ABSTRACT
Multiple sclerosis (MS) is an autoimmune disorder causing demyelination and neurodegeneration in the central nervous system. MS is characterized by disturbed motor performance and cognitive impairment. Current MS treatments delay disease progression and reduce relapse rates with general immunomodulation, yet curative therapies are still lacking. Regulatory T cells (Tregs) are able to suppress autoreactive immune cells, which drive MS pathology. However, Tregs are functionally impaired in people with MS. Interestingly, Tregs were recently reported to also have regenerative capacity. Therefore, experts agree that Treg cell therapy has the potential to ameliorate the disease. However, to perform their local anti-inflammatory and regenerative functions in the brain, they must first migrate across the blood-brain barrier (BBB). This review summarizes the reported results concerning the migration of Tregs across the BBB and the influence of Tregs on migration of other immune subsets. Finally, their therapeutic potential is discussed in the context of MS.
Introduction
Multiple sclerosis (MS) is an autoimmune disease characterized by demyelinating lesions and neurodegeneration in the central nervous system (CNS). MS affects close to 2.8 million people worldwide, with women having a higher prevalence than men (3:1 ratio).Citation1–3 Demyelination results from chronic neuroinflammation caused by autoreactive immune cells and CNS resident cells.Citation4 Symptoms of the disease manifest as a heterogeneous spectrum covering motor, sensory, visual, autonomic, and cognitive features depending on the spatiotemporal distribution of the lesions.Citation2,Citation5 Current therapies are found effective in delaying disease progression and decreasing relapse rates while symptomatic treatments manage acute relapses and symptoms related to neuroinflammation. These disease-modifying treatments (DMT) are available, yet these are associated with many side effects and are not curative.Citation5,Citation6 In addition, they generally focus on the inflammatory component, which is mainly present in early, relapsing-remitting MS (RR-MS).Citation7 Indeed, the only DMT available for people with primary progressive MS (PP-MS) is ocrelizumab.Citation8 Siponimod is the only approved treatment for secondary progressive MS (SP-MS).Citation9 Therefore, research about novel therapeutic strategies for MS is of great importance and the topic of extensive research. Since regulatory T cells (Tregs) suppress autoreactive T cells, they are currently under investigation as a novel cell therapy for MS.Citation10,Citation11 Furthermore, they show interesting reparative properties such as remyelination,Citation12–15 mediated by growth-regulatory protein cellular communication network factor 3 (CCN3),Citation12 and neural stem cell proliferation.Citation16 In addition, they suppress neurotoxic astrogliosisCitation17 and microglia.Citation18 However, to exert their functions at the site of inflammation, these cells must first cross the blood-brain barrier (BBB). The migratory capacity of Tregs and their influence on migration of other immune cells to the brain are discussed in this review, in light of their therapeutic potential for MS.
MS immunopathology
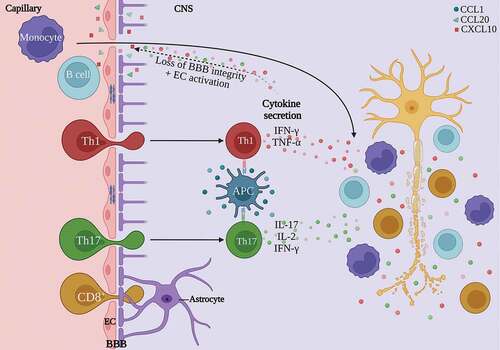
An important hallmark of the early inflammatory phase of MS is breakdown of the BBB.Citation19 The BBB mainly consists of endothelial cells, connected by tight junctions; the glia limitans, produced by astrocytic endfeet; and pericytes. This structure normally prevents the entry of immune cells and nonspecific molecules into the CNS, while tightly controlled exchange of nutrients and waste products is ensured.Citation20 Due to the neuroinflammation in MS, the BBB becomes leaky. Junctional proteins are disturbed and adhesion molecules are upregulated ().Citation21 Subsequently, immune cells infiltrate the CNS and effector CD4+ T cells are locally reactivated by antigen-presenting cells (APCs) presenting their cognate autoantigen. The main effector cells are the CD4+ T helper cell (Th) subsets Th1 and Th17, and CD8+ cytotoxic T cells. Th1 cells were found to be increased in people with MS, from which the myelin-reactive compartment was mostly memory cells.Citation22,Citation23 This indicates a previous activation of these Th1 cells reactive to myelin components. Increased frequencies of Th17 cells and interleukin (IL)-17 were detected in MS lesions, which were also correlated with disease activity.Citation24–26 Other Th subsets also contribute to MS pathology. Th1-like Th17 cells are increased in the CNS of EAE and people with MSCitation27 and the blood during a relapse.Citation28 This T cell population expresses both interferon gamma (IFN-γ) and IL-17 and possibly contributes to disease activity.Citation29 Th22 cells are a major source of IL-22 production.Citation30,Citation31 This cytokine is increased in the serumCitation32 and active lesionsCitation33 of people with MS while it is reduced during the recovery phase in EAE.Citation34 It is also involved in BBB disruption and CNS inflammation.Citation35 All of these cell types cause an inflammatory cascade in the CNS by releasing chemokines and pro-inflammatory cytokines such as IFN-γ, IL-2, tumor necrosis factor alpha (TNF-α) and oncostatin M (OSM).Citation2,Citation36,Citation37 As a consequence, the BBB becomes inflamed and other immune cells like B cells and macrophages are recruited and activated. In MS, activated B cells were shown to excessively produce the cytokines lymphotoxin, TNF-α and granulocyte macrophage-colony stimulating factor (GM-CSF), next to their extensive antibody production marking the myelin for phagocytosis.Citation38,Citation39 Additionally, B cells present antigens that induce proliferation of autoreactive CD4+ T cells.Citation40 However, the most convincing evidence for the importance of B cells in MS pathology is the efficacy of B cell depleting therapies in MS. Ocrelizumab, a humanized monoclonal antibody that selectively targets and depletes CD20+ B cells, is approved for the treatment of active RR-MS and PP-MS.Citation41 This therapy depletes B cells in most stages, thereby eliminating B and T cell interactions and reducing T cell activation and proliferation.Citation40,Citation42 B cell depletion is associated with significantly lower rates of disease activity and progression, suggesting a key role of B cells in MS.Citation8,Citation43 Macrophages infiltrating the inflamed CNS were shown to predominantly adopt the highly inflammatory M1 phenotype.Citation44 Here, they have an increased secretion of inflammatory cytokines and toxic mediators, hence contributing to disease severity and progression.Citation45,Citation46 Mast cells are also involved in MS pathology as they promote demyelination, present myelin antigens to T cells and disrupt the BBB.Citation47 This immune cascade ultimately cumulates in extensive demyelination, axonal and neuronal injury and eventually brain atrophy ().Citation36
Figure 1. Summary of the immunopathology of MS. Peripherally-activated CD4+ T cell subsets Th1 and Th17, together with CD8+ T cells migrate over the BBB. Here, CD4+ T cells are reactivated by APCs. They secrete pro-inflammatory cytokines responsible for BBB breakdown and endothelial cell activation. Activated endothelial cells will produce inflammatory cytokines and chemokines, which will enhance neuroinflammation. Additionally, B cells and macrophages are recruited and activated. Altogether, this neuroinflammatory response causes demyelination and neurodegeneration in the CNS. APC, antigen-presenting cell; BBB, blood-brain barrier; CCL, C-C motif chemokine ligand; CNS, central nervous system; CXCL, C-X-C motif chemokine ligand; EC, endothelial cell; IFN-γ, interferon-gamma; IL, interleukin; Th, T helper; TNF-α, tumor necrosis factor-alpha. Figure created with Biorender.com.
Treg (dys)functionality in MS
Thymus-derived Tregs (tTregs) are CD4+ CD25+ Forkhead box P3+ (FOXP3+) cells that, in healthy conditions, suppress effector T cells and other immune cells from reacting against self-antigens (reviewed by Janssens et al.Citation48). They maintain peripheral self-tolerance by expressing co-inhibitory molecules and secreting anti-inflammatory cytokines. When transforming growth factor beta (TGF-β) and IL-2 are available in the microenvironment, Tregs can develop in the periphery (pTregs) from CD4+ T cells.Citation49 One subtype of pTregs is Tr1 cells, which are characterized by high levels of IL-10 secretion without expressing FOXP3.Citation50 Under inflammatory circumstances, Tregs can adapt a Th17 phenotype and become exFOXP3 effector cells.Citation51 For example, IL-17 producing FOXP3+ Tregs have undergone inflammatory conversion and either transiently or stably express FOXP3 together with IL-17.Citation50,Citation52 These CD4+ Treg subtypes demonstrate the extensive phenotype plasticity of Tregs (reviewed by Baeten et al.Citation10). Another Treg subset, CD8+ Tregs, have a regulatory function by killing antigen-activated CD4+ T cells by the use of perforin.Citation53 Lastly, regulatory B cells (Bregs) have a similar function to Tregs, suppressing the inflammatory immune response in healthy individuals.Citation54 In this review, the focus is on CD4+CD25+FOXP3+ Tregs. The suppressive capacity of Tregs can also differ due to alternative splicing of FOXP3.Citation55 There are two main isoforms, a full-length FOXP3 and a FOXP3 lacking exon 2.Citation56 Alterations in splice variants can have implications in the development of autoimmunity and MS.Citation56,Citation57 For instance, Tregs from people with MS have a reduced expression of the epitope encoded by exon 2, which normally prevents the development of Th17 cells.Citation56 Tregs expressing this FOXP3 lacking exon 2 were therefore found to be unstable and less suppressive.Citation58
It was shown that Treg numbers and function are disturbed in people with MS. Lower numbers of Tregs have been detected in MS peripheral blood samples.Citation59–61 Depending on the treatment or disease status, Tregs could be identified in some, yet not all MS lesions.Citation26,Citation62 In contrast, in the cerebrospinal fluid (CSF) of people with MS, an increased number of Tregs was identified, although these were mostly apoptosis-prone CD95high cells.Citation61–63 Tregs from people with MS are less immunosuppressive compared to healthy controls, unleashing autoreactivity and inflammation in the CNS.Citation61–67 One crucial factor related to this suppressive dysfunction is the decreased expression of the transcription factor FOXP3 in Tregs.Citation68 Besides, dysfunction of Bregs has been indicated in MS.Citation69 This is characterized by reduced IL-10 production in vitro and a reduced number of IL-10-producing Bregs.Citation69,Citation70 Th1 effector cell suppression by Bregs was also dysfunctional in people with MS.Citation71 Accordingly, Breg dysfunction possibly contributes to MS development or maintenance.
Next to the peripheral effects of Tregs, once migrated through the BBB, they can perform their suppressive properties in the CNS. However, in a preclinical murine model for MS, called experimental autoimmune encephalomyelitis (EAE), Tregs were found to become unstable after entry in the CNS due to the downregulation of FOXP3 expression, leading to a reduced suppressive capacity.Citation72,Citation73 In a different study, myelin-specific Tregs accumulate in the CNS and do effectively suppress naïve myelin oligodendrocyte glycoprotein (MOG)-specific T cells.Citation74 CNS-derived encephalitogenic T effector cells, however, were found to be resistant to this suppression during the active phase of the disease.Citation74 This effect is likely due to the production of IL-6 and TNF-α in inflamed tissue, making T cells less sensitive to Tregs. In contrast, it has been demonstrated that Treg accumulation has suppressive properties in the CNS of EAE mice, mediated by the production of IL-10.Citation75 This effect is primarily responsible for the remission phase of EAE. It was also shown that Tregs that have entered the CNS are more activated and have a more protective role compared to Tregs derived from the lymph nodes.Citation75,Citation76 Although studies in EAE models have brought on much knowledge on autoimmune pathology and neuroinflammation, caution should be taken when translating the results to MS. EAE is a mainly T cell driven pathology, hence largely ignoring the role of B cells.Citation77,Citation78 Additionally, the progressive phases of MS are only limitedly represented by EAE models.Citation79,Citation80 Despite their disadvantages, EAE and MS share several key features such as neuroinflammation and BBB disruption.Citation81,Citation82 These animal models are crucial in acquiring knowledge on MS immunopathology and developing novel therapeutics.
In addition to their immunosuppressive properties, Tregs were found to have regenerative abilities as well. Importantly, Dombrowski et al.Citation12 discovered that Tregs mediate remyelination in the CNS. Whether this process is affected in MS similarly to the suppressive capacity of Tregs, is not known yet. Overall, functional Tregs could have high potential of ameliorating disease in people with MS, even in progressive stages.Citation12,Citation83 The functional consequences of Treg migration across the BBB remains a black box that urgently needs to be explored.
Diapedesis of immune cells
For immune cells to exert effector functions in the CNS, they must migrate through the BBB; a process called diapedesis. Leukocytes can undergo transendothelial migration through the BBB in a series of sequential steps.Citation84,Citation85 Mediated by endothelial E- and P-selectins, cells adhere weakly to the vascular endothelial wall and start rolling. Chemokines produced by the endothelial cells increase the affinity of integrins very late antigen (VLA)-4 and leukocyte function associated antigen (LFA)-1 on leukocytes. Binding their ligands on the endothelial cells, vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1, leads to a complete arrest.Citation21,Citation36,Citation86 Immune cells start crawling against the blood flow in search for a suitable site to transmigrate,Citation87 which is also influenced by endothelial cell surface levels of ICAM-1.Citation88 Infiltrating immune cells enter the perivascular space and specifically T cells are here reactivated by local APCs. Cytokines produced by these T cells trigger BBB dysfunction and expression of matrix metalloproteinases (MMP) to degrade the glia limitans.Citation21 Together with this cytokine-induced compromised BBB, chemokine-dependent attraction of other immune cells leads to a massive leukocyte infiltration into the CNS parenchyma (). In MS, changes in protein expression are found for the adhesion molecule platelet endothelial cell adhesion molecule-1 (PECAM-1) and MMP-9 amongst others, contributing to higher immune cell migration through the BBB.Citation89,Citation90 Furthermore, research suggests that oxidative stress and nitric oxide play a role in the loss of BBB integrity in MS.Citation91,Citation92 Lastly, Nishihara et al. recently showed an intrinsic impairment of BBB-EC-like cells derived from people with MS.Citation93 Altogether, BBB impairment is a key component of MS pathology early in the disease. Full understanding of BBB disturbances and immune cell migration, including interactions between both compartments, is of high importance to develop novel, more specific treatments for MS.
Migration process of Tregs across BBB
Tregs are crucial in moderating the neuroinflammatory response and show remyelinating functions in MS animal models. Hence, it is interesting to investigate how these cells migrate through the BBB in MS and whether they exert certain effects on the migration of other immune cells through the BBB.
Transendothelial migration of Tregs in MS
Tregs are hypoproliferative in vitro and form only a small section of the peripheral CD4+ T cell subset.Citation94,Citation95 As a result, Tregs must have higher migration rates to the target site for them to influence effector cells.Citation96,Citation97 This feature is confirmed in both murine and human Tregs from healthy individuals in non-inflammatory conditions, where Tregs showed enhanced migratory abilities compared to non-Treg cells.Citation96 Nevertheless, Tregs derived from people with RR-MS showed significantly impaired migration under non-inflammatory conditions, possibly explaining the following unleashed CNS inflammation. In inflammatory conditions, the proportion of migrated Tregs was restored, similar to control conditions. During the remission phase of EAE, Tregs accumulate in the murine CNS.Citation86,Citation96 In this phase, ICAM-1 was shown to be upregulated ().Citation86 Inhibition of ICAM-1 during this phase aggravated the clinical symptoms in EAE animals, suggesting a role for LFA-1/ICAM-1 interaction in Treg infiltration in the CNS. The molecular interactions between T cells and the BBB are similar for T effector cells and Tregs. Yet, they possibly occur at different stages of the disease course given that inhibition of ICAM-1 at the stage of disease progression had a tempered disease course.Citation86 Additionally, LFA-1 is highly expressed on FOXP3+ Tregs.Citation96 Mice deficient in LFA-1 showed an increased demyelination and increased numbers of autoreactive T cells, leading to more severe EAE.Citation98,Citation99 This suggests that Tregs make use of LFA-1 for their entry into the CNS during EAE.Citation100
Figure 2. Summary of Treg migration in healthy/remission state vs. relapsing/progressive MS. (a) in healthy conditions or remitting MS, Tregs cause decreased autoreactive T cell migration by downregulating the CXCR3 receptor. Additionally, Treg IL-10 and IL-35 downregulate the CCR2 receptor on mast cells and consequently cause decreased mast cell transendothelial migration. Tregs mainly migrate through interactions of the LFA-1 and CCR6 receptor. (b) in relapsing MS, autoreactive T cells and mast cells show an increased migration. Tregs migrate in response to chemokines derived from the inflamed CNS (CCL1, CCL20, CXCL10). CCR, C-C chemokine receptor; CNS, central nervous system; CXCL, C-X-C motif chemokine ligand; CXCR, C-X-C motif chemokine receptor; EAE, experimental autoimmune encephalomyelitis; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; LFA-1, leukocyte function associated antigen-1; MS, multiple sclerosis; Treg, regulatory T cell. Figure created with Biorender.com.
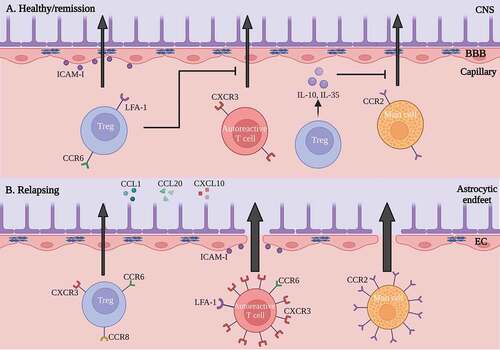
During inflammation, Tregs express transcription factors that define Th subsets and cytokine production.Citation101,Citation102 It is suggested that this enables Tregs to also express typical chemokine receptors of Th1 and Th17 cells to follow them to the site of inflammation.Citation101 Examples are chemokine receptor C-X-C motif chemokine receptor 3 (CXCR3) for Th1 cells and C-C motif chemokine receptor type 6 (CCR6) for Th17 cells. The CXCR3 is a plausible mediator for Treg chemotaxis ().Citation103 In the broad context of inflammation, CXCR3 is expressed on Tregs,Citation104,Citation105 while one of the ligands for this receptor, C-X-C motif chemokine ligand 10 (CXCL10), is upregulated in EAE and MS lesions.Citation103 Inhibition of CXCR3 led to a decrease in Tregs present in EAE lesions and failure to recover from the disease. This suggests a role for this receptor-ligand interaction in the migration of Tregs across the BBB. On the other hand, CXCR3 is also an important mediator in the accumulation of Th1 cells in MS, as CXCR3+ T cells are increased in the CSF of people with MS and in active MS lesions.Citation106–108
Furthermore, CCR6 is also involved in the migration of Tregs ().Citation108,Citation109 In EAE, both Tregs and Th17 cells express CCR6, attracting them to the inflamed CNS in response to the ligand C-C motif chemokine ligand 20 (CCL20). Indeed, CCR6+ Tregs are effector-memory cells which accumulate in the CNS during inflammation after induction of EAE.Citation110 CCR6-deficient mice show an abnormal EAE disease course with a delayed onset, yet a higher clinical score in later disease stages.Citation111 This could be attributed to the decreased Treg migration to the CNS at the peak phase of the disease. These results suggest the role of CCR6 as an important chemokine receptor for Th17 cell recruitment at onset of EAE, while CCR6 is important for Treg migration at later time points. These findings highlight the complexity of these molecular interactions at different stages of the disease.
In addition, CCR8 is expressed on Tregs, guiding these cells to the CNS in EAE ().Citation112 Ligands of this receptor, e.g. CCL1, are produced by activated T cells and APCs in inflamed tissues, creating a chemotactic gradient. Interactions between CCR8 and CCL1 increase the suppressive capacity of human Tregs by upregulating FOXP3, CD39, IL-10 and granzyme B.Citation113 When administering a CCL1 antibody fusion protein to the EAE model, CCR8+ Treg proliferation was enhanced, leading to an effective suppression of the disease. This role of CCR8 was also confirmed in graft-versus-host disease (GvHD), where CCR8-/- Tregs showed to be incompetent in the prevention of this disease.Citation114 Although they are not Treg specific, all of these molecules might be interesting targets for manipulation of Treg migration in future Treg-based therapies.Citation115
Finally, it is important to realize that not all Tregs are immunomodulatory. A small part of the Treg population consists of pro-inflammatory CD49d+ cells.Citation116 CD49d is the α4-β1 integrin receptor, also known as VLA-4, which is an important adhesion molecule for the diapedesis of leukocytes into inflamed tissue.Citation117 In people with RR-MS, a higher frequency of Tregs in peripheral blood expressed CD49d compared to healthy controls or people with SP-MS.Citation61 These results might partially explain the therapeutic efficacy of natalizumab in people with RR-MS, an antibody treatment targeted against this α4-β1 integrin receptor.Citation118–120 This treatment will cease the transmigration of pro-inflammatory effector cells to the inflamed CNS without affecting CD49d- Treg migration, leading to a restored effector/suppressor balance in the CNS.Citation116
Tregs influence migration of pathogenic cells in MS
Next to affecting the local immune response, Tregs can also influence other immune cells in the diapedesis process. Tischner et al.Citation121 describe that polyclonal expansion of Tregs inhibits the infiltration of effector T cells in the CNS of EAE animals without migrating themselves (). Tregs rather implemented their suppressive effect in the secondary lymphoid organs, where they suppressed the secretion of IFN-γ by encephalitogenic T cells, reducing their expression of CXCR3. This leads to a compromised migration of these Th1 cells in the CNS. Additionally, with the expansion of Tregs, the BBB was limitedly damaged and remained more intact, indicating a Treg-driven protective effect on the BBB. Enhancement of Tregs also decreases the migration of mast cells through the BBB via a down-regulation of CCR2 and several adhesion molecules.Citation122 Interestingly, Tregs were shown to inactivate mast cells through cell-to-cell interactions and secretion of IL-10 and IL-35 (). In the CNS, mast cells can attract other leukocytes to the site of inflammation by secreting the cytokines IL-1β and IL-8. Hence, an increase in Tregs indirectly leads to a decrease in damaging leukocytes in the CNS and a milder disease course.
Once T effector cells cross the BBB and infiltrate the brain parenchyma, Tregs also influence their local motility. In the absence of Tregs, T effector cells showed a decreased velocity and were more stationary in the CNS of EAE mice.Citation123 In the presence of Tregs, increased velocities of Th17 cells were observed, indicating decreased Th17 cell-APC interactions.Citation124
Therapeutic potential of Tregs
Since current therapies are primarily based on delaying disease progression and reducing relapse rate, there is a high need for new and curative treatments specifically targeted at the disease pathology of MS. As previously mentioned, Tregs are of great importance in lowering damaging effects of T effector cells and even show regenerative functions, making them an interesting target for the development of new MS therapies. Most clinical trials using Treg-based therapies have been performed in people with GvHD. Both people suffering from acute and chronic GvHD benefited from this therapy.Citation125,Citation126 Symptoms of people with chronic GvHD stabilized or even improved after the treatment.Citation127 People with leukemia experienced reduced posttransplant relapse rates after Treg therapy.Citation128 For other (auto)immune diseases such as Crohn’s diseaseCitation129 (and NCT03185000), type 1 diabetesCitation130,Citation131 (and NCT02691247 and NCT02932826), amyotrophic lateral sclerosis (ALS)Citation132 (and NCT04055623 and NCT03241784) and coronavirus disease 2019 (COVID-19)Citation133 (and NCT04468971), Treg-based therapies are also in clinical trials. Results from these studies demonstrated safety and tolerability for the therapy. In the field of MS research, only one clinical trial testing the effect of a Treg-based therapy has been conducted. A phase 1b/2a clinical trial including 14 people with RR-MS was recently performed.Citation11 Eleven participants received ex vivo expanded Tregs intravenously, while three others received freshly isolated Tregs intrathecally. None of them showed adverse effects after administration. Interestingly, disease progression was halted in all people who received Tregs intrathecally. On the other hand, people who received Tregs intravenously still experienced relapses or deterioration of their disability.Citation11 Although the trial only included a low participant number, these results suggest that in people with MS, Tregs might not get into the brain in high enough amounts regardless the expression of important chemokine receptors and adhesion molecules. This is in line with the observation that not always FOXP3+ Tregs can be found in the brain lesions of people with MS.Citation62 In addition, Treg functionality in the brain is also still under investigation and might also explain the lack of FOXP3 expression in the CNS. When these questions are resolved, Treg-based therapies could have great potential in treating MS.
Like natalizumab, other therapies inhibiting the migration of B and T cells exist. Ozanimod, a sphingosine-1-phosphate receptor-1 (S1P1) modulator, has recently been approved for the treatment of RR-MS.Citation134 S1P1 is important in the migration of T and B cells into the blood circulation. Additional research is being conducted, where ozanimod is tested on its ability to prevent migration of immune cells through the BBB and reduce cytokine-mediated breakdown of the BBB (NCT05245344).
A possible approach for Treg-based therapy is to expand and adapt the patient’s Tregs ex vivo, followed by adoptive transfer of these cells to the patient.Citation75 A myelin-specific, single-chain Fv chimeric antigen receptor (CAR) was designed which can be expressed by Tregs.Citation135 These Tregs are specific for myelin basic protein (MBP) and myelin oligodendrocyte glycoprotein (MOG). Administration of these CAR Tregs ameliorated disease progression of EAE in mice. Another alternative is to increase the functionality of Tregs in vivo. In preclinical studies, the neutralization of IL-6 was disease-reducing, possibly resulting from a higher Treg activity.Citation74,Citation136 Many other possibilities of enhancing Treg functionality ex vivo have been proposed, including viral vector-mediated approaches and CRISPR-Cas9 gene-editing tools.Citation10
Many preclinical studies in EAE have already shown the therapeutic potential of Tregs.Citation86,Citation121,Citation137 None of these preclinical therapies thus far consider Treg migration across the BBB. To increase the number of Tregs in the brain, one could consider increasing the expression of chemokine receptors (CXCR3, CCR6, CCR8) on Tregs, while ensuring their stability in vivo.Citation10,Citation138 This would result in an increased local T effector cell suppression and increased remyelination.
Conclusion
Not much is known about the specific interactions between the endothelial cells of the BBB and Tregs in MS. This might be interesting when talking about a potential Treg cell-based therapy, since functional Tregs may ameliorate disease course once they have entered the CNS. Some studies indicated a dysfunctional Treg migratory capacity in people with RR-MS. In EAE, an accumulation of Tregs but lack of suppression in the CNS lesions was observed. Different receptors, such as ICAM-1, CXCR3, CCR6, and CCR8 have been proposed regarding the chemotaxis of Tregs, leading them across the BBB. It was also shown that different interactions may play a role in the transendothelial migration of Tregs at different times in the disease course. This leads to the realization that there is still a huge lack of knowledge regarding different phases and time points of the disease and how important these may be in finding novel therapies for MS. Additionally, Tregs have the potential to decrease the migratory capacity of effector cells to the CNS. These results lead to believe that Tregs could be used as a therapeutic opportunity, while bearing in mind the difficulties of Tregs crossing the BBB when administering them intravenously. That being said, many knowledge gaps still exist in this domain, such as elucidation about the migratory capacity of Tregs in MS, the capacity of Tregs to possibly repair the BBB disruption during remitting phases of MS, Tregs affecting B cell migration, and many other topics. Addressing these have great potential to further the quest toward a curative MS treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, et al. Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult Scler. 2020;26(14):1816–10. doi:10.1177/1352458520970841.
- Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–58. doi:10.1038/nri3871.
- Bishop M, Rumrill PD, Bishop M, Rumrill PD. Multiple sclerosis: etiology, symptoms, incidence and prevalence, and implications for community living and employment. Work. 2015;52(4):725–34. doi:10.3233/wor-152200.
- Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol. 2018;9(3116). doi:10.3389/fimmu.2018.03116.
- Doshi A, Chataway J. Multiple sclerosis, a treatable disease. Clin Med (Lond). 2016;16(Suppl 6):s53–59. doi:10.7861/clinmedicine.16-6-s53.
- Duffy SS, Keating BA, Moalem-Taylor G. Adoptive transfer of regulatory T cells as a promising immunotherapy for the treatment of multiple sclerosis. Front Neurosci. 2019;13(1107). doi:10.3389/fnins.2019.01107.
- Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases. 2015;3(7):545–55. doi:10.12998/wjcc.v3.i7.545.
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–20. doi:10.1056/NEJMoa1606468.
- Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, Vermersch P, Arnold DL, Arnould S, Scherz T, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (expand): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–73. doi:10.1016/s0140-6736(18)30475-6.
- Baeten P, Van Zeebroeck L, Kleinewietfeld M, Hellings N, Broux B. Improving the efficacy of regulatory T cell therapy. Clin Rev Allergy Immunol. 2022;62:1–19. doi:10.1007/s12016-021-08866-1.
- Chwojnicki K, Iwaszkiewicz-Grześ D, Jankowska A, Zieliński M, Łowiec P, Gliwiński M, Grzywińska M, Kowalczyk K, Konarzewska A, Glasner P, et al. Administration of CD4+CD25highCD127−FOXP3+ Regulatory T Cells for Relapsing-Remitting Multiple Sclerosis: a Phase 1 Study. BioDrugs. 2021;35(1):47–60. doi:10.1007/s40259-020-00462-7.
- Dombrowski Y, O’Hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, Fleville S, Eleftheriadis G, Zhao C, Naughton M, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat Neurosci. 2017;20(5):674–80. doi:10.1038/nn.4528.
- McIntyre LL, Greilach SA, Othy S, Sears-Kraxberger I, Wi B, Ayala-Angulo J, Vu E, Pham Q, Silva J, Dang K, et al. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol Dis. 2020;140:104868. doi:10.1016/j.nbd.2020.104868.
- Plaisted WC, Zavala A, Hingco E, Tran H, Coleman R, Lane TE, Loring JF, Walsh CM, Chen HCI. Remyelination is correlated with regulatory T cell induction following human embryoid body-derived neural precursor cell transplantation in a viral model of multiple sclerosis. PLoS One. 2016;11(6):e0157620. doi:10.1371/journal.pone.0157620.
- Dittmer M, Young A, O’Hagan T, Eleftheriadis G, Bankhead P, Dombrowski Y, Medina RJ, Fitzgerald DC. Characterization of a murine mixed neuron-glia model and cellular responses to regulatory T cell-derived factors. Mol Brain. 2018;11(1):25. doi:10.1186/s13041-018-0367-6.
- Wang J, Xie L, Yang C, Ren C, Zhou K, Wang B, Zhang Z, Wang Y, Jin K, Yang GY. Activated regulatory T cell regulates neural stem cell proliferation in the subventricular zone of normal and ischemic mouse brain through interleukin 10. Front Cell Neurosci. 2015;9:361. doi:10.3389/fncel.2015.00361.
- Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature. 2019;2019565(7738):246–50. doi:10.1038/s41586-018-0824-5.
- Kipnis J, Avidan H, Caspi RR, Schwartz M. Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: a dialogue with microglia. Proc Natl Acad Sci U S A. 2004;101(Suppl 2(Suppl 2)):14663–69. doi:10.1073/pnas.0404842101.
- Cramer SP, Simonsen H, Frederiksen JL, Rostrup E, Larsson HBW. Abnormal blood–brain barrier permeability in normal appearing white matter in multiple sclerosis investigated by mri. NeuroImage. 2014;4:182–89. doi:10.1016/j.nicl.2013.12.001.
- Engelhardt B, Coisne C. Fluids and barriers of the CNS establish immune privilege by confining immune surveillance to a two-walled castle moat surrounding the cns castle. Fluids Barriers CNS. 2011;8(1):4. doi:10.1186/2045-8118-8-4.
- Ortiz GG, Pacheco-Moisés FP, Macías-Islas M, Flores-Alvarado LJ, Mireles-Ramírez MA, González-Renovato ED, Hernández-Navarro VE, Sánchez-López AL, Alatorre-Jiménez MA. Role of the blood-brain barrier in multiple sclerosis. Arch Med Res. 2014;45(8):687–97. doi:10.1016/j.arcmed.2014.11.013.
- Burns J, Bartholomew B, Lobo S. Isolation of myelin basic protein-specific t cells predominantly from the memory T-cell compartment in multiple sclerosis. Ann Neurol. 1999;45(1):33–39. doi:10.1002/1531-8249(199901)45:1<33:AID-ART7>3.0.CO;2-G.
- Olsson T, Zhi WW, Höjeberg B, Kostulas V, Jiang YP, Anderson G, Ekre HP, Link H. Autoreactive T lymphocytes in multiple sclerosis determined by antigen-induced secretion of interferon-gamma. J Clin Invest. 1990;86(3):981–85. doi:10.1172/jci114800.
- Montes M, Zhang X, Berthelot L, Laplaud DA, Brouard S, Jin J, Rogan S, Armao D, Jewells V, Soulillou JP, et al. Oligoclonal myelin-reactive T-cell infiltrates derived from multiple sclerosis lesions are enriched in Th17 cells. Clin Immunol. 2009;130(2):133–44. doi:10.1016/j.clim.2008.08.030.
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8(5):500–08. doi:10.1038/nm0502-500.
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172(1):146–55. doi:10.2353/ajpath.2008.070690.
- Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-gamma-expressing Th17 cells in multiple sclerosis. Ann Neurol. 2009;66(3):390–402. doi:10.1002/ana.21748.
- Edwards LJ, Robins RA, Constantinescu CS. Th17/Th1 phenotype in demyelinating disease. Cytokine. 2010;50(1):19–23. doi:10.1016/j.cyto.2009.12.003.
- van Langelaar J, van der Vuurst de Vries RM, Janssen M, Wierenga-Wolf AF, Spilt IM, Siepman TA, Dankers W, Verjans G, de Vries HE, Lubberts E, et al. T helper 17.1 cells associate with multiple sclerosis disease activity: perspectives for early intervention. Brain. 2018;141(5):1334–49. doi:10.1093/brain/awy069.
- Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10(8):857–63. doi:10.1038/ni.1767.
- Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10(8):864–71. doi:10.1038/ni.1770.
- Perriard G, Mathias A, Enz L, Canales M, Schluep M, Gentner M, Schaeren-Wiemers N, Du Pasquier RA. Interleukin-22 is increased in multiple sclerosis patients and targets astrocytes. J Neuroinflammation. 2015;12(119). doi:10.1186/s12974-015-0335-3.
- Wing AC, Hygino J, Ferreira TB, Kasahara TM, Barros PO, Sacramento PM, Andrade RM, Camargo S, Rueda F, Alves-Leon SV, et al. Interleukin-17- and interleukin-22-secreting myelin-specific CD4 + T cells resistant to corticoids are related with active brain lesions in multiple sclerosis patients. Immunology. 2016;147(2):212–20. doi:10.1111/imm.12552.
- Almolda B, Costa M, Montoya M, González B, Castellano B, Villoslada P. Increase in Th17 and T-reg lymphocytes and decrease of IL22 correlate with the recovery phase of acute EAE in rat. PLoS One. 2011;6(11):e27473. doi:10.1371/journal.pone.0027473.
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human th17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13(10):1173–75. doi:10.1038/nm1651.
- Garg N, Smith TW. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015;5(9):e00362. doi:10.1002/brb3.362.
- Hermans D, Houben E, Baeten P, Slaets H, Janssens K, Hoeks C, Hosseinkhani B, Duran G, Bormans S, Gowing E, et al. Oncostatin M triggers brain inflammation by compromising blood–brain barrier integrity. Acta Neuropathol. 2022;144(2):259–81. doi:10.1007/s00401-022-02445-0.
- Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010;67(4):452–61. doi:10.1002/ana.21939.
- Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human b cell subsets and implication in multiple sclerosis. J Immunol. 2007;178(10):6092–99. doi:10.4049/jimmunol.178.10.6092.
- Jelcic I, Al Nimer F, Wang J, Lentsch V, Planas R, Jelcic I, Madjovski A, Ruhrmann S, Faigle W, Frauenknecht K, et al. Memory B cells activate brain-homing, autoreactive CD4(+) T cells in multiple sclerosis. Cell. 2018;175(1):85–100.e123. doi:10.1016/j.cell.2018.08.011.
- Cencioni MT, Mattoscio M, Magliozzi R, Bar-Or A, Muraro PA. B cells in multiple sclerosis - from targeted depletion to immune reconstitution therapies. Nat Rev Neurol. 2021;17(7):399–414. doi:10.1038/s41582-021-00498-5.
- Mancinelli CR, Rossi N, Capra R. Ocrelizumab for the treatment of multiple sclerosis: safety, efficacy, and pharmacology. Ther Clin Risk Manag. 2021;17:765–76. doi:10.2147/tcrm.S282390.
- Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–34. doi:10.1056/NEJMoa1601277.
- Vogel DYS, Vereyken EJF, Glim JE, Heijnen PDAM, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation. 2013;10(1):809. doi:10.1186/1742-2094-10-35.
- Kouwenhoven M, Teleshova N, Ozenci V, Press R, Link H. Monocytes in multiple sclerosis: phenotype and cytokine profile. J Neuroimmunol. 2001;112(1–2):197–205. doi:10.1016/s0165-5728(00)00396-9.
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14(9):1142–49. doi:10.1038/nn.2887.
- Elieh-Ali-Komi D, Cao Y. Role of mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Rev Allergy Immunol. 2017;52(3):436–45. doi:10.1007/s12016-016-8595-y.
- Janssens I, Cools N. Regulating the regulators: is introduction of an antigen-specific approach in regulatory T cells the next step to treat autoimmunity? Cell Immunol. 2020;358:104236. doi:10.1016/j.cellimm.2020.10423610.1016/j.cellimm.2020.104236.
- Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. Il-2 is essential for tgf-beta to convert naive CD4+CD25- cells to CD25+FOXP3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178(4):2018–27. doi:10.4049/jimmunol.178.4.2018.
- Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105(3):1162–69. doi:10.1182/blood-2004-03-1211.
- Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-Hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of FOXP3+ T cells into th17 cells in autoimmune arthritis. Nat Med. 2014;20(1):62–68. doi:10.1038/nm.3432.
- Bhaskaran N, Cohen S, Zhang Y, Weinberg A, Pandiyan P. Tlr-2 signaling promotes IL-17a production in CD4+CD25+FOXP3+ regulatory cells during oropharyngeal candidiasis. Pathogens. 2015;4(1):90–110. doi:10.3390/pathogens4010090.
- Mishra S, Srinivasan S, Ma C, Zhang N. CD8(+) regulatory T cell - a mystery to be revealed. Front Immunol. 2021;12(708874). doi:10.3389/fimmu.2021.708874.
- Hasan MM, Thompson-Snipes L, Klintmalm G, Demetris AJ, O’Leary J, Oh S, Joo H. CD24(hi)CD38(hi) and CD24(hi)CD27(+) human regulatory b cells display common and distinct functional characteristics. J Immunol. 2019;203(8):2110–20. doi:10.4049/jimmunol.1900488.
- Seitz C, Joly A-L, Fang F, Frith K, Gray P, Andersson J. The FOXP3 full-length isoform controls the lineage-stability of CD4+FOXP3+ regulatory T cells. Clinical Immunology. 2022;237(108957). doi:10.1016/j.clim.2022.108957.
- Sambucci M, Gargano F, De Rosa V, De Bardi M, Picozza M, Placido R, Ruggieri S, Capone A, Gasperini C, Matarese G, et al. FOXP3 isoforms and pd-1 expression by T regulatory cells in multiple sclerosis. Sci Rep. 2018;8(1):3674. doi:10.1038/s41598-018-21861-5.
- De Rosa V, Galgani M, Porcellini A, Colamatteo A, Santopaolo M, Zuchegna C, Romano A, De Simone S, Procaccini C, La Rocca C, et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat Immunol. 2015;16(11):1174–84. doi:10.1038/ni.3269.
- Du J, Wang Q, Yang S, Chen S, Fu Y, Spath S, Domeier P, Hagin D, Anover-Sombke S, Haouili M, et al. FOXP3 exon 2 controls T reg stability and autoimmunity. Sci Immunol. 2022;7(72):eabo5407. doi:10.1126/sciimmunol.abo5407.
- Li YF, Zhang SX, Ma XW, Xue YL, Gao C, Li XY, Xu AD. The proportion of peripheral regulatory T cells in patients with multiple sclerosis: a meta-analysis. Mult Scler Relat Disord. 2019;28:75–80. doi:10.1016/j.msard.2018.12.019.
- Kouchaki E, Salehi M, Reza Sharif M, Nikoueinejad H, Akbari H. Numerical status of CD4(+)CD25(+)FOXP3(+) and CD8(+)CD28(-) regulatory T cells in multiple sclerosis. Iran J Basic Med Sci. 2014;17(4):250–55. doi:10.22038/ijbms.2014.2582.
- Venken K, Hellings N, Thewissen M, Somers V, Hensen K, Rummens JL, Medaer R, Hupperts R, Stinissen P. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123(1):79–89. doi:10.1111/j.1365-2567.2007.02690.x.
- Fritzsching B, Haas J, König F, Kunz P, Fritzsching E, Pöschl J, Krammer PH, Brück W, Suri-Payer E, Wildemann B, et al. Intracerebral human regulatory T cells: analysis of CD4+ CD25+ FOXP3+ T cells in brain lesions and cerebrospinal fluid of multiple sclerosis patients. PLoS One. 2011;6(3):e17988. doi:10.1371/journal.pone.0017988.
- Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147(3):412–18. doi:10.1111/j.1365-2249.2006.03271.x.
- Danikowski KM, Jayaraman S, Prabhakar BS. Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation. 2017;14(1):117. doi:10.1186/s12974-017-0892-8.
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199(7):971–79. doi:10.1084/jem.20031579.
- Haas J, Hug A, Viehöver A, Fritzsching B, Falk CS, Filser A, Vetter T, Milkova L, Korporal M, Fritz B, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35(11):3343–52. doi:10.1002/eji.200526065.
- Kumar M, Putzki N, Limmroth V, Remus R, Lindemann M, Knop D, Mueller N, Hardt C, Kreuzfelder E, Grosse-Wilde H. CD4+CD25+FOXP3+ t lymphocytes fail to suppress myelin basic protein-induced proliferation in patients with multiple sclerosis. J Neuroimmunol. 2006;180(1–2):178–84. doi:10.1016/j.jneuroim.2006.08.003.
- Costantino CM, Baecher-Allan C, Hafler DA. Multiple sclerosis and regulatory T cells. J Clin Immunol. 2008;28(6):697–706. doi:10.1007/s10875-008-9236-x.
- Okada Y, Ochi H, Fujii C, Hashi Y, Hamatani M, Ashida S, Kawamura K, Kusaka H, Matsumoto S, Nakagawa M, et al. Signaling via toll-like receptor 4 and CD40 in b cells plays a regulatory role in the pathogenesis of multiple sclerosis through interleukin-10 production. J Autoimmun. 2018;88:103–13. doi:10.1016/j.jaut.2017.10.011.
- Knippenberg S, Peelen E, Smolders J, Thewissen M, Menheere P, Cohen Tervaert JW, Hupperts R, Damoiseaux J. Reduction in IL-10 producing b cells (breg) in multiple sclerosis is accompanied by a reduced naïve/memory breg ratio during a relapse but not in remission. J Neuroimmunol. 2011;239(1–2):80–86. doi:10.1016/j.jneuroim.2011.08.019.
- Cencioni MT, Ali R, Nicholas R, Muraro PA. Defective cd19+cd24(hi)cd38(hi) transitional b-cell function in patients with relapsing-remitting MS. Mult Scler. 2021;27(8):1187–97. doi:10.1177/1352458520951536.
- Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity. 2013;39(5):949–62. doi:10.1016/j.immuni.2013.10.016.
- Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, FOXP3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17(6):673–75. doi:10.1038/nm.2389.
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB, et al. Myelin-specific regulatory T cells accumulate in the cns but fail to control autoimmune inflammation. Nat Med. 2007;13(4):423–31. doi:10.1038/nm1564.
- McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175(5):3025–32. doi:10.4049/jimmunol.175.5.3025.
- Ronin E, Pouchy C, Khosravi M, Hilaire M, Grégoire S, Casrouge A, Kassem S, Sleurs D, Martin GH, Chanson N, et al. Tissue-restricted control of established central nervous system autoimmunity by tnf receptor 2–expressing Treg cells. Proc Natl Acad Sci. 2021;118(13):e2014043118. doi:10.1073/pnas.2014043118.
- Kurschus FC. T cell mediated pathogenesis in EAE: molecular mechanisms. Biomed J. 2015;38(3):183–93. doi:10.4103/2319-4170.155590.
- Lassmann H, Ransohoff RM. The CD4-Th1 model for multiple sclerosis: a critical [correction of crucial] re-appraisal. Trends Immunol. 2004;25(3):132–37. doi:10.1016/j.it.2004.01.007.
- Ransohoff RM. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat Neurosci. 2012;15(8):1074–77. doi:10.1038/nn.3168.
- Lassmann H, Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 2017;133(2):223–44. doi:10.1007/s00401-016-1631-4.
- Bennett J, Basivireddy J, Kollar A, Biron KE, Reickmann P, Jefferies WA, McQuaid S. Blood-brain barrier disruption and enhanced vascular permeability in the multiple sclerosis model EAE. J Neuroimmunol. 2010;229(1–2):180–91. doi:10.1016/j.jneuroim.2010.08.011.
- Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (ms). Br J Pharmacol. 2011;164(4):1079–106. doi:10.1111/j.1476-5381.2011.01302.x.
- Jin S, Sonobe Y, Kawanokuchi J, Horiuchi H, Cheng Y, Wang Y, Mizuno T, Takeuchi H, Suzumura A. Interleukin-34 restores blood-brain barrier integrity by upregulating tight junction proteins in endothelial cells. PLoS One. 2014;9(12):e115981. doi:10.1371/journal.pone.0115981.
- Lopes Pinheiro MA, Kooij G, Mizee MR, Kamermans A, Enzmann G, Lyck R, Schwaninger M, Engelhardt B, de Vries HE. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochim Biophys Acta. 2016;1862(3):461–71. doi:10.1016/j.bbadis.2015.10.018.
- Broux B, Gowing E, Prat A. Glial regulation of the blood-brain barrier in health and disease. Semin Immunopathol. 2015;37(6):577–90. doi:10.1007/s00281-015-0516-2.
- Doerck S, Göbel K, Weise G, Schneider-Hohendorf T, Reinhardt M, Hauff P, Schwab N, Linker R, Mäurer M, Meuth SG, et al. Temporal pattern of ICAM-I mediated regulatory T cell recruitment to sites of inflammation in adoptive transfer model of multiple sclerosis. PLoS One. 2010;5(11):e15478. doi:10.1371/journal.pone.0015478.
- Steiner O, Coisne C, Cecchelli R, Boscacci R, Deutsch U, Engelhardt B, Lyck R. Differential roles for endothelial ICAM-1, ICAM-2, and VCAM-1 in shear-resistant T cell arrest, polarization, and directed crawling on blood-brain barrier endothelium. J Immunol. 2010;185(8):4846–55. doi:10.4049/jimmunol.0903732.
- Abadier M, Haghayegh Jahromi N, Cardoso Alves L, Boscacci R, Vestweber D, Barnum S, Deutsch U, Engelhardt B, Lyck R. Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood-brain barrier. Eur J Immunol. 2015;45(4):1043–58. doi:10.1002/eji.201445125.
- Losy J. Is MS an inflammatory or primary degenerative disease? J Neural Transm (Vienna). 2013;120(10):1459–62. doi:10.1007/s00702-013-1079-9.
- Blecharz KG, Haghikia A, Stasiolek M, Kruse N, Drenckhahn D, Gold R, Roewer N, Chan A, Förster CY. Glucocorticoid effects on endothelial barrier function in the murine brain endothelial cell line cend incubated with sera from patients with multiple sclerosis. Mult Scler. 2010;16(3):293–302. doi:10.1177/1352458509358189.
- Giovannoni G, Heales SJ, Land JM, Thompson EJ. The potential role of nitric oxide in multiple sclerosis. Mult Scler. 1998;4(3):212–16. doi:10.1177/135245859800400323.
- Ortiz GG, Macías-Islas MÁ, Pacheco-Moisés FP, Cruz-Ramos JA, Sustersik S, Barba EA, Aguayo A. Oxidative stress is increased in serum from Mexican patients with relapsing-remitting multiple sclerosis. Dis Markers. 2009;26(1):35–39. doi:10.3233/DMA-2009-0602.
- Nishihara H, Perriot S, Gastfriend BD, Steinfort M, Cibien C, Soldati S, Matsuo K, Guimbal S, Mathias A, Palecek SP, et al. Intrinsic blood–brain barrier dysfunction contributes to multiple sclerosis pathogenesis. Brain. 2022. doi:10.1093/brain/awac019.
- Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26.
- Fisson S, Darrasse-Jèze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon BL. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198(5):737–46. doi:10.1084/jem.20030686.
- Schneider-Hohendorf T, Stenner M-P, Weidenfeller C, Zozulya AL, Simon OJ, Schwab N, Wiendl H. Regulatory t cells exhibit enhanced migratory characteristics, a feature impaired in patients with multiple sclerosis. Eur J Immunol. 2010;40(12):3581–90. doi:10.1002/eji.201040558.
- Lee JH, Kang SG, Kim CH. FOXP3+ T cells undergo conventional first switch to lymphoid tissue homing receptors in thymus but accelerated second switch to nonlymphoid tissue homing receptors in secondary lymphoid tissues. J Immunol. 2007;178(1):301–11. doi:10.4049/jimmunol.178.1.301.
- Gültner S, Kuhlmann T, Hesse A, Weber JP, Riemer C, Baier M, Hutloff A. Reduced Treg frequency in LFA-1-deficient mice allows enhanced T effector differentiation and pathology in EAE. Eur J Immunol. 2010;40(12):3403–12. doi:10.1002/eji.201040576.
- Welsh CT, Rose JW, Hill KE, Townsend JJ. Augmentation of adoptively transferred experimental allergic encephalomyelitis by administration of a monoclonal antibody specific for LFA-1 alpha. J Neuroimmunol. 1993;43(1–2):161–67. doi:10.1016/0165-5728(93)90087-f.
- Glatigny S, Duhen R, Arbelaez C, Kumari S, Bettelli E. Integrin alpha l controls the homing of regulatory T cells during cns autoimmunity in the absence of integrin alpha 4. Sci Rep. 2015;5:7834. doi:10.1038/srep07834.
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control Th17 responses in a stat3-dependent manner. Science. 2009;326(5955):986–91. doi:10.1126/science.1172702.
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10(6):595–602. doi:10.1038/ni.1731.
- Müller M, Carter SL, Hofer MJ, Manders P, Getts DR, Getts MT, Dreykluft A, Lu B, Gerard C, King NJ, et al. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179(5):2774–86. doi:10.4049/jimmunol.179.5.2774.
- Eksteen B, Miles A, Curbishley SM, Tselepis C, Grant AJ, Walker LS, Adams DH. Epithelial inflammation is associated with ccl28 production and the recruitment of regulatory T cells expressing ccr10. J Immunol. 2006;177(1):593–603. doi:10.4049/jimmunol.177.1.593.
- Debes GF, Dahl ME, Mahiny AJ, Bonhagen K, Campbell DJ, Siegmund K, Erb KJ, Lewis DB, Kamradt T, Hamann A. Chemotactic responses of IL-4-, IL-10-, and IFN-γ-producing CD4+ T cells depend on tissue origin and microbial stimulus. J Immunol. 2006;176(1):557–66. doi:10.4049/jimmunol.176.1.557.
- Sørensen TL, Trebst C, Kivisäkk P, Klaege KL, Majmudar A, Ravid R, Lassmann H, Olsen DB, Strieter RM, Ransohoff RM, et al. Multiple sclerosis: a study of cxcl10 and cxcr3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127(1–2):59–68. doi:10.1016/s0165-5728(02)00097-8.
- Balashov KE, Rottman JB, Weiner HL, Hancock WW. Ccr5(+) and cxcr3(+) T cells are increased in multiple sclerosis and their ligands mip-1alpha and ip-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A. 1999;96(12):6873–78. doi:10.1073/pnas.96.12.6873.
- Kivisäkk P, Trebst C, Liu Z, Tucky BH, Sørensen TL, Rudick RA, Mack M, Ransohoff RM. T-cells in the cerebrospinal fluid express a similar repertoire of inflammatory chemokine receptors in the absence or presence of cns inflammation: implications for cns trafficking. Clin Exp Immunol. 2002;129(3):510–18. doi:10.1046/j.1365-2249.2002.01947.x.
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181(12):8391–401. doi:10.4049/jimmunol.181.12.8391.
- Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rötzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25+CD4+ T-cell subset. Blood. 2005;105(7):2877–86. doi:10.1182/blood-2004-07-2505.
- Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martínez AC, Varona R. CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol. 2009;39(6):1671–81. doi:10.1002/eji.200839123.
- Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors ccr4 and ccr8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194(6):847–53. doi:10.1084/jem.194.6.847.
- Barsheshet Y, Wildbaum G, Levy E, Vitenshtein A, Akinseye C, Griggs J, Lira SA, Karin N. Ccr8(+)FOXP3(+) t(reg) cells as master drivers of immune regulation. Proc Natl Acad Sci U S A. 2017;114(23):6086–91. doi:10.1073/pnas.1621280114.
- Coghill JM, Fowler KA, West ML, Fulton LM, van Deventer H, McKinnon KP, Vincent BG, Lin K, Panoskaltsis-Mortari A, Cook DN, et al. Cc chemokine receptor 8 potentiates donor Treg survival and is critical for the prevention of murine graft-versus-host disease. Blood. 2013;122(5):825–36. doi:10.1182/blood-2012-06-435735.
- Jatczak-Pawlik I, Wolinski P, Książek-Winiarek D, Pietruczuk M, Glabinski A. CCR6 blockade on regulatory T cells ameliorates experimental model of multiple sclerosis. Cent Eur J Immunol. 2020;45(3):256–66. doi:10.5114/ceji.2020.101241.
- Kleinewietfeld M, Starke M, Di Mitri D, Borsellino G, Battistini L, Rötzschke O, Falk K. CD49d provides access to “untouched” human FOXP3+ Treg free of contaminating effector cells. Blood. 2009;113(4):827–36. doi:10.1182/blood-2008-04-150524.
- Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature. 1992;356(6364):63–66. doi:10.1038/356063a0.
- Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910. doi:10.1056/NEJMoa044397.
- Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O’Connor PW. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003;348(1):15–23. doi:10.1056/NEJMoa020696.
- O’Connor PW, Goodman A, Willmer-Hulme AJ, Libonati MA, Metz L, Murray RS, Sheremata WA, Vollmer TL, Stone LA. Randomized multicenter trial of natalizumab in acute MS relapses: clinical and mri effects. Neurology. 2004;62(11):2038–43. doi:10.1212/01.wnl.0000128136.79044.d6.
- Tischner D, Weishaupt A, van den Brandt J, Müller N, Beyersdorf N, Ip CW, Toyka KV, Hünig T, Gold R, Kerkau T, et al. Polyclonal expansion of regulatory T cells interferes with effector cell migration in a model of multiple sclerosis. Brain. 2006;129(Pt 10):2635–47. doi:10.1093/brain/awl213.
- Hong GU, Kim NG, Jeoung D, Ro JY. Anti-CD40 Ab-or 8-oxo-dG-enhanced Treg cells reduce development of experimental autoimmune encephalomyelitis via down-regulating migration and activation of mast cells. J Neuroimmunol. 2013;260(1–2):60–73. doi:10.1016/j.jneuroim.2013.04.002.
- Koutrolos M, Berer K, Kawakami N, Wekerle H, Krishnamoorthy G. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the cns. Acta Neuropathol Commun. 2014;2(163). doi:10.1186/s40478-014-0163-1.
- Othy S, Jairaman A, Dynes JL, Dong TX, Tune C, Yeromin AV, Zavala A, Akunwafo C, Chen F, Parker I, et al. Regulatory T cells suppress Th17 cell Ca 2+ signaling in the spinal cord during murine autoimmune neuroinflammation. Proc Natl Acad Sci U S A. 2020;117(33):20088–99. doi:10.1073/pnas.2006895117.
- Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, Curtsinger J, Verneris MR, MacMillan ML, Levine BL, et al. Umbilical cord blood–derived T regulatory cells to prevent gvhd: kinetics, toxicity profile, and clinical effect. Blood. 2016;127(8):1044–51. doi:10.1182/blood-2015-06-653667.
- Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, Myśliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clinical Immunology. 2009;133(1):22–26. doi:10.1016/j.clim.2009.06.001.
- Theil A, Tuve S, Oelschlägel U, Maiwald A, Döhler D, Oßmann D, Zenkel A, Wilhelm C, Middeke JM, Shayegi N, et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy. 2015;17(4):473–86. doi:10.1016/j.jcyt.2014.11.005.
- Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, Pierini A, Massei MS, Amico L, Urbani E, et al. Hla-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124(4):638–44. doi:10.1182/blood-2014-03-564401.
- Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory crohn’s disease. Gastroenterology. 2012;143(5):1207–17.e1202. doi:10.1053/j.gastro.2012.07.116.
- Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7(315):315ra189. doi:10.1126/scitranslmed.aad4134.
- Dong S, Hiam-Galvez KJ, Mowery CT, Herold KC, Gitelman SE, Esensten JH, Liu W, Lares AP, Leinbach AS, Lee M, et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight. 2021;6(18). doi:10.1172/jci.insight.147474
- Thonhoff JR, Beers DR, Zhao W, Pleitez M, Simpson EP, Berry JD, Cudkowicz ME, Appel SH. Expanded autologous regulatory T-lymphocyte infusions in ALS: a phase I, first-in-human study. Neurol Neuroimmunol Neuroinflamm. 2018;5(4):e465. doi:10.1212/nxi.0000000000000465.
- Gladstone DE, Kim BS, Mooney K, Karaba AH, D’Alessio FR. Regulatory T cells for treating patients with covid-19 and acute respiratory distress syndrome: two case reports. Ann Intern Med. 2020;173(10):852–53. doi:10.7326/l20-0681.
- Lassiter G, Melancon C, Rooney T, Murat AM, Kaye JS, Kaye AM, Kaye RJ, Cornett EM, Kaye AD, Shah RJ, et al. Ozanimod to treat relapsing forms of multiple sclerosis: a comprehensive review of disease, drug efficacy and side effects. Neurol Int. 2020;12(3):89–108. doi:10.3390/neurolint12030016.
- De Paula Pohl A, Schmidt A, Zhang AH, Maldonado T, Königs C, Scott DW. Engineered regulatory T cells expressing myelin-specific chimeric antigen receptors suppress EAE progression. Cell Immunol. 2020;358:104222. doi:10.1016/j.cellimm.2020.104222.
- Gijbels K, Brocke S, Abrams JS, Steinman L. Administration of neutralizing antibodies to interleukin-6 (IL-6) reduces experimental autoimmune encephalomyelitis and is associated with elevated levels of IL-6 bioactivity in central nervous system and circulation. Mol Med. 1995;1(7):795–805. doi:10.1007/BF03401894.
- Beyersdorf N, Gaupp S, Balbach K, Schmidt J, Toyka KV, Lin CH, Hanke T, Hünig T, Kerkau T, Gold R. Selective targeting of regulatory T cells with cd28 superagonists allows effective therapy of experimental autoimmune encephalomyelitis. J Exp Med. 2005;202(3):445–55. doi:10.1084/jem.20051060.
- Baeten P, Hellings N, Broux B. In vitro tailoring of regulatory T cells prior to cell therapy. Trends Mol Med. 2020;26(11):1059–60. doi:10.1016/j.molmed.2020.08.008.
