COVID-19 variant landscape as it evolves. Clinical evaluation of a multivalent COVID-19 mRNA-based booster platform is currently underway, with bivalent booster candidates that include mRNA-1273.211, mRNA-1273.214, mRNA-1273.351, Pfizer-BioNTech’s omicron-adaptive vaccine, and mRNA-based vaccines containing the BA.4/BA.5 variant.Citation30,Citation99,Citation114,Citation115
mRNA-1273.211 contains equal amounts of two spike protein sequences from WT SARS-CoV-2 and the beta variant (the beta variant shares key antibody escape mutations as omicron); mRNA-1273.214 contains equal amounts of two spike protein sequences from WT SARS-CoV-2 infection, including omicron. Interim results from a phase 2/3 study of mRNA-1273.211 (NCT04927065; ) in adults have demonstrated increased neutralizing antibody titers with mRNA-1273.211 as a booster (dose 3) against the beta, delta, and omicron variants 4 weeks after administration compared with an mRNA-1273 booster.Citation98 This superior immunogenicity continued for beta and omicron variants 6 months following booster administration. In addition, the safety and tolerability profile were consistent with the authorized 50 μg mRNA-1273 booster.Citation98 Another phase 2/3 study evaluating the mRNA-1273.214 booster vaccine in adults (dose 3) is underway, with full results expected in late 2022 (NCT05249829; ). Preliminary results (1 month following booster dose) showed that mRNA-1273.214 administered as a heterologous booster (dose 3) exhibited a 5.4-fold increase in neutralizing antibody response (95% CI, 5.0–5.9) against omicron BA.4/BA.5 above baseline in all individuals regardless of prior SAR-2 COV-2 infection, and a 6.3-fold increase (95% CI, 5.7–6.9) in the subset of seronegative individuals.Citation115 The mRNA-1273.214 booster was generally well tolerated, and the reactogenicity and safety profiles were consistent with that of homologous boosting with mRNA-1273.Citation115 Pfizer-BioNTech’s omicron adaptive vaccines are also being evaluated as heterologous boosters (dose 3 or 4) in a phase 2/3 trial in individuals ≥56 years of age ().Citation114 Interim results showed that these vaccines candidates (monovalent and bivalent) elicited substantially higher responses against the omicron BA.1 subvariant compared with homologous boosting with BNT1626b and that both vaccines were generally well tolerated.Citation114
With the greater protection that mRNA-based vaccines strategies may offer against emerging VoCs, increasing accessibility will aid in optimizing global coverage. Supply of any vaccine can greatly depend on available storage facilities, and for future mRNA-based vaccines, improving ease of storage will further enhance vaccine options at the practical level. Currently, mRNA-1273 and BNT1626b must be stored at low temperatures (−50°C to −15°C; −90°C to −60°C, respectively) over a long period of time, although both can be stored undiluted in the refrigerator for up to 1 month.Citation37,Citation116 To enhance the storage supply, the refrigerator stable (2°C to 5°C) mRNA-based vaccine mRNA-1283 has been developed and is currently being evaluated as a next-generation mRNA-based vaccine in clinical trials (NCT04813796 and NCT05137236).Citation33,Citation117
Discussion
As various heterologous booster permutations continue to evolve, it remains important to assess the immunogenicity of these vaccination regimens; these data can then inform national vaccination recommendations. Available studies show that heterologous mRNA-based boosting, specifically with mRNA-1273, induces stronger humoral and cellular immune responses compared with homologous or heterologous boosts with BNT162b2 or ChAd-Ox1.S.Citation20,Citation21,Citation94 Currently, there is no agreed upon correlate of protection against SARS-CoV-2, although cellular and humoral responses are commonly used. Results from real-world studies also demonstrate that an mRNA-1273 or BNT162b2 heterologous booster following primary ChAd-Ox1.S vaccination provides strong protection against symptomatic COVID-19.Citation93,Citation100,Citation104 Additionally, for certain VoCs, heterologous ChAd-Ox1.S-Ox1/mRNA-1273 and ChAd-Ox1.S/BNT162b2 vaccination schedules have shown strong neutralization capacity.Citation21,Citation100,Citation104 Although a higher immunogenic response to mRNA-1273 may evoke greater protection, it may also be associated with more reactogenicity.Citation118 However, local and systemic adverse events are transient in nature (with a 7-day window following injection), and the safety and tolerability profile is generally acceptable, especially in the context of mRNA-1273, providing immune responses that ultimately help prevent serious outcomes associated with severe COVID-19 disease.
Currently, there are limited data investigating heterologous boosting against asymptomatic SARS-CoV-2 infection. During the emergence of omicron, a higher rate of asymptomatic infection was reported compared with during delta dominance (~23–26% and 8%, respectively), with results from a few studies that assessed booster vaccination, reporting that a higher proportion of individuals presented with asymptomatic infection and non-severe disease compared with individuals who were unvaccinated or received a primary vaccination series only.Citation119,Citation120 As populations are exposed to different circulating VoCs over time and receive various primary vaccination regimens, future heterologous mRNA-based boosting strategies might also assess the impact of asymptomatic infection in addition to symptomatic infection.
To help keep pace with the evolving COVID-19 pandemic, heterologous primary and booster vaccine strategies can offer safe and improved prophylactic options compared with homologous vaccine schedules in terms of both immunogenicity and reactogenicity.Citation121 Clinical and real-world evidence to date supports heterologous vaccination schedules among both COVID-19-convalescent and -naive populations, although the optimal booster combination and interval after completing the primary vaccination series are still being examined.Citation20,Citation21,Citation32,Citation100,Citation104,Citation122 In addition, the seasonal timing of a booster dose, use in children and adolescents, concomitant use with other routine vaccines, and ability to combat the emergence of current and future VoCs should also be further considered.
With 40 COVID-19 vaccines approved by the World Health Organization (WHO) and more likely to be approved in the future, considerations of heterologous boosters after region-specific primary vaccination regimens can simplify future booster strategies.Citation15 The current evidence strongly supports the administration of one booster (>1 booster dose for immunocompromised or older adults) to significantly reduce the risk of severe COVID-19 disease especially with the emergence of VoCs such as omicron.Citation23,Citation24,Citation101,Citation102 As of mid-July 2022, boost-ers have been administered to only 53%, 11%, and 1.2% of individuals (who have already received a primary COVID-19 vaccine regimen) in high-income, low- to middle-income and low-income countries, respectively.Citation123 Indeed, there remains a huge proportion of these populations who would benefit from the administration of a booster, and with various primary regimens supplied around the globe, heterologous boosting strategies can support the practicalities of supply and superior effectiveness within an acceptable safety profile to include protection against current and future VoCs.
The studies summarized in this review suggest that heterologous boosting with mRNA-based vaccines shows superior vaccine effectiveness, which may complement the various primary regimens already administered globally. Several studies have suggested that the addition of mRNA-1273 to vaccine regimens results in higher immunogenicity and observed protection even to “non-vaccine-matched” VoCs. In addition, the administration of an adenoviral vector-based vaccine within a heterologous mRNA-based vaccine regimen appears to increase overall CD8+ T-cell response. In a practical context, such “mix and match” strategies can serve to simplify the implementation of mass vaccination programs, which will continue to evolve as VoCs emerge across the COVID-19 landscape. A limitation of currently published studies is the absence of long-term data to assess the association between waning antibody response and overall vaccine protection.
Conclusions
Heterologous vaccine booster strategies following a primary vaccine series facilitate a safe, effective, and pragmatic approach toward preventing severe outcomes from COVID-19. In particular, flexible mRNA-based vaccine platforms provide the advantage of rapidly designing new vaccines against emerging SARS-CoV-2 variants that can be promptly deployed to mitigate COVID-19. It is also crucial to consider the primary regimens already admin-istered or still to be administered worldwide to determine how heterologous priming and boosting regimens may be optimized within this framework. Data from future clinical and real-world observational studies across the globe will further strengthen decisive COVID-19 booster vaccination strategies.
Author contributions
RNH was involved in the literature searches and the drafting of this manuscript. RD, RNH, PB, JMM, and BJK were involved with the critical review of this manuscript. All authors give final approval of the published version.
Acknowledgments
Medical writing and editorial assistance were provided by Clare Lee, PhD, of MEDiSTRAVA in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, Inc., and under the direction of the authors. This work was funded by Moderna, Inc.
Disclosure statement
RD, PB, and JMM are employees of Moderna, Inc., and hold stock/stock options. BJK is a consultant for Moderna, Inc., RNH was an employee of Moderna, Inc., at the time of writing the manuscript and held stock/stock options at the company.
Data availability statement
The data summarized in this review are from published articles and are publicly available.
Additional information
Funding
References
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–19. doi:10.1056/NEJMoa2034577.
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. doi:10.1056/NEJMoa2035389.
- Sadoff J, Gray G, Vandebosch A, Cardenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Fennema H, Spiessens B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–201. doi:10.1056/NEJMoa2101544.
- Falsey AR, Frenck RW, Walsh EE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Bailey R, Swanson KA, Xu X, et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med. 2021;385(17):1627–29. doi:10.1056/NEJMc2113468.
- World Health Organization. COVID-19 vaccine tracker and landscape; 2022.
- World Health Organization. Vaccines – COVID-19 vaccine tracker; 2022.
- World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern; 2021.
- Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, Dushoff J, Mlisana K, Moultrie H. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. Science. 2022;376(6593):eabn4947. doi:10.1126/science.abn4947.
- Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, Metzler M, Kohmer N, Hoehl S, Helfritz FA, et al. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. medRxiv 2021:2021.12.07.21267432.
- Cele S, Jackson L, Khan K, Khoury DS, Moyo-Gwete T, Tegally H, San JE, Cromer D, Scheepers C, Amoako D, et al. SARS-CoV-2 omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv 2021:2021.12.08.21267417.
- Dejnirattisai W, Shaw RH, Supasa P, Liu C, Stuart ASV, Pollard AJ, Liu X, Lambe T, Crook D, Stuart DI, et al. Reduced neutralisation of SARS-COV-2 Omicron-B.1.1.529 variant by post-immunisation serum. Lancet. 2022;399(10321):234–36. doi:10.1016/S0140-6736(21)02844-0.
- Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021;600(7889):367–68. doi:10.1038/d41586-021-03672-3.
- Lambrou AS, Shirk P, Steele MK, Paul P, Paden CR, Cadwell B, Reese HE, Aoki Y, Hassell N, Zheng X-Y, et al. Genomic surveillance for SARS-CoV-2 variants: predominance of the delta (B.1.617.2) and omicron (B.1.1.529) variants — United States, June 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(6):206–11. doi:10.15585/mmwr.mm7106a4.
- United States Food and Drug Administration. Coronavirus (COVID-19) update: FDA to hold advisory committee meeting on COVID-19 vaccines to discuss future boosters; 2022.
- World Health Organization. COVID-19 vaccine tracker, approved vaccines; 2022.
- World Health Organization. Tracking SARS-CoV-2 variants; 2022.
- Centers for Disease Control and Prevention. COVID-19 vaccine booster shots; 2021.
- Our World in Data. Coronavirus (COVID-19) vaccinations; 2022.
- United States Food and Drug Administration. Coronavirus (COVID-19) update: FDA takes additional actions on the use of a booster dose for COVID-19 vaccines; 2021.
- Stuart ASV, Shaw RH, Liu X, Greenland M, Aley PK, Andrews NJ, Cameron JC, Charlton S, Clutterbuck EA, Collins AM, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. The Lancet. 2022;399(10319):36–49. doi:10.1016/S0140-6736(21)02718-5.
- Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, Dodd K, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. The Lancet. 2021;398(10318):2258–76. doi:10.1016/S0140-6736(21)02717-3.
- Janssen C, Cachanado M, Ninove L, Lachatre M, Michon J, Epaulard O, Maakaroun-Vermesse Z, Chidiac C, Laviolle B, Aumaitre H, et al. Immunogenicity and reactogenicity of heterologous and homologous mRNA-1273 and BNT162b2 vaccination: a multicenter non-inferiority randomized trial. Preprints with the Lancet. 2021 Dec 20;48:101444. doi:10.1016/j.eclinm.2022.101444.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–46. doi:10.1056/NEJMoa2119451.
- Natarajan K, Prasad N, Dascomb K, Irving SA, Yang DH, Gaglani M, Klein NP, DeSilva MB, Ong TC, Grannis SJ, et al. Effectiveness of homologous and heterologous COVID-19 booster doses following 1 Ad.26.COV2.S (Janssen [Johnson & Johnson]) vaccine dose against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults - VISION network, 10 States, December 2021-March 2022. MMWR Morb Mortal Wkly Rep. 2022;71(13):495–502. doi:10.15585/mmwr.mm7113e2.
- Jara A, Undurraga EA, Zubizarreta JR, González C, Pizarro A, Acevedo J, Leo K, Paredes F, Bralic T, Vergara V, et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: a large-scale prospective cohort study. Lancet Glob Health. 2022;10(6):e798–806. doi:10.1016/S2214-109X(22)00112-7.
- Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, Rostad CA, Martin JM, Johnston C, Rupp RE, et al. Homologous and heterologous Covid-19 booster vaccinations. N Engl J Med. 2022;386(11):1046–57. doi:10.1056/NEJMoa2116414.
- Jamshidi E, Asgary A, Shafiekhani P, Khajeamiri Y, Mohamed K, Esmaily H, Jamal Rahi S, Mansouri N. Longevity of immunity following COVID-19 vaccination: a comprehensive review of the currently approved vaccines. Hum Vaccin Immunother. 2022;18(5):1–10. doi:10.1080/21645515.2022.2037384.
- Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. 2022;23(2):186–93. doi:10.1038/s41590-021-01122-w.
- Astrazeneca. Understanding risk: benefit; 2022.
- ClinicalTrials.gov. A study to evaluate the immunogenicity and safety of omicron variant vaccines in comparison with mRNA-1273 booster vaccine for COVID-19; 2022.
- Advisory Committee on Immunization Practices (ACIP). COVID-19 ACIP vaccine recommendations; 2022.
- Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, Rostad CA, Martin JM, Johnston C, Rupp RE, et al. Heterologous SARS-CoV-2 booster vaccinations - preliminary report. medRxiv. 2021.
- ClinicalTrials.gov. A study to evaluate the immunogenicity and safety of mRNA-1283 COVID-19 vaccine boosters; 2022.
- United States Food and Drug Administration. COVID-19 vaccines; 2022.
- European Medicines Agency. COVID-19 vaccines; 2021.
- United States Food and Drug Administration. Fact sheet for healthcare providers administering vaccine emergency use authorization of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019; 2022.
- Pfizer-BioNTech. Prescribing information COMIRNATY; 2021.
- Janssen Pharmaceutical Companies. Fact sheet for healthcare providers administering vaccine (vaccination providers) Emergency Use Authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19); 2022.
- European Medicines Agency. Vaxzevria, COVID-19 vaccine (ChAdOx1-S [recombinant]); 2021.
- European Medicines Agency. Jcovden (previously COVID-19 vaccine Janssen); 2022.
- European Medicines Agency. EMA recommends Nuvaxovid for authorisation in the EU; 2021.
- Dunkle LM, Kotloff KL, Gay CL, Áñez G, Adelglass JM, Barrat Hernández AQ, Harper WL, Duncanson DM, McArthur MA, Florescu DF, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2021;386(6):531–43. doi:10.1056/NEJMoa2116185.
- United States Food and Drug Administration. Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum - mRNA-1273; 2020.
- United States Food and Drug Administration. Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum - BNT162b2; 2020.
- United States Food and Drug Administration. FDA Approves First COVID-19 Vaccine; 2021.
- United States Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Takes Key Action by Approving Second COVID-19 Vaccine; 2022.
- United States Food and Drug Administration. Coronavirus (COVID-19) Update: FDA authorizes Moderna and Pfizer-BioNtech COVID-19 vaccines for children down to 6 months of age; 2022.
- European Medicines Agency. COVID-19 vaccine Janssen EPAR product information; 2021.
- United States Food and Drug Administration. Novovax (NVX-CoV2373) COVID-19 vaccine adjuvant. 2022.
- United States Food and Drug Administration. Janssen COVID-19 vaccine; 2022.
- United States Food and Drug Administration. Novavax COVID-19 vaccine, adjuvanted; 2022.
- United States Food and Drug Administration. Janssen LOA limit use due to TTS; 2022.
- European Medicines Agency. COVID-19 vaccine AstraZeneca; 2021.
- Government of Canada. Approved COVID-19 vaccines; 2022.
- Government of Australia. Approved COVID-19 vaccines Australia; 2022.
- Centers for Disease Control and Prevention. COVID data tracker; 2022.
- Bruxvoort KJ, Sy LS, Qian L, Ackerson BK, Luo Y, Lee GS, Tian Y, Florea A, Takhar HS, Tubert JE, et al. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: interim results from a prospective observational cohort study. Lancet Reg Health Am. 2021:100134. doi:10.2139/ssrn.3916094.
- Andrejko KL, Pry J, Myers JF, Jewell NP, Openshaw J, Watt J, Jain S, Lewnard JA, Samani H, Li SS, et al. Prevention of coronavirus disease 2019 (COVID-19) by mRNA-based vaccines within the general population of California. Clin Infect Dis. 2022;74(8):1382–89. doi:10.1093/cid/ciab640.
- Chung H, He S, Nasreen S, Sundaram ME, Buchan SA, Wilson SE, Chen B, Calzavara A, Fell DB, Austin PC, et al. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ. 2021;374(n1943):n1943. doi:10.1136/bmj.n1943.
- Fowlkes A, Gaglani M, Groover K, Groover K, Thiese MS, Tyner H, Ellingson K. Effectiveness of COVID-19 vaccines in preventing SARS-CoV-2 infection among frontline workers before and during B.1.617.2 (Delta) variant predominance — eight U.S. locations, December 2020–august 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1167–69. doi:10.15585/mmwr.mm7034e4.
- Nanduri S, Pilishvili T, Derado G, Soe MM, Dollard P, Wu H, Li Q, Bagchi S, Dubendris H, Link-Gelles R, et al. Effectiveness of Pfizer-BioNtech and Moderna vaccines in preventing SARS-CoV-2 infection among nursing home residents before and during widespread circulation of the SARS-CoV-2 B.1.617.2 (Delta) variant - National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1163–66. doi:10.15585/mmwr.mm7034e3.
- Rosenberg ES, Holtgrave DR, Dorabawila V, Conroy M, Greene D, Lutterloh E, Backenson B, Hoefer D, Morne J, Bauer U, et al. New COVID-19 cases and hospitalizations among adults, by vaccination status — New York, May 3–July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(37):1306–11. doi:10.15585/mmwr.mm7037a7.
- Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, Sabo RT, Hall N, Foreman A, Schubert PL, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1059–62. doi:10.15585/mmwr.mm7031e2.
- Goldberg Y, Mandel M, Bar-On YM, Bodenheimer O, Freedman L, Ash N, Alroy-Preis S, Huppert A, Milo R. Protection and waning of natural and hybrid COVID-19 immunity. N Engl J Med. 2021;386(23):2201–12. doi:10.1056/NEJMoa2118946.
- Naranbhai V, Garcia-Beltran WF, Chang CC, Berrios Mairena C, Thierauf JC, Kirkpatrick G, Onozato ML, Cheng J, St Denis KJ, Lam EC, et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 vaccines. J Infect Dis. 2022;225(7):1141–50. doi:10.1093/infdis/jiab593.
- Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, Bruxvoort KJ, Tubert JE, Florea A, Ku JH, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28(5):1063–71. doi:10.1038/s41591-022-01753-y.
- Hansen CH, Schelde AB, Moustsen-Helm IR, Emborg H-D, Krause TG, Mølbak K, Valentiner-Branth P. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv 2021:2021.12.20.21267966.
- United States Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes second booster dose of two COVID-19 vaccines for older and immunocompromised individuals; 2022.
- Baden LR, El Sahly HM, Essink B, Follmann D, Neuzil KM, August A, Clouting H, Fortier G, Deng W, Han S, et al. Covid-19 in the phase 3 trial of mRNA-1273 during the delta-variant surge. medRxiv 2021:2021.09.17.21263624.
- Tanriover MD, Akova M. COVID-19 vaccine booster strategy: striving for best practice. Lancet Glob Health. 2022;10(6):e774–5. doi:10.1016/S2214-109X(22)00204-2.
- Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, Gallagher E, Chand M, Brown K, Ladhani SN, et al. Vaccine effectiveness and duration of protection of Comirnaty, Vaxzevria and Spikevax against mild and severe COVID-19 in the UK. medRxiv 2021:2021.09.15.21263583.
- Bar-On YM, Goldberg Y, Mandel M, Bodenheimer O, Freedman L, Kalkstein N, Mizrahi B, Alroy-Preis S, Ash N, Milo R, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–400. doi:10.1056/NEJMoa2114255.
- Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, Reis BY, Balicer RD. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. The Lancet. 2021;398(10316):2093–100. doi:10.1016/S0140-6736(21)02249-2.
- Drawz PE, DeSilva M, Bodurtha P, Benitez GV, Murray A, Chamberlain AM, Dudley RA, Waring S, Kharbanda AB, Murphy D, et al. Effectiveness of BNT162b2 and mRNA-1273 second doses and boosters for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and SARS-CoV-2–related hospitalizations: a statewide report from the Minnesota electronic health record consortium. Clin Infect Dis. 2022;75(5):890–92. Online ahead of print. doi:10.1093/cid/ciac110.
- Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta K-D, House T, Hay J, Bell JI, Newton JN, et al. Impact of delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. medRxiv 2021:2021.08.18.21262237.
- Chu L, Montefiori D, Huang W, Nestorova B, Chang Y, Carfi A, Edwards DK, Oestreicher J, Legault H, Girard B, et al. Immune memory response after a booster injection of mRNA-1273 for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). medRxiv 2021:2021.09.29.21264089.
- Khandker SS, Godman B, Jawad MI, Meghla BA, Tisha TA, Khondoker MU, Haq MA, Charan J, Talukder AA, Azmuda N, et al. A systematic review on COVID-19 vaccine strategies, their effectiveness, and issues. Vaccines (Basel). 2021;9(12):1387. doi:10.3390/vaccines9121387.
- Rzymski P, Camargo CA Jr., Fal A, Flisiak R, Gwenzi W, Kelishadi R, Leemans A, Nieto JJ, Ozen A, Perc M, et al. COVID-19 vaccine boosters: the good, the bad, and the ugly. Vaccines (Basel). 2021;9(11):1299. doi:10.3390/vaccines9111299.
- Shekhar R, Garg I, Pal S, Kottewar S, Sheikh AB. COVID-19 vaccine booster: to boost or not to boost. Infect Dis Rep. 2021;13(4):924–29. doi:10.3390/idr13040084.
- Cheng H, Peng Z, Si S, Alifu X, Zhou H, Chi P, Zhuang Y, Mo M, Yu Y. Immunogenicity and safety of homologous and heterologous prime–boost immunization with COVID-19 vaccine: systematic review and meta-analysis. Vaccines (Basel). 2022;10(5):798. doi:10.3390/vaccines10050798.
- Chu L, Vrbicky K, Montefiori D, Huang W, Nestorova B, Chang Y, Carfi A, Edwards DK, Oestreicher J, Legault H, et al. Immune response to SARS-CoV-2 after a booster of mRNA-1273: an open-label phase 2 trial. Nat Med. 2022;28(5):1042–49. doi:10.1038/s41591-022-01739-w.
- United States Food and Drug Administration. FDA authorizes booster dose of Pfizer-BioNtech COVID-19 vaccine for certain populations; 2021.
- United States Food and Drug Administration. Coronavirus (COVID-19) update: FDA expands eligibility for COVID-19 vaccine boosters; 2021.
- European Centre for Disease Prevention and Control. EMA and ECDC recommendations on heterologous vaccination courses against COVID-19; 2021.
- United States Food and Drug Administration. Do I qualify for a COVID-19 vaccine booster and which one? 2022.
- Lu S. Heterologous prime–boost vaccination. Curr Opin Immunol. 2009;21(3):346–51. doi:10.1016/j.coi.2009.05.016.
- Barry H, Mutua G, Kibuuka H, Anywaine Z, Sirima SB, Meda N, Anzala O, Eholie S, Bétard C, Richert L, et al. Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in healthy and HIV-infected adults: a randomised, placebo-controlled phase II clinical trial in Africa. PLoS Med. 2021;18(10):e1003813. doi:10.1371/journal.pmed.1003813.
- United States Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Moderna and Pfizer-BioNtech bivalent COVID-19 vaccines for use as a booster dose in younger age groups; 2022.
- Gilbert PB, Montefiori DC, McDermott AB, Fong Y, Benkeser D, Deng W, Zhou H, Houchens CR, Martins K, Jayashankar L, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. doi:10.1126/science.abm3425.
- Perry J, Osman S, Wright J, Richard-Greenblatt M, Buchan SA, Sadarangani M, Bolotin S. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS One. 2022;17(4):e0266852. doi:10.1371/journal.pone.0266852.
- Paramithiotis E, Sugden S, Papp E, Bonhomme M, Chermak T, Crawford SY, Demetriades SZ, Galdos G, Lambert BL, Mattison J, et al. Cellular immunity is critical for assessing COVID-19 vaccine effectiveness in immunocompromised individuals. Front Immunol. 2022;13:880784. doi:10.3389/fimmu.2022.880784.
- Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, Dold C, Fuskova M, Gilbert SC, Hirsch I, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–40. doi:10.1038/s41591-021-01540-1.
- Munro APS, Feng S, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, Bula M, Cathie K, Chatterjee K, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdox1 nCov-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22(8):1131–41. doi:10.1016/S1473-3099(22)00271-7.
- Janssen C, Cachanado M, Ninove L, Lachatre M, Michon J, Epaulard O, Maakaroun-Vermesse Z, Chidiac C, Laviolle B, Aumaitre H, et al. Immunogenicity and reactogenicity of heterologous and homologous mRNA-1273 and BNT162b2 vaccination: a multicenter non-inferiority randomized trial. EClinicalMedicine. 2022;48:101444. doi:10.1016/j.eclinm.2022.101444.
- Sablerolles RSG, Rietdijk WJR, Goorhuis A, Postma DF, Visser LG, Geers D, Schmitz KS, Garcia Garrido HM, Koopmans MPG, Dalm VASH, et al. Immunogenicity and reactogenicity of vaccine boosters after Ad26.COV2.S priming. N Engl J Med. 2022;386(10):951–63. doi:10.1056/NEJMoa2116747.
- Lai KT, Lai Wan Loong EY, Fung TL, Luk LW, Lau CC, Zee JS, Ma ESK, Tang BSF. Safety and immunogenicity of a booster vaccination by CoronaVac or BNT162b2 in previously two-dose inactivated virus vaccinated individuals with negative neutralizing antibody. Vaccines (Basel). 2022;10(4):556. doi:10.3390/vaccines10040556.
- Poh XY, Tan CW, Lee IR, Chavatte JM, Fong SW, Prince T, Hartley C, Yeoh AYY, Rao S, Chia PY, et al. Antibody response of heterologous vs homologous mRNA vaccine boosters against the SARS-CoV-2 Omicron variant: interim results from the PRIBIVAC study, a randomized clinical trial. Clin Infect Dis. 2022;345. doi:10.2139/ssrn.4056669.
- Chalkias S, Eder F, Essink B, Khetan S, Nestorova B, Feng J, Chen X, Chang Y, Zhou H, Montefiori D, et al. 2022. Safety, immunogenicity and antibody persistence of a bivalent beta-containing booster vaccine. Preprint research square. https://www.researchsquare.com/article/rs-1555201/v1.
- Anderson E, Jackson L, Rouphael N, Widge A, Montefiori D, Doria-Rose N, Suthar M, Cohen K, O’Connell S, Makowski M, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 mRNA vaccine — an interim analysis. Res Sq. 2022.
- Nordström P, Ballin M, Nordström A. Effectiveness of heterologous ChAdox1 nCov-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: a nationwide cohort study. Lancet Reg Health – Eu. 2021;11:11. doi:10.1016/j.lanepe.2021.100249.
- Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, Miller J, Schrag SJ, Verani JR. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–51. doi:10.1001/jama.2022.0470.
- Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, Ramsay M, Lopez Bernal J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831–37. doi:10.1038/s41591-022-01699-1.
- Monge S, Rojas-Benedicto A, Olmedo C, Mazagatos C, José Sierra M, Limia A, Martín-Merino E, Larrauri A, Hernán MA, Moreno D, et al. Effectiveness of mRNA vaccine boosters against infection with the SARS-CoV-2 omicron (B.1.1.529) variant in Spain: a nationwide cohort study. Lancet Infect Dis. 2022;22(9):1313–20. doi:10.1016/S1473-3099(22)00292-4.
- Fabricius D, Ludwig C, Scholz J, Rode I, Tsamadou C, Jacobsen EM, Winkelmann M, Grempels A, Lotfi R, Janda A, et al. mRNA vaccines enhance neutralizing immunity against SARS-CoV-2 variants in convalescent and ChAdox1-primed subjects. Vaccines (Basel). 2021;9(8):918. doi:10.3390/vaccines9080918.
- Clemens JQ. Infection and inflammation of the genitourinary tract. J Urol. 2022;207(1):209. doi:10.1097/JU.0000000000002269.
- Willett BJ, Grove J, MacLean OA, Wilkie C, Logan N, Lorenzo GD, Furnon W, Scott S, Manali M, Szemiel A, et al. The hyper-transmissible SARS-CoV-2 Omicron variant exhibits significant antigenic change, vaccine escape and a switch in cell entry mechanism. medRxiv 2022:2022.01.03.21268111.
- Thompson MG, Natarajan K, Irving SA, Rowley EA, Griggs EP, Gaglani M, Klein NP, Grannis SJ, DeSilva MB, Stenehjem E, et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):139–45. doi:10.15585/mmwr.mm7104e3.
- Pajon R, Doria-Rose NA, Shen X, Schmidt SD, O’Dell S, McDanal C, Feng W, Tong J, Eaton A, Maglinao M, et al. SARS-CoV-2 omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med. 2022;386(11):1088–91. doi:10.1056/NEJMc2119912.
- Qu P, Faraone J, Evans JP, Zou X, Zheng Y-M, Carlin C, Bednash JS, Lozanski G, Mallampalli RK, Saif LJ, et al. Neutralization of the SARS-CoV-2 Omicron BA.4/5 and BA.2.12.1 subvariants. N Engl J Med. 2022;386(26):2526–28. doi:10.1056/NEJMc2206725.
- Young-Xu Y, Zwain GM, Izurieta HS, Korves C, Powell EI, Smith J, Balajee A, Holodniy M, Beenhouwer DO, Rodriguez-Barradas MC, et al. Effectiveness of mRNA COVID-19 vaccines against omicron and delta variants in a matched test-negative case–control study among US veterans. BMJ Open. 2022;12(8):e063935. doi:10.1136/bmjopen-2022-063935.
- Assawakosri S, Kanokudom S, Suntronwong N, Auphimai C, Nilyanimit P, Vichaiwattana P, Thongmee T, Duangchinda T, Chantima W, Pakchotanon P, et al. Neutralizing activities against the omicron variant after a heterologous booster in healthy adults receiving two doses of coronavac vaccination. J Infect Dis. 2022:jiac092.
- Straus W, Urdaneta V, Esposito DB, Mansi JA, Rodriguez CS, Burton P, Vega JM. Analysis of myocarditis among 252 million mRNA-1273 recipients worldwide. Clin Infect Dis. 2022. doi:10.1093/cid/ciac446.
- Hause AM, Baggs J, Marquez P, Abara WE, Olubajo B, Myers TR, Su JR, Thompson D, Gee J, Shimabukuro TT, et al. Safety monitoring of COVID-19 vaccine booster doses among persons aged 12–17 Years — United States, December 9, 2021–February 20, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(9):347–51. doi:10.15585/mmwr.mm7109e2.
- Pfizer-BioNTech. Pfizer and BioNtech announce omicron-adapted COVID-19 vaccine candidates demonstrate high immune response against omicron; 2022.
- Moderna, Inc. Moderna announces bivalent booster mRNA-1273.214 demonstrates potent neutralizing antibody response against omicron subvariants BA.4 and BA.5; 2022.
- United States Food and Drug Administration. Fact sheet for healthcare providers administering vaccine (vaccination providers) Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19); 2021.
- ClinicalTrials.gov. A study to evaluate safety, reactogenicity, and immunogenicity of mRNA-1283 and mRNA-1273 vaccines in healthy adults between 18 years and 55 years of age to prevent COVID-19; 2022.
- Siangphoe U, Baden LR, El Sahly HM, Essink B, Ali K, Berman G, Tomassini JE, Deng W, Pajon R, McPhee R, et al. Associations of immunogenicity and reactogenicity after SARS-CoV-2 mRNA-1273 vaccine in COVE and TeenCOVE trials. Clin Infect Dis. 2022. doi:10.1093/cid/ciac780.
- Garrett N, Tapley A, Andriesen J, Seocharan I, Fisher LH, Bunts L, Espy N, Wallis CL, Randhawa AK, Miner MD, et al. High asymptomatic carriage with the omicron variant in South Africa. Clin Infect Dis. 2022;75(1):e289–92. doi:10.1093/cid/ciac237.
- Yu W, Guo Y, Zhang S, Kong Y, Shen Z, Zhang J. Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 omicron variant: a systematic review and analysis. J Med Virol. 2022;94(12):5790–801. doi:10.1002/jmv.28066.
- Chiu NC, Chi H, Tu YK, Huang YN, Tai YL, Weng SL, Chang L, Huang DTN, Huang F-Y, Lin C-Y. To mix or not to mix? A rapid systematic review of heterologous prime–boost covid-19 vaccination. Expert Rev Vaccines. 2021;20(10):1211–20. doi:10.1080/14760584.2021.1971522.
- Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, Cameron JC, Charlton S, Clutterbuck EA, Collins AM, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–69. doi:10.1016/S0140-6736(21)01694-9.
- Our world in data. Coronavirus pandemic (COVID-19); 2022.
